- Department of Neurology, Shijiazhuang People's Hospital, Shijiazhuang, Hebei, China
Background: As one of the most common causes of stroke, symptomatic intracranial artery stenosis (sICAS) is a great threat to public health, and its financial burden is substantial, with annual direct high medical costs particularly in China. Currently, the long-term use of conventional dual antiplatelet therapy (DAPT) as the primary modality of treatment for sICAS decreases the risk of stroke recurrence but increases the risk of bleeding. We aimed to evaluate the efficacy and safety of low dose aspirin plus clopidogrel for the treatment of sICAS in the elderly population.
Methods: This randomized, controlled study included 181 older patients with transient ischemic attack (TIA) or ischemic stroke (IS) attributed to sICAS, who were recruited between April 2015 and November 2020. The 90 patients assigned to the low dose therapy group included aspirin, 75 mg, plus clopidogrel, 50 mg, daily for 90 and 91 patients assigned to the conventional group included aspirin, 100 mg, plus clopidogrel, 75 mg, daily for 90 days (aspirin or clopidogrel alone daily thereafter) were included in this intention-to-treat analysis. Efficacy and safety analyses were done in this trial.
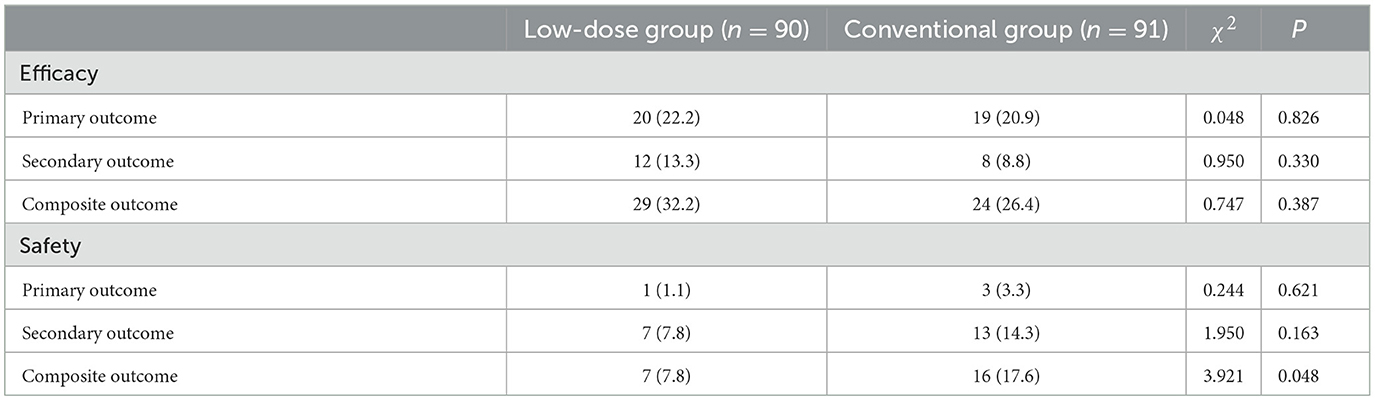
Results: One hundred eighty-one eligible elderly patients with sICAS were enrolled in this trial. The median age was 70 years ranged 60–83 years. Seventy-five participants were with TIA and 106 with IS. The median time of follow-up was 30 months ranged 1–36 months. Ninety patients were assigned randomly to the low dose group and 91 patients to the conventional group. The rate of primary, secondary and composite efficacy were not significantly different between the low dose and conventional group (P > 0.05). The rate of composite safety outcome was 7.8% (7/90) in the low dose group, which was lower than 17.6% (16/91) in the conventional group (χ2 = 3.921, P = 0.048). At the time of last follow-up, 17 (9.4%) of 181 patients developed GI injuries, which occurred in four (4.4%) of 90 patients in the low dose group and in 13 (14.3%) of 91 patients in the conventional group (χ2 = 4.058, P = 0.044). The primary efficacy outcome occurred in six (18.2%) of 33 patients with severe sICAS and in 22 (38.6%) of 57 patients with moderate sICAS (χ2 = 4.064, P = 0.044) in the low dose group.
Conclusion: In this study, the safety of low dose aspirin combined with clopidogrel proved to be equally efficient and significantly safer than those of conventional dose within 24 months in elderly patients with sICAS. However, the small size of this study limits the validity of the results. Further larger longitudinal and randomized controlled trials are necessary to evaluate the role of low dose DAPT in the patients with sICAS.
Introduction
As one of most common causes of stroke, intracranial atherosclerotic stenosis (ICAS) is prevalent among the Asians, especially in China (1). The data from the Chinese study about transient ischemic attack (TIA) and ischemic (IS) caused by symptomatic (sICAS) with a high risk recurrence indicated that the prevalence is as high as 30−46% (2, 3). Actually, ICAS is also an independent risk factor for IS in Asian and African-American patients, with high stroke recurrence rate for some pathological reasons (4). The therapeutic methods of sICAS nowadays primarily include medical management and interventional therapy with control of risk factors (5).
The mechanism of action of DAPT as medical treatment included reducing the synthesis of thromboxane A2, blocking ADP binding and inhibition of P2Y12 receptors. Through these mechanisms, antiplatelet medical therapy can prevent TIA or IS. As an immediate resuscitative therapy for sICAS, percutaneous transluminal angioplasty and stenting (PTAS) is mainly applicable to some vascular lesions with distal ischemia caused by arterial stenosis (6), with the aim to achieve the revascularization of target lesion (7). Additionally, this technique is used extensively in the revascularization of coronary artery, lower extremity artery (8, 9). PTAS is considered to modify stenosis, improve hemodynamics and further reduce stroke recurrence, but its efficacy and safety remains controversial in patients with sICAS (10). The Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) (11) and The China Angioplasty and Stenting for Symptomatic Intracranial Severe Stenosis (CASSISS) (12) provided an updated strategy that medical treatement is still the main therapeutic strategy for secondary prevention for sICAS, and do not support the addition of PTAS to medical therapy.
Moreover, despite medical therapy reducing stroke recurrence in sICAS, some patients still face a high bleeding rate. At present, there are few study on the analysis of different antiplatelet doses to evaluate the efficacy and safety in elderly patients with sICAS (10), and many researchers have attempted to improve current DAPT dose to improve safety. Based on this premise, this study aimed to evaluate the low-dose DAPT might enhance the preventive effects of conventional DAPT and address the prevalence with the risk of increasing bleeding of conventional DAPT therapy among Chinese population with sICAS.
Materials and methods
Study participants
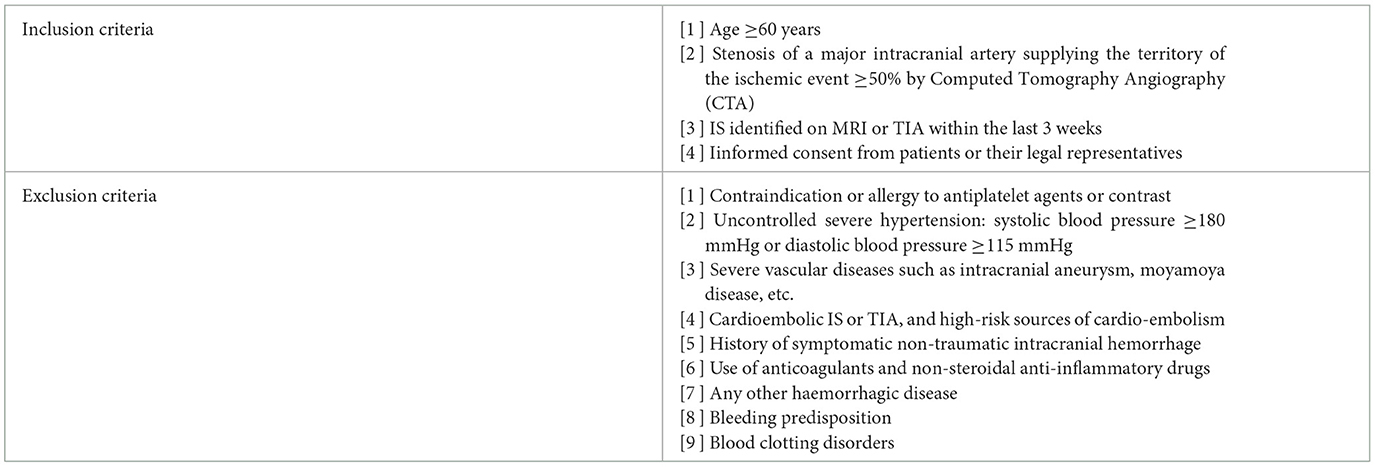
The population of this retrospective randomiazed study was enrolled in the people's hospital in Shijiazhuang, China from April 2015 to November 2020. All patients were diagnosed with sICAS measured according to the North American Carotid Endarterectomy Test criteria (13). Inclusion and exclusion criteria for the participant in the study were listed in Table 1.
Finally, a total of 181 patients diagnosed with sICAS were recruited to this study. The protocolwas reviewed and approved by the ethics committee of Shijiazhuang people's hospital (20211280). All patients or their legal representatives provided their written informed consent to participate in this study, and changes in medications were not permitted after informed consent was obtained.
Treatment
All patients were randomly assigned to the low dose group (aspirin (Bayer Healthcare Ltd.) 75 mg/day and clopidogrel (Shenzhen Xinlitai Pharmaceutical Co., Ltd.) 50 mg/day) and the conventional group (aspirin 100 mg/day and clopidogrel 75 mg/day) in a 1:1 ratio assigned by a random number table, and all patients were masked to the treatment allocation. The protocol treatment of DAPT was started after expeditious evaluation and definitive diagnosis of sICAS, for a duration of 90 days from the latest ischemic symptom onset. After that, patients were taking monotherapy antiplatelet therapy. To reduce the risk of recurrent stroke and vascular events, clinicians recommend patients enrolled in this study that high-intensity statin therapy to achieve a goal LDL < 70 mg/dl and a long-term BP target of < 140/90 mmHg in clinically stable patients. Aggressive medical management also comprises life-style management that incorporates exercise, smoking cessation and weight management.
Follow-up
Patients had to be able to visit the study department throughout the follow-up period. The visits were conducted by a combination of outpatient and telephone review. The patients all underwent CTA, transcranial Doppler ultrasound (TCD), and carotid ultrasound. The efficacy and safety outcomes were always evaluated by the physicians who were masked to treatment allocation. Follow-up intervals were at 30 days, 3 months, 6 months, and every 6 months thereafter. Patients who stopped taking the trial drugs for longer than 2 weeks were withdrawn from the trial. Participants who did not develop any event or were lost to follow-up at the last observational date were treated as censored.
Definitions and measurement
According to World Health Organization criteria, TIA was defined as acute onset of neurologic deficit, persisting for < 24 h. IS was defined as persisting >24 h, confirmed by diffusion-weighted imaging on MRI. According to American Academy of Neurology, sICAS is defined as TIA or ischemic stroke attributed to 50%−99% atherosclerotic stenosis of a major intracranial artery (14). Moderate sICAS was defined as stenosis of 50%−69% and severe ICAS was defined as stenosis of 70%−99%. A major intracranial artery was defined as the level of A2 (the postcommunicating segment of the anterior cerebral artery), M2 (the Sylvian segment of the middle cerebral artery) or P2 (the ambient segment of the posterior cerebral artery) (15). The primary efficacy outcome was defined as the first recurrence of TIA or IS. The secondary efficacy outcome was defined as hemorrhagic stroke, death from any cause, and other vascular events including myocardial infarction, pulmonary embolism, heart failure, aortic dissection and peripheral artery disease requiring hospital admission. The Composite efficacy outcome included both primary and secondary efficacy outcome. The primary safety outcome was defined as major bleeding resulting in substantial haemodynamic instability requiring hospitalization and/or blood transfusion. The secondary safety outcome was defined as gastrointestinal (GI) injury excluded major bleeding, and minor bleeding included skin mucosa gingival bleeding. GI injury was defined as dyspepsia, gastroduodenal ulcers, perforation and bleeding. The Composite safety outcome included both primary and secondary safety outcome. Coronary artery disease was defined history of angina, myocardial infarction, or prior coronary artery revascularization (9). Any event related to the outcomes was reviewed by professionals who were masked to DAPT medications. All workers who were provided access to the results were asked to sign a confidentiality agreement to ensure that the results were not disclosed before presentation of the results.
Statistical analysis
All data provided in the statistical analysis plan were analyzed using SPSS 26.0 software (SPSS, Inc., Chicago, USA). Results are summarized as means ± standard deviation and range for continuous variables and, and categorical data are summarized as counts or percentages. The data was analyzed using χ2 test for categoric variables and the t-test or F-test for continuous variables. All data were tested for normality using the K–S test. If data were not normally distributed, a M–W U-test was applied. The Kaplan–Meier method was used to calculate estimated cumulative event rates, and the treatment groups are compared using the log-rank test. A P-value of < 0.05 was considered significant.
Results analysis
Baseline characteristics
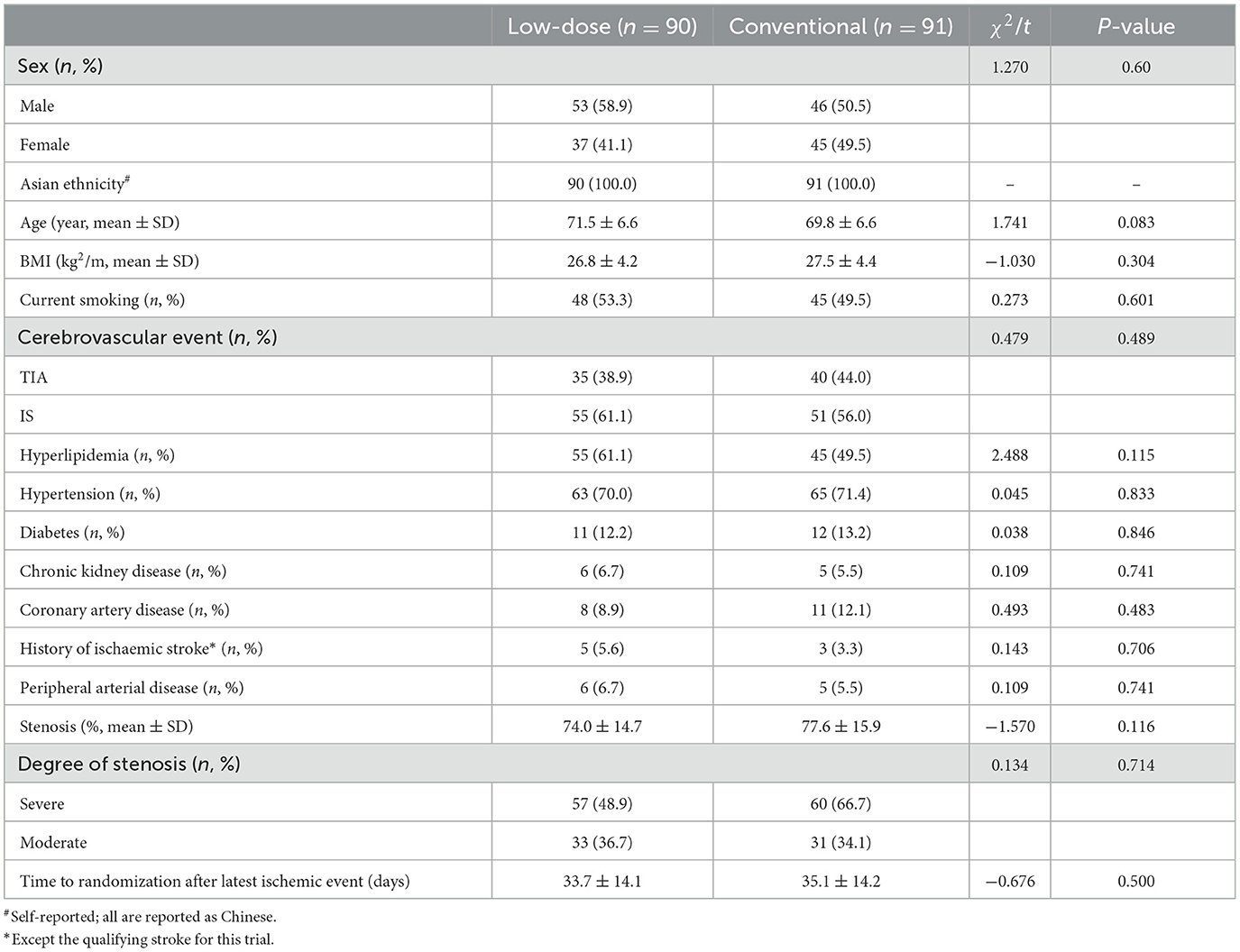
One hundred eighty-one eligible patients with sICAS, between 12 days and 61 days before the start of the protocol treatment were enrolled. The median age was 70 years (60–83). Ninety patients were assigned randomly to the low dose group and 91 patients to the conventional group. Risk factors included current smoking, hypertension, diabetes mellitus, hyperlipidemia, chronic kidney disease, peripheral arterial disease, history of ischaemic stroke, and coronary heart disease. There was no significant difference in clinical baseline characteristics between the conventional group and the low dose group (P > 0.05). Characteristics at baseline are shown in Table 2.
The median time of follow-up was 30 months (1–36). At the last time, follow-up data were available for 171 patients. Ten cases were lost during follow-up, included 6 cases in the low dose group and four cases in the conventional group. Median time to randomization after index events was 35.5 days (12–59) in low dose group and 33.0 days (12–61) in conventional group.
Efficacy and safety outcomes
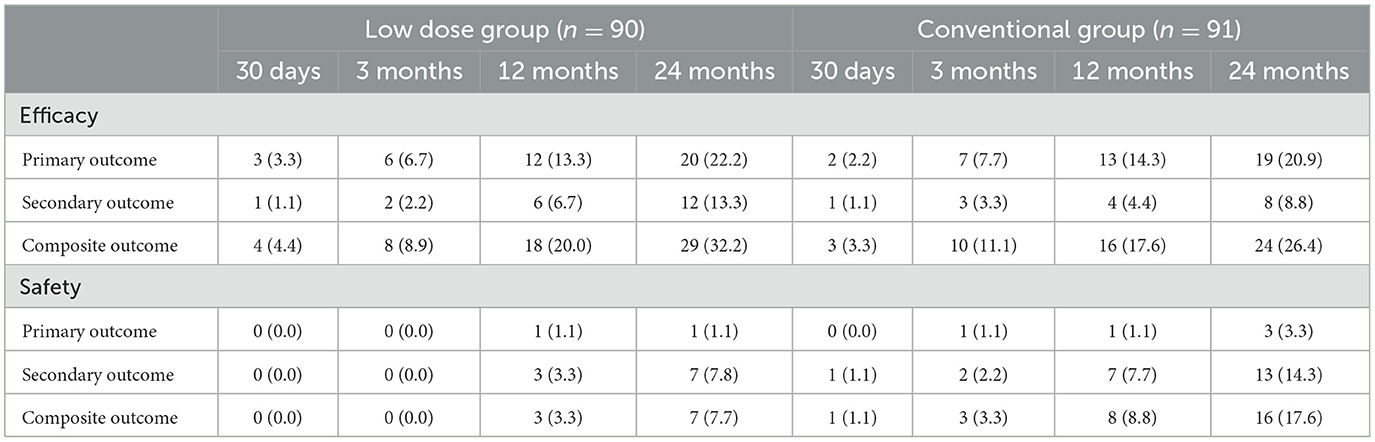
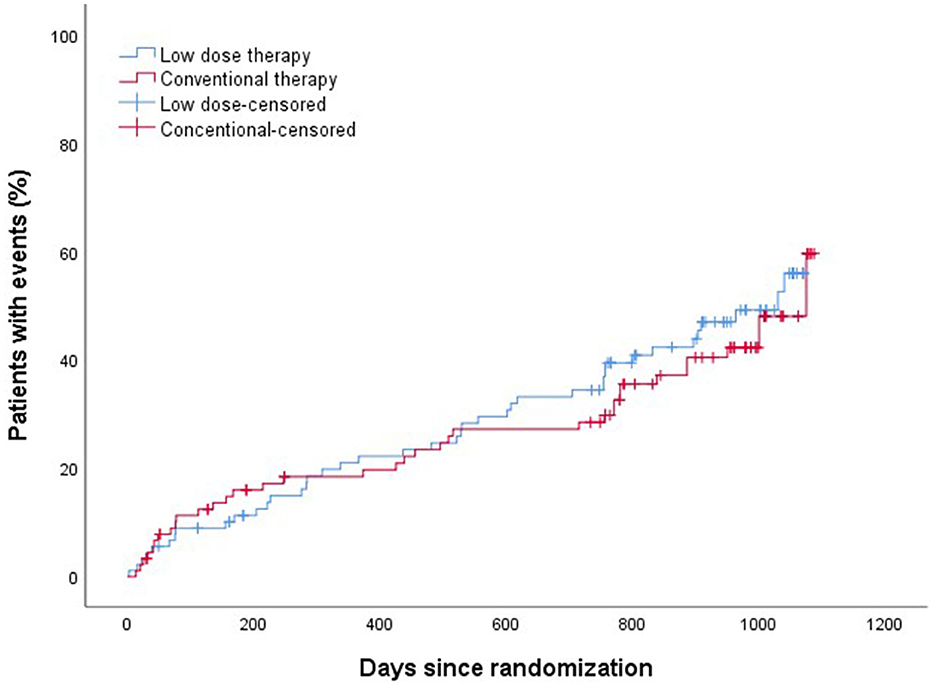
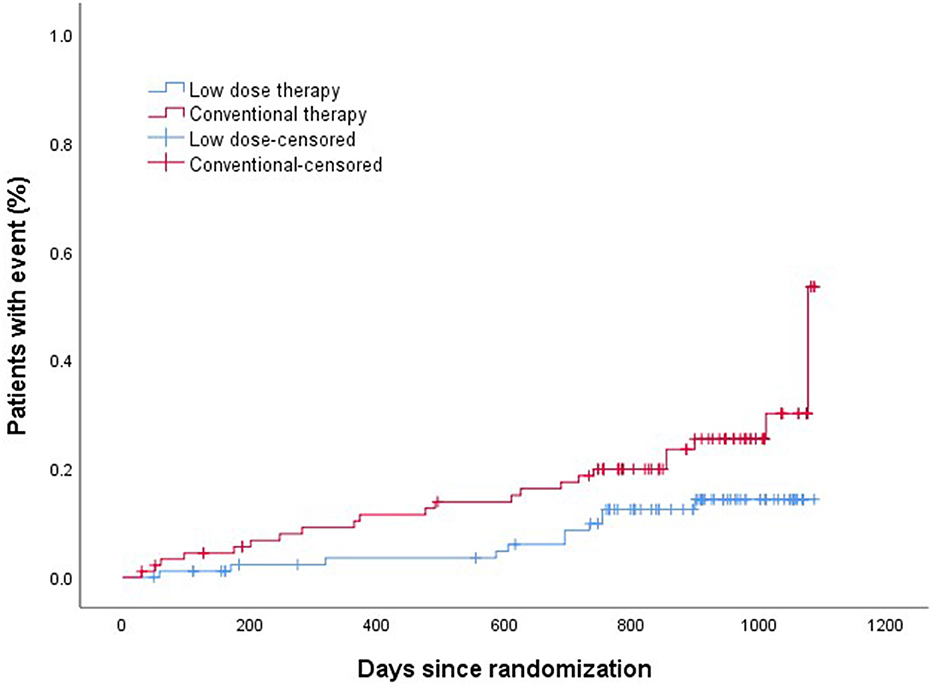
The efficacy and safety outcomes a at the 24 months of follow up were shown in Table 2. The efficacy and safety outcomes in the low dose group compared with the conventional group at the 24 months were shown in Table 3 and Figures 1, 2. The rate of primary, secondary and composite efficacy were not significantly different between the low dose and conventional group (P > 0.05). The rate of composite safety outcome was 7.8% (7/90) in the low dose group, which was lower than 17.6% (16/91) in the conventional group (χ2 = 3.921, P = 0.048).
Evaluation of gastrointestinal injuries
At the time of last follow-up, 17 (9.4%) of 181 patients developed GI injuries, which occurred in four (4.4%) of 90 patients in the low dose group and in 13 (14.3%) of 91 patients in the conventional group (χ2 = 4.058, P = 0.044). Major bleeding occurred in 1 (1.1%) of 90 patients than in the low dose group and in three (3.3%) of 91 patients in the conventional group, all of them recovered from their illness. In addition to this, two (2.2%) cases of minor bleeding and one (1.1%) case of gastric ulcer occurred in the low dose group (Table 4). Six cases of minor bleeding, two (2.2%) cases of gastric ulcer, one (1.1%) case of gastric perforation because of ulcer, one (1.1%) case of dyspepsia and one (1.1%) case of abdominal pain occurred in the conventional group.
Efficacy and safety outcomes of sICAS with different stenosis
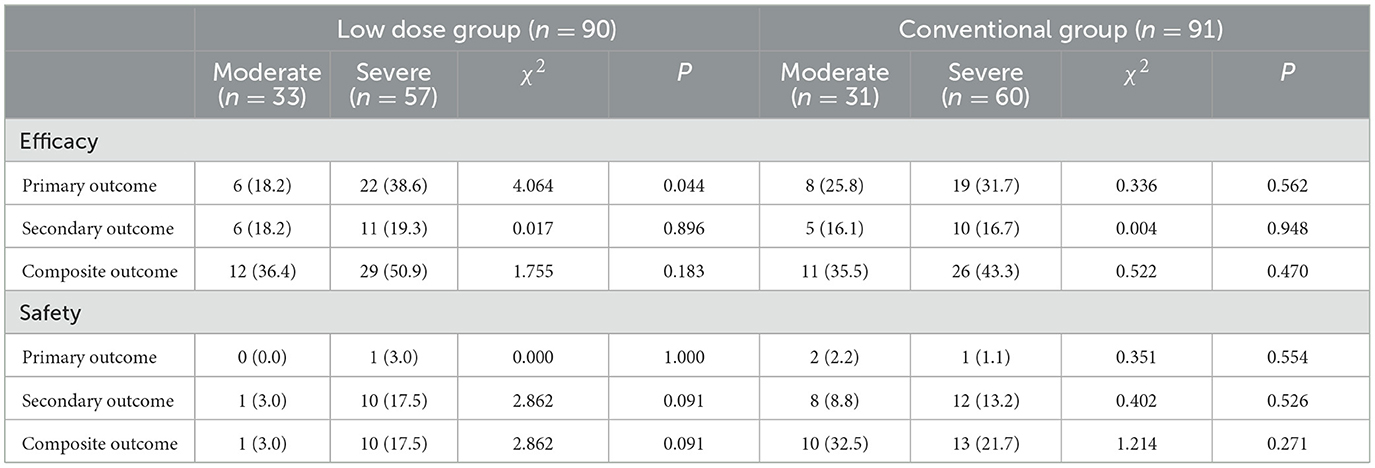
At the time of last follow-up, in the low dose group, the primary efficacy outcome occurred in six (18.2%) of 33 patients with severe sICAS and in 22 (38.6%) of 57 patients with moderate sICAS (χ2 = 4.064, P = 0.044). The rate of secondary and composite efficacy outcomes were higher in severe sICAS group (P > 0.05). In the conventional group, the rate of primary, secondary and composite efficacy outcomes were higher in severe sICAS group (P > 0.05). The rate of composite safety outcome with severe sICAS was higher in the low dose group and lower in the conventional group (P > 0.05; Table 5).
Discussion
The treatment of sICASis a hot issue of clinical research, but the choice between medical therapy and PTAS has been controversial. Studies found that PTAS can partially restore neurological function and reduce mortality in patients with sICAS, but complications related to ischemia-reperfusion, embolic dislodgement, and bleeding can increase recurrence and mortality, seriously affecting the clinical outcomes (16). The CASSISS trial (12) concluded that the addition of PTAS to medical therapy, compared with medical therapy alone, resulted in no significant difference in the risk of stroke or death within 30 days among patients with TIA or IS due to sICAS. To date, studies have demonstrated the benefit of DAPT for primary and secondary prevention of TIA or IS, and for patients with sICAS, it is recommended that DAPT should be applied in early stage and long-term after index events (5). Given the comparable benefit of aspirin in the range of 50–325 mg/day for the prevention of IS (17), Liu et al. (18) found the greatest benefit of DAPT for 90 days in patients with sICAS. In combination with the advanced age of patients, this single-center observational study was to evaluate the efficacy and safety outcomes of low dose DAPT for the treatment of elderly patients with sICAS. Our aim was to test the efficacy and safety of the low dose DPAT treatment in a controlled analysis of patients with sICAS, and this study shown that the recurrence of TIA or IS was similar with the low dose therapy than conventional dose, and safety outcome of the low dose group are better than conventional group. In this study of secondary stroke prevention in patients with sICAS, we found no evidence of a higher risk of recurrence of adverse events with low dose than conventional therapy. The incidence of the efficacy outcomes in the low dose and conventional group was almost same, and the reoccurrence rates of TIA or IS at 12 and 24 months were 13.3% (12/90) and 22.2% (20/90) in the low dose group and 14.3% (13/91) and 20.9% (19/91) in the conventional group, respectively, both of which and other efficacy outcomes were higher than those previously reported (15, 19). This outcome can be attributed to the following: first, all patients in this trial were elderly. Persoon et al. (20) reported that age was a risk factor for the first or recurrence of sICAS, which was considered that the blood vessels of elderly patients become both sclerotic and stenotic. Second, the patients with TIA were selected for the present study that increased the rate of adverse event rates. Finally, conventional DAPT could prevent stenosis progression of patients with sICAS, and it may have no this effect by the low dose DAPT in this study, although the change in arterial stenosis during the follow-up period was not assessed in this trial. Bleeding from DAPT use most frequently occurred in the digestive system (21). Long-term use the antiplatelet therapy and aging itself can increase risk of GI bleeding (22). It is know that GI bleeding and ulceration from DAPT use increase in severity and frequency with increasing age, and NSAID use as exclusion criteria in this trial increases the risk of GI bleeding in the elderly four folds (23). In this analysis, the incidence of bleeding as a core safety outcome rate in low dose group was significantly lower than that in the conventional group, although their low incidences might weaken statistical power. Another common adverse event of DAPT in elderly patients isGI injury. The incidence of GI injury in this study was 14.3% (13/91) in the conventional group compared to4.4 (4/90) in the low dose group (P < 0.05), which may be associated with increased GI toxicity due to high dosage of DAPT. Tegos et al. (24) and Kasner et al. (25) reported a linear positive correlation between the severity of ICAS and the incidence of stroke. Our result was highly consistent with the findings in above mentioned studies.
The evolution of other medical treatment has advanced improving the efficacy and safety outcomes of sICAS. Toyoda et al. (15) reported that the combination of cilostazol with aspirin or clopidogrel had a reduced incidence of IS recurrence and a similar risk of severe or life-threatening bleeding. Amarenco et al. (26) concluded that ticagrelor was superior to aspirin at preventing stroke in a prespecified exploratory analysis. These findings provided effective therapeutic options to prevent adverse events. However, guidelines do not prove the final of submitted version of taking these drug until now.
Patients with sICAS are often comorbid with risk factors correlated with stroke recurrence, including hypertension, metabolic disorders, dyslipidaemia (4). Our study also support the management of vascular risk factors to reduce the risk of recurrent stroke and vascular events. Evidence for the use of high-intensity statins is applicable to patients with sICAS (27, 28). Analyses form Chinese Intracranial Atherosclerosis have demonstrated that a mean systolic BP < 140 mm Hg was associated with a good follow-up outcome, among clinically stable patients with sICAS (29).
Despite the emerging research results, there still persist lots of problems and concerns in clinical practice. For example, this medical therapy for secondary prevention of sICAS in this study is mainly concentrated on population of urban areas. To better balance medical resources, the population of countryside patients should be included in this study. However, the majority of this patients with are TIA or IS has poor compliance with the DAPT secondary prevention (30), and neurological care services need to be enhanced at the country-level hospitals to improve health care delivery.
Study limitations
There are several limitations in this study. First, this was a retrospective observational study involving a small cohort and the risk of all sorts of bias and confounding is substantial, which limited external validity of any finding or conclusion. Multivariate analysis could not be done to the relatively small size in a single center. Second, the trial treatment was not masked. Thus, the trial investigators were aware of the study drug allocation. Third, long-term of follow-up data which is important indicator of therapy success are not available. Finally, generalizability of the present finding to other countries is uncertain because the ethnicity was limited to Chinese people within this research.
Conclusions
This study represents a conceptual advance over previously published work. The low dose of DAPT involving aspirin plus clopidogrel was similar effective but safer than the conventional dose during non-cardioembolic IS or TIA with sICAS. The results of the present study support the use of the low dose of DAPT in elderly patients with sICAS. In addition, the study found that the recurrence rate of IS or TIA in patients with severe symptoms of ICAS is higher than that in elderly patients with moderate symptoms of ICAS in the two groups during fellow-up. Further studies should be performed to evaluate the efficacy and safety of low dose DAPT in a long-term follow-up.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Shijiazhuang People Hospital of the Ethics Committee (No. 2021031). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Z-yW and Z-cS participated in the design of this study. H-xS drafted and wrote the main manuscript text. BZ and SL collected clinical and demographic data. JY and JC performed the statistical analysis. H-lL prepared Tables 1–5 and Figures 1, 2. All authors critically reviewed the manuscript and agreed on this final version to be submitted to the journal.
Funding
This study was funded by the Medical Science Research Project of the Health Commission of Hebei Province, China (No. 20211280). The funding body played no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
DAPT, dual antiplatelet therapy; BP, blood pressure; PTAS, percutaneous transluminal angioplasty and stenting; sICAS, symptomatic intracranial atherosclerotic arterial stenosis; IS, ischemic stroke; TIA, transient ischemic attack; GI, gastrointestinal.
References
1. Wu S, Wu B, Liu M, Chen Z, Wang W, Anderson CS, et al. Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. (2019) 18:394–405. doi: 10.1016/S1474-4422(18)30500-3
2. Derdeyn CP, Chimowitz MI, Lynn MJ, Fiorella D, Turan TN, Janis LS, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet. (2014) 383:333–41. doi: 10.1016/S0140-6736(13)62038-3
3. Liu D, Liu J, Cai Y, Wong KSL, Liu L. Is the future of symptomatic intracranial atherosclerotic stenosis management promising? J Neurol Neurosurg Psychiatry. (2020) 91:122–4. doi: 10.1136/jnnp-2019-321564
4. Waters MF, Hoh BL, Lynn MJ, Kwon HM, Turan TN, Derdeyn CP, et al. Factors associated with recurrent ischemic stroke in the medical group of the SAMMPRIS trial. JAMA Neurol. (2016) 73:308–15. doi: 10.1001/jamaneurol.2015.4315
5. Kim JS, Bang OY. Medical treatment of intracranial atherosclerosis: an update. J Stroke. (2017) 19:261–70. doi: 10.5853/jos.2017.01830
6. Zhou R, Zhai H, Yin Z, Cui J, Hu N. Virtual reality-assisted percutaneous transluminal angioplasty for interventional treatment of lower-extremity arteriosclerosis obliterans. J Healthc Eng. (2021) 2021:9975583. doi: 10.1155/2021/9975583
7. Schroeder H, Werner M, Meyer DR, Reimer P, Krüger K, Jaff MR, et al. Low-dose paclitaxel-coated versus uncoated percutaneous transluminal balloon angioplasty for femoropopliteal peripheral artery disease: one-year results of the ILLUMENATE European randomized clinical trial (randomized trial of a novel paclitaxel-coated percutaneous angioplasty balloon). Circulation. (2017) 135:2227–36. doi: 10.1161/CIRCULATIONAHA.116.026493
8. Heinrich A, Engler MS, Güttler FV, Matthäus C, Popp J, Teichgräber UK. Systematic evaluation of particle loss during handling in the percutaneous transluminal angioplasty for eight different drug-coated balloons. Sci Rep. (2020) 10:17220. doi: 10.1038/s41598-020-74227-1
9. Liu P, Zheng L-H, He X-Q, Yang Y, Zhang L-K, Zhang L, et al. Midterm outcomes of endovascular therapy for TASC II D femoropopliteal lesions with critical limb ischemia: a retrospective analysis. Ann Vasc Surg. (2023) 88:182–90. doi: 10.1016/j.avsg.2022.08.004
10. Turan TN, Cotsonis G, Lynn MJ, Wooley RH, Swanson S, Williams JE, et al. Intracranial stenosis: impact of randomized trials on treatment preferences of US neurologists and neurointerventionists. Cerebrovasc Dis. (2014) 37:203–11. doi: 10.1159/000358120
11. Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. (2011) 365:993–1003. doi: 10.1056/NEJMoa1105335
12. Gao P, Wang T, Wang D, Liebeskind DS, Shi H, Li T, et al. Effect of stenting plus medical therapy vs medical therapy alone on risk of stroke and death in patients with symptomatic intracranial stenosis: the CASSISS randomized clinical trial. JAMA. (2022) 328:534–42. doi: 10.1001/jama.2022.18918
13. Ross EG, Mell MW. Evaluation of regional variations in length of stay after elective, uncomplicated carotid endarterectomy in North America. J Vasc Surg. (2020) 71:536–44.e7. doi: 10.1016/j.jvs.2019.02.071
14. Turan TN, Zaidat OO, Gronseth GS, Chimowitz MI, Culebras A, Furlan AJ, et al. Stroke prevention in symptomatic large artery intracranial atherosclerosis practice advisory: report of the AAN guideline subcommittee. Neurology. (2022) 98:486–98. doi: 10.1212/WNL.0000000000200030
15. Toyoda K, Uchiyama S, Yamaguchi T, Easton JD, Kimura K, Hoshino H, et al. Dual antiplatelet therapy using cilostazol for secondary prevention in patients with high-risk ischaemic stroke in Japan: a multicentre, open-label, randomised controlled trial. Lancet Neurol. (2019) 18:539–48. doi: 10.1016/S1474-4422(19)30148-6
16. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. (2015) 372:1009–18. doi: 10.1056/NEJMoa1414792
17. Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. (2002) 324:71–86. doi: 10.1136/bmj.324.7329.71
18. Liu L, Wong KS, Leng X, Pu Y, Wang Y, Jing J, et al. Dual antiplatelet therapy in stroke and ICAS: subgroup analysis of chance. Neurology. (2015) 85:1154–62. doi: 10.1212/WNL.0000000000001972
19. Jin H, Peng Q, Nan D, Lv P, Liu R, Sun W, et al. Prevalence and risk factors of intracranial and extracranial artery stenosis in asymptomatic rural residents of 13 villages in China. BMC Neurol. (2017) 17:136. doi: 10.1186/s12883-017-0924-0
20. Persoon S, Luitse MJA, de Borst GJ, van der Zwan A, Algra A, Kappelle LJ, et al. Symptomatic internal carotid artery occlusion: a long-term follow-up study. J Neurol Neurosurg Psychiatry. (2011) 82:521–6. doi: 10.1136/jnnp.2010.208330
21. Selak V, Kerr A, Poppe K, Wu B, Harwood M, Grey C, et al. Annual risk of major bleeding among persons without cardiovascular disease not receiving antiplatelet therapy. JAMA. (2018) 319:2507–20. doi: 10.1001/jama.2018.8194
22. Wongrakpanich S, Wongrakpanich A, Melhado K, Rangaswami J. A comprehensive review of non-steroidal anti-inflammatory drug use in the elderly. Aging Dis. (2018) 9:143–50. doi: 10.14336/AD.2017.0306
23. Sabzwari SR, Qidwai W, Bhanji S. Polypharmacy in elderly: a cautious trail to tread. J Pak Med Assoc. (2013) 63:624–7.
24. Tegos TJ, Kalodiki E, Daskalopoulou SS, Nicolaides AN. Stroke: epidemiology, clinical picture, and risk factors–part I of III. Angiology. (2000) 51:793–808. doi: 10.1177/000331970005101001
25. Kasner SE, Lynn MJ, Chimowitz MI, Frankel MR, Howlett-Smith H, Hertzberg VS, et al. Warfarin vs aspirin for symptomatic intracranial stenosis: subgroup analyses from WASID. Neurology. (2006) 67:1275–8. doi: 10.1212/01.wnl.0000238506.76873.2f
26. Amarenco P, Albers GW, Denison H, Easton JD, Evans SR, Held P, et al. Efficacy and safety of ticagrelor versus aspirin in acute stroke or transient ischaemic attack of atherosclerotic origin: a subgroup analysis of SOCRATES, a randomised, double-blind, controlled trial. Lancet Neurol. (2017) 16:301–10. doi: 10.1016/S1474-4422(17)30038-8
27. Kasner SE, Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. (2006) 113:555–63. doi: 10.1161/CIRCULATIONAHA.105.578229
28. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart association Task Force on clinical practice guidelines. Circulation. (2019) 139:e1082–143. doi: 10.1016/j.jacc.2018.11.002
29. Yu DD, Pu YH, Pan YS, Zou XY, Soo Y, Leung T, et al. High blood pressure increases the risk of poor outcome at discharge and 12-month follow-up in patients with symptomatic intracranial large artery stenosis and occlusions: subgroup analysis of the CICAS study. CNS Neurosci Ther. (2015) 21:530–5. doi: 10.1111/cns.12400
Keywords: low dose DAPT, elderly, symptomatic intracranial artery stenosis, efficacy, safety
Citation: Song H-x, Zhang B, Liu S, Shi Z-c, Wang Z-y, Lu H-l, Yao J and Chen J (2023) Efficacy and safety of low dose aspirin plus clopidogrel in the treatment of elderly patients with symptomatic intracranial artery stenosis. Front. Neurol. 14:1060733. doi: 10.3389/fneur.2023.1060733
Received: 05 October 2022; Accepted: 06 February 2023;
Published: 02 March 2023.
Edited by:
Linxin Li, University of Oxford, United KingdomReviewed by:
Fathi M. Sherif, University of Tripoli, LibyaMichele Romoli, Maurizio Bufalini Hospital, Italy
Huaizhang Shi, First Affiliated Hospital of Harbin Medical University, China
Copyright © 2023 Song, Zhang, Liu, Shi, Wang, Lu, Yao and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Yao, WmhhbmdmZW5nMjAyMjA1MjhAMTYzLmNvbQ==; Juan Chen, MTg2MzM4ODg2MTJAMTYzLmNvbQ==
 Hai-xia Song
Hai-xia Song Bin Zhang
Bin Zhang