
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 07 March 2023
Sec. Endovascular and Interventional Neurology
Volume 14 - 2023 | https://doi.org/10.3389/fneur.2023.1023475
 Ping Zhang†
Ping Zhang† Lei Chen†
Lei Chen† Yi Jiang
Yi Jiang Hui Yuan
Hui Yuan Xuan Zhu
Xuan Zhu Minmin Zhang
Minmin Zhang Tao Wu
Tao Wu Benqiang Deng
Benqiang Deng Pengfei Yang*
Pengfei Yang* Yongwei Zhang*
Yongwei Zhang* Jianmin Liu
Jianmin LiuObjective: The purpose of the study was to assess the risk factors for poststroke pneumonia (PSP) and its association with the outcomes in patients with acute ischemic stroke (AIS) due to large artery occlusion treated with mechanical thrombectomy (MT).
Methods: Consecutive patients with AIS who underwent MT from January 2019 to December 2019 in the stroke center of Changhai Hospital were identified retrospectively. All of the patients were evaluated for the occurrence of PSP while in the hospital, and their modified Rankin scale (mRS) scores were assessed 90 days after having a stroke. Logistic regression analysis was conducted to determine the independent predictors of PSP, and the associations between PSP and clinical outcomes were analyzed.
Results: A total of 248 patients were enrolled, of whom 33.47% (83) developed PSP. Logistic regression analysis revealed that body mass index (BMI) [unadjusted odds ratio (OR) 1.200, 95% confidence interval (CI) 1.038–1.387; p = 0.014], systemic immune-inflammation index (SII) (OR 1.001, 95% CI 1.000–1.002; p = 0.003), dysphagia (OR 9.498, 95% CI 3.217–28.041; p < 0.001), and intubation after MT (OR 4.262, 95% CI 1.166–15.581; p = 0.028) were independent risk factors for PSP. PSP was a strong predictor of clinical outcomes: it was associated with functional independence (mRS score ≤ 2) (OR 0.104, 95% CI 0.041–0.260; p < 0.001) and mortality at 90 days (OR 3.010, 95% CI 1.068–8.489; p = 0.037).
Conclusion: More than one in three patients with AIS treated with MT developed PSP. Dysphagia, intubation, higher BMI, and SII were associated with PSP in these patients. Patients with AIS who develop PSP are more likely to experience negative outcomes. The prevention and identification of PSP are necessary to reduce mortality and improve clinical outcomes.
Mechanical thrombectomy (MT) has been proven to be effective for patients with acute ischemic stroke (AIS) due to large artery occlusion (1). Although most of these patients achieve complete recanalization after MT, many patients with AIS die of complications (2). The most common complication is pneumonia, which negatively affects clinical outcomes and increases the cost and duration of hospitalization (3). The prediction of poststroke pneumonia (PSP) remains challenging. No single biomarker pattern predicting PSP or outcome has been identified (3). The severity of stroke and dysphagia are considered risk factors for PSP (4). However, there is still no conclusive evidence regarding the risks and effects of PSP after MT (5). Whether thrombolysis before MT or anesthesia adds potential health risks for patients and increases PSP rates is still uncertain (6, 7). Therefore, it is necessary to investigate the risk factors for PSP and its association with outcomes in patients with AIS treated with MT. The goal of this study was to evaluate the predictive factors of PSP in patients with AIS with MT and the association between PSP and clinical outcomes.
In this retrospective study, patients who presented at the Department of Stroke Center at Changhai Hospital between January 2019 and December 2019 were included. The inclusion criteria were as follows: (1) diagnosis of acute ischemic stroke; (2) age ≥ 18 years; (3) large artery occlusion confirmed by computed tomographic angiography or digital subtraction angiography; (4) treatment with mechanical thrombectomy with or without intravenous alteplase according to the American Heart Association (AHA)–American Stroke Association (ASA) guidelines (1); and (5) hospitalization in Changhai Hospital after MT. The exclusion criteria were as follows: (1) diagnosis with community-acquired pneumonia; (2) other infectious diseases or treatment with broad-spectrum antibiotics or corticosteroid therapy for the 2 weeks before MT; (3) cancer; and (4) loss to follow-up. Written informed consent was obtained from all the participants. The study was approved by the Shanghai ethical committee.
Poststroke pneumonia was defined as a clinical diagnosis of pneumonia within 7 days after stroke onset during the hospital stay that did not fulfill the criteria for community-acquired pneumonia (3, 5), regardless of whether the patient was intubated. The clinical diagnosis of pneumonia was made based on the following findings: a new or progressive infiltrate, consolidation, or ground glass opacity revealed on chest computed tomography (CT) or radiography plus two or more of the following three criteria: (1) fever (>38°C) without another cause; (2) leukopenia (<4,000 leukocytes/mm3) or leukocytosis (>10,000 leukocytes/mm3); and (3) for patients older than 70 years old, at least two of the following: (a) a positive sputum culture; (b) new onset or worsening cough, or respiratory rate; (c) rales, crackles, or bronchial breath sounds; and (d) worsening gas exchange.
Patient demographics, medical histories, laboratory findings, and clinical characteristics were extracted from the clinical records. These data included age, sex, body mass index (BMI = weight/height2), pre-stroke modified Rankin Scale (mRS) score, comorbidity, smoking, and drinking status, National Institutes of Health Stroke Scale (NIHSS) score, Glasgow Coma Scale (GCS) score, and systemic immune-inflammation index (SII, platelet × neutrophil/lymphocyte ratio) on admission, stroke onset to revascularization time (ORT), location of the lesion, the occlusion site, Alberta Stroke Program Early Computed Tomography Score (ASPECTS), treatment with intravenous alteplase, general anesthesia (GA) in the MT (including using GA at the beginning or converting from sedation during the MT operation), the presence of dysphagia, intubation after MT, and the degree of vessel recanalization after MT. The mRS at 90 days was used to evaluate the functional outcome, and an mRS score of ≤2 was considered a good outcome. Modified thrombolysis in cerebral infarction (mTICI) was used to measure vessel recanalization, of which mTICI ≥2b was defined as successful recanalization. All intracranial hemorrhage and symptomatic intracranial hemorrhage were diagnosed according to the Heidelberg criteria (8). Overall, two physicians blindly evaluated the imaging and procedural characteristics. If there was a disagreement, a third experienced physician made the final decision.
The patients were followed up by outpatient clinics or telephone at 90 days after stroke (within a window of ±14 days). The mRS at 90 days was used to evaluate the functional outcome, and mRS ≤2 was considered a good outcome. Trained physicians conducted interviews blindly. Mortality in the hospital and mortality at 90 days were included as safety outcomes.
Statistical analysis was performed with SPSS Statistics version 25. Continuous variables with normal distribution were expressed as the means (SE), categorical variables were expressed as counts (%), and continuous variables not normally distributed were expressed as median (P25, P45) values. Differences between the groups were calculated using the t-test, the χ2-test, or the Mann–Whitney U-test as needed. Univariate analysis was used to identify the predictive parameters at a p-value of < 0.05. Variables with a p-value of < 0.05 in univariate analysis were included in the logistic regression analysis. In addition, p < 0.05 was considered statistically significant.
There were 265 patients with AIS treated with MT between January 2019 and December 2019 in our stroke center. In total, six patients were excluded because they were diagnosed with community-acquired pneumonia on admission, eight patients were excluded because they were diagnosed with other infectious diseases or cancer, and three patients were lost to follow-up. A total of 248 patients were included in the study (Figure 1), and 83 (33.47%) patients developed PSP. Of the 248 patients, the mean age was 67.19 ± 11.47 years, and 160 (64.5%) patients were men. The median time from stroke onset until the clinical diagnosis of PSP (marked by the prescription of antibiotics) was 2 days. The comparison of the patients with or without PSP is shown in Table 1. The univariate analysis suggested that BMI, hypertension history, serum glucose, SII, NIHSS score, GCS score, ASPECTS, white blood cell (WBC) count on admission, stroke ORT, NIHSS score at 24 h, median volume of lesions with CBF <30% on CT, dysphagia, intubation after MT, the posterior circulation AIS, and the use of sedatives after MT were significantly different between the PSP and non-PSP groups. The MT-related factors, stroke ORT, the median volume of lesions with CBF <30% on CT, and NIHSS score at 24 h after MT were significantly different between the two groups.
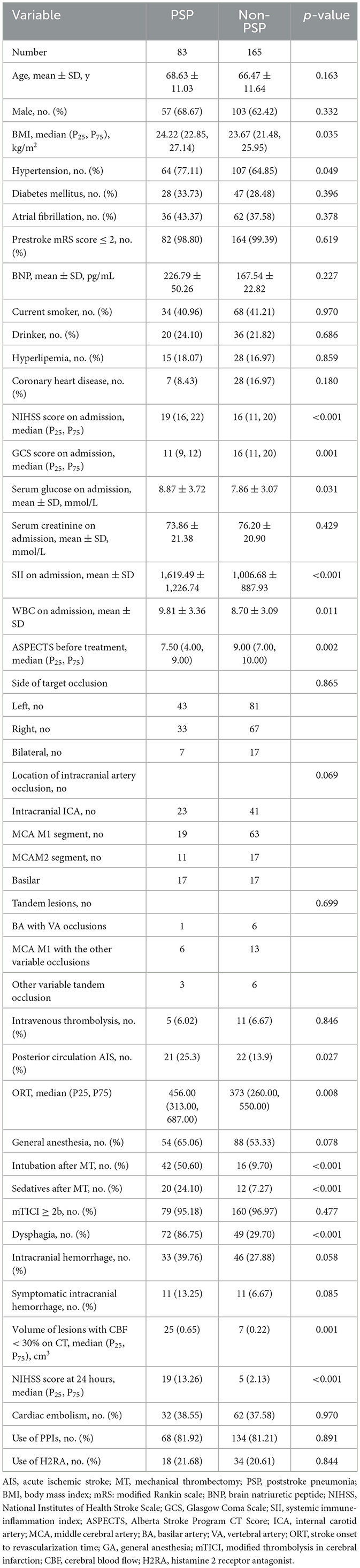
Table 1. Univariate analysis of predictive factors for developing poststroke pneumonia after mechanical thrombectomy.
Variables with p < 0.05 in univariate analysis were included in the logistic regression model. The independent predictors of PSP were BMI (OR 1.200, 95% CI 1.038–1.387; p = 0.014), SII (OR 1.001, 95% CI 1.000–1.002; p = 0.003), dysphagia (OR 9.498, 95% CI 3.217–28.041; p < 0.001), and intubation after MT (OR 4.262, 95% CI 1.166–15.581; p = 0.028). The details are shown in Table 2.
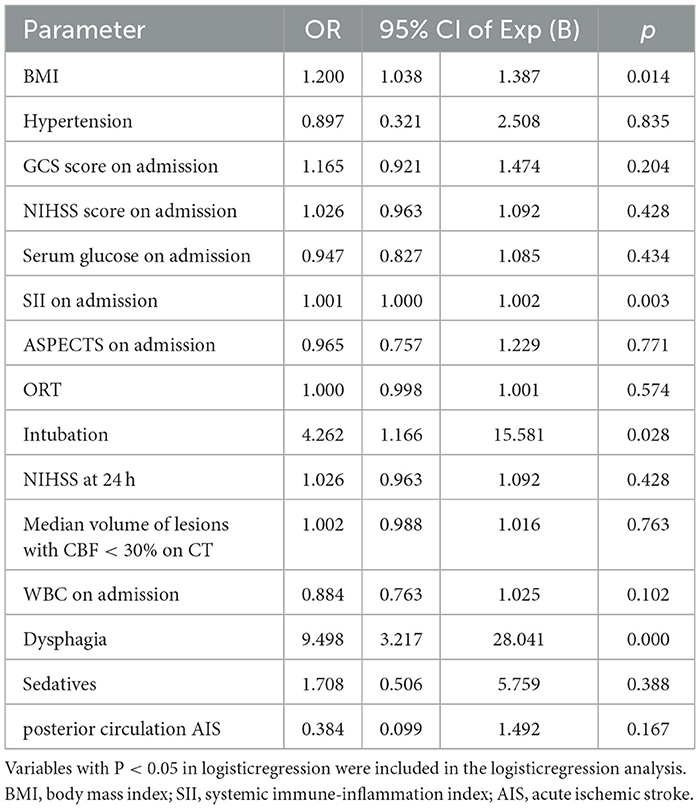
Table 2. Logistic regression analysis of possible predictive factors of developing poststroke pneumonia after mechanical thrombectomy.
The overall 90-day mortality of the cohort was 18.6%, and this rate was associated with the presence of PSP. Compared with the patients with non-PSP, the patients with PSP had a higher in-hospital mortality (18.07 vs. 9.09%; p = 0.061) and 90-day mortality (33.73 vs. 10.30%; p < 0.001) and had a lower functional independence rate (mRS score ≤2) (13.25 vs. 68.48%; p < 0.001) (Figures 2, 3). PSP was negatively associated with functional independence (mRS score ≤2) (OR 0.104, 95% CI 0.041–0.260; p < 0.001) and positively associated with 90-day mortality (OR 3.010, 95% CI 1.068–8.489; p = 0.037) (Table 3).
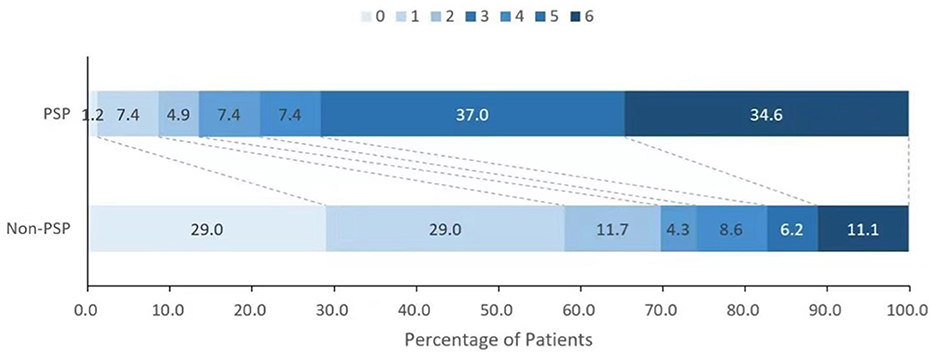
Figure 2. Distribution of the modified Rankin scale scores between patients with and without poststroke pneumonia.
The incidence of PSP after AIS varies from 5.4 to 44% depending on the clinical setting and the definition based on the literature (9, 10). In our study, 33.47% of the patients with AIS treated with MT developed PSP, which was significantly higher than the ~ 12% incidence of pneumonia in patients who have had AIS of any type (9).
Moreover, our study demonstrated that PSP was negatively associated with functional independence (mRS ≤2) and was positively associated with mortality rates in patients with AIS who received MT. Similar findings were also reported in many other studies (11). Infection is already considered a determinant of outcomes after stroke (12). Among all infections, pneumonia had the greatest impact on the outcome of patients with stroke. The proportion of deaths attributed to pneumonia occurring within the 1st week after stroke onset accounted for one-third of all deaths in patients with acute ischemic stroke. A nationwide 4-year study confirmed that the patients with hospital-acquired pneumonia (HAP) after the stroke had an elevated risk of death (OR, 1.2; 95% CI, 1.1–1.3) (13). Our research subjects had PSP, including not only HAP but also ventilator-associated pneumonia (VAP) after stroke. PSP significantly elevated 90-day mortality (OR 3.010; 95% CI, 1.068–8.489) in our study. Therefore, identifying risk factors and the early identification of PSP are important for patients with AIS treated with MT.
This study demonstrated that patients with higher BMI, higher SII on admission, dysphagia, and intubation after MT were more likely to develop PSP. As risk factors for PSP, dysphagia and intubation after MT have been frequently reported by various studies (8, 11). This indicates that the predictive risk factors remain mostly identical in patients with AIS treated with MT. However, they suffer more severe stroke symptoms and have an overall higher risk of developing PSP. Intubation due to the severity of the stroke, not due to GA, is an independent risk factor for PSP, which indicates that the severity of the stroke itself is the key point for PSP. The NIHSS of patients with PSP was higher than that of patients with non-PSP (p < 0.001) but was not an independent factor for PSP according to logistic regression. This may be because other stroke factors affecting respiration directly, such as dysphagia and intubation, are more closely associated with PSP in patients with AIS with MT (5). Another reason may be that the sample size in our study was also small. The effect of anesthesia on pneumonia has always been a controversial issue. Our study did not find that GA was an independent risk factor for PSP. Many previous studies and meta-analyses have confirmed that there is no difference in complications and outcomes after stroke between patients who underwent GA and those who underwent conscious sedation (7, 14, 15).
Interestingly, we found that BMI was also an independent predictor of PSP. One study reported that obesity was a predictor of an increased risk of in-hospital complications in patients with cerebral hemorrhage (16). Another study reported that for patients treated with MT, a high BMI was independently associated with lower rates of functional independence among recanalized patients (17). A meta-analysis also demonstrated an association between obesity and increased postoperative complications (18). However, the relationship between obesity and stroke outcomes is still unclear. A post-hoc analysis of the MR CLEAN trial found that obesity was associated with better functional outcomes after a stroke in patients treated with MT (19). The NIH FAST-MAG study reported that obesity was associated with increased survival but had a U-shaped or J-shaped relation to disability and stroke-related quality of life (20). Although BMI was an independent factor affecting PSP, the BMI values did not differ between the patients with good and poor outcomes in our study. Higher quality evidence is needed to clarify the relationship between obesity and outcome in patients with stroke.
The SII, which combines platelets, lymphocytes, and neutrophils to reflect thrombosis and inflammation, was also an independent predictor of PSP based on our study. The inflammatory mechanism after stroke plays a critical role in the development of AIS ischemic stroke (21). Stroke induces the activation of the inflammatory cascade in both the CNS and PNS, which is called stroke-induced immunosuppression (SIIS). It occurs within hours of stroke onset and increases the host's susceptibility to poststroke infection. The inflammatory response of AIS is complex. The damage caused by neutrophils causes systemic inflammation and damages the blood–brain barrier. Platelets become excessively active and begin to accumulate. Inflammatory cytokines trigger lymphocyte apoptosis (22). The infiltration of leukocytes and the release of various inflammatory mediators may also result in adverse outcomes. A high SII was an independent risk factor for poor outcomes at 3 months in patients with AIS (23, 24). Ahmet Adiguzel et al. reported that the SII reached statistical significance in terms of discriminability for pneumonia development (25) based on daily measurement of the SII. Our study only recorded the SII on admission but still indicated an association with PSP. Therefore, the SII, which is a relatively integrated index that can be quickly calculated from blood, maybe a potential prognostic factor for PSP in clinical practice. Large-scale population data are needed to verify the reliability of SII in predicting PSP.
The study had some limitations. First, as a retrospective single-center study, the sample size was small, which may lead to selection bias. Second, the degree of dysphagia and feeding actions were not analyzed. Third, only SII on admission was calculated. The correlation between the dynamic monitoring of SII and PSP was unclear. The associations between PSP and other inflammatory markers, such as C-reactive protein, were not discussed in this study. Finally, our study included all patients with pneumonia except the patients with community-acquired pneumonia and did not distinguish ventilator-associated pneumonia.
This study found that nearly one in three patients with stroke with AIS treated with MT developed PSP during acute care. Patients with AIS who developed PSP had worse outcomes than those without PSP. Dysphagia, intubation after MT, higher BMI, and SII on admission were all associated with PSP in these patients. The findings of the current study may help to prevent the development of PSP by identifying patients who are at risk.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Naval Medical University (M2019-010-2019-12-10). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
PZ and LC contributed to draft the manuscript. HY and YJ contributed to collect the data. XZ and MZ contributed to follow the patients. TW, BD, and JL contributed to polish the language. PY and YZ contributed to revise the manuscript. All authors contributed to the article and approved the submitted version.
This study was funded by Deep Blue 123 free exploration project of The First Affiliated Hospital of Naval Military Medical University (2020SLZ001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. American Heart Association Stroke Council 2018. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2018) 49:e46–110. doi: 10.1161/STR.0000000000000158
2. Zhu Y, Gao J, Lv Q, Yin Q, Yang D. Risk factors and outcomes of stroke-associated pneumonia in patients with stroke and acute large artery occlusion treated with mechanical thrombectomy. J Stroke Cerebrovasc Dis. (2020) 29:105223. doi: 10.1016/j.jstrokecerebrovasdis.2020.105223
3. De Jonge JC, Takx RAP, Kauw F, de Jong PA, Dankbaar JW, van der Worp HB. Signs of pulmonary infection on admission chest computed tomography are associated with pneumonia or death in patients with acute stroke. Stroke. (2020) 51:1690–5. doi: 10.1161/STROKEAHA.120.028972
4. Li Y, Zhang Y, Ma L, Niu X, Chang J. Risk of stroke-associated pneumonia during hospitalization: predictive ability of combined A(2)DS(2) score and hyperglycemia. BMC Neurol. (2019) 19:298. doi: 10.1186/s12883-019-1497-x
5. Schaller-Paule MA, Foerch C, Bohmann FO, Lapa S, Misselwitz B, Kohlhase K, et al. Predicting poststroke pneumonia in patients with anterior large vessel occlusion: a prospective, population-based stroke registry analysis. Front Neurol. (2022) 13:824450. doi: 10.3389/fneur.2022.824450
6. Ren C, Xu G, Liu Y, Liu G, Wang J, Gao J. Effect of conscious sedation vs. general anesthesia on outcomes in patients undergoing mechanical thrombectomy for acute ischemic stroke: a prospective randomized clinical trial. Front Neurol. (2020) 11:170. doi: 10.3389/fneur.2020.00170
7. Schönenberger S, Hendén PL, Simonsen CZ, Uhlmann L, Klose C, Pfaff JAR, et al. Association of general anesthesia vs. procedural sedation with functional outcome among patients with acute ischemic stroke undergoing thrombectomy: a systematic review and meta-analysis. JAMA. (2019) 322:1283–93. doi: 10.1001/jama.2019.11455
8. Von Kummer R, Broderick JP, Campbell BC, Demchuk A, Goyal M, Hill MD, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. (2015) 46:2981–6. doi: 10.1161/STROKEAHA.115.010049
9. Badve MS, Zhou Z, van de Beek D, Anderson CS, Hackett ML. Frequency of post-stroke pneumonia: systematic review and meta-analysis of observational studies. Int J Stroke. (2019) 14:125–36. doi: 10.1177/1747493018806196
10. Cugy E, Sibon I. Stroke-associated pneumonia risk score: validity in a French stroke unit. J Stroke Cerebrovasc Dis. (2017) 26:225–9. doi: 10.1016/j.jstrokecerebrovasdis.2016.09.015
11. Patel UK, Kodumuri N, Dave M, Lekshminarayanan A, Khan N, Kavi T, et al. Stroke-associated pneumonia: a retrospective study of risk factors and outcomes. Neurologist. (2020) 25:39–48. doi: 10.1097/NRL.0000000000000269
12. Elkind MSV, Boehme AK, Smith CJ, Meisel A, Buckwalter MS. Infection as a stroke risk factor and determinant of outcome after stroke. Stroke. (2020) 51:3156–68. doi: 10.1161/STROKEAHA.120.030429
13. Gonçalves-Pereira JC, Marino F, Mergulhão P, Nunes B, Froes F. Hospital-acquired pneumonia is more frequent and lethal in stroke patients: a nationwide 4-year study. Infect Control Hosp Epidemiol. (2021) 29:1–3. doi: 10.1017/ice.2021.398
14. Löwhagen Hendén P, Rentzos A, Karlsson JE, Rosengren L, Leiram B, Sundeman H, et al. General anesthesia versus conscious sedation for endovascular treatment of acute ischemic stroke: the anstroke trial (Anesthesia During Stroke). Stroke. (2017) 48:1601–7. doi: 10.1161/STROKEAHA.117.016554
15. Simonsen CZ, Yoo AJ, Sørensen LH, Juul N, Johnsen SP, Andersen G, Rasmussen M. Effect of general anesthesia and conscious sedation during endovascular therapy on infarct growth and clinical outcomes in acute ischemic stroke: a randomized clinical trial. JAMA Neurol. (2018) 75:470–7. doi: 10.1001/jamaneurol.2017.4474
16. Cao Z, Liu X, Li Z, Gu H, Jiang Y, Zhao X, et al. Body mass index and clinical outcomes in patients with intracerebral haemorrhage: results from the China Stroke Center Alliance. Stroke Vasc Neurol. (2021) 6:424–32. doi: 10.1136/svn-2020-000534
17. Chen SH, McCarthy D, Saini V, Brunet MC, Peterson EC, Yavagal D, et al. Effect of body mass index on outcomes of mechanical thrombectomy in acute ischemic stroke. World Neurosurg. (2020) 143:e503–15. doi: 10.1016/j.wneu.2020.07.220
18. Saravana-Bawan B, Goplen M, Alghamdi M, Khadaroo RG. The relationship between visceral obesity and post-operative complications: a meta-analysis. J Surg Res. (2021) 267:71–81. doi: 10.1016/j.jss.2021.04.034
19. Pirson FAV, Hinsenveld WH, Staals J, de Greef BTA, van Zwam WH, Dippel DWJ, et al. The effect of body mass index on outcome after endovascular treatment in acute ischemic stroke patients: a post hoc analysis of the MR CLEAN trial. Cerebrovasc Dis. (2019) 48:200–6. doi: 10.1159/000504744
20. Liu Z, Sanossian N, Starkman S, Avila-Rinek G, Eckstein M, Sharma LK, et al. Adiposity and outcome after ischemic stroke: obesity paradox for mortality and obesity parabola for favorable functional outcomes. Stroke. (2021) 52:144–51. doi: 10.1161/STROKEAHA.119.027900
21. Hou D, Wang C, Luo Y, Ye X, Han X, Feng Y, et al. Systemic immune inflammation index (SII) but not platelet-albumin-bilirubin (PALBI) grade is associated with severity of acute ischemic stroke (AIS). Int J Neurosci. (2020) 131:1203–8. doi: 10.1080/00207454.2020.1784166
22. Weng Y, Zeng T, Huang H, Ren J, Wang J, Yang C, et al. Systemic immune-inflammation index predicts 3-month functional outcome in acute ischemic stroke patients treated with intravenous thrombolysis. Clin Interv Aging. (2021) 16:877–86. doi: 10.2147/CIA.S311047
23. Anrather J, Iadecola C. Inflammation and stroke: an overview. Neurotherapeutics. (2016) 13:661–70. doi: 10.1007/s13311-016-0483-x
24. Zhou YX, Li WC, Xia SH, Xiang T, Tang C, Luo JL, et al. Predictive value of the systemic immune inflammation index for adverse outcomes in patients with acute ischemic stroke. Front Neurol. (2022) 13:836595. doi: 10.3389/fneur.2022.836595
Keywords: pneumonia, ischemic stroke, thrombectomy, outcome, inflammation
Citation: Zhang P, Chen L, Jiang Y, Yuan H, Zhu X, Zhang M, Wu T, Deng B, Yang P, Zhang Y and Liu J (2023) Risk factors for and outcomes of poststroke pneumonia in patients with acute ischemic stroke treated with mechanical thrombectomy. Front. Neurol. 14:1023475. doi: 10.3389/fneur.2023.1023475
Received: 05 September 2022; Accepted: 06 February 2023;
Published: 07 March 2023.
Edited by:
Diogo C. Haussen, Emory University, United StatesReviewed by:
Sibel Canbaz Kabay, Kutahya Health Sciences University, TürkiyeCopyright © 2023 Zhang, Chen, Jiang, Yuan, Zhu, Zhang, Wu, Deng, Yang, Zhang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongwei Zhang, emhhbmd5b25nd2VpQDE2My5jb20=; Pengfei Yang, MTU5MjExOTYzMTJAMTYzLmNvbQ==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.