
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol., 10 November 2022
Sec. Dementia and Neurodegenerative Diseases
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.999820
This article is part of the Research TopicNeural Immuno-Inflammatory Response in Neurodegenerative DiseasesView all 6 articles
Background: Several studies showed inconsistencies in the relationships between inflammatory rheumatic diseases (IRDs) and the risk of Parkinson's disease (PD). Therefore, we carried out a meta-analysis to investigate the associations between different IRDs and PD risk.
Methods: A comprehensive search was undertaken on PubMed, Embase, Cochrane Library, and Web of Science databases up to June 2022. Studies reporting the relationships between IRDs and PD risk were included. Pooled relative risks (RRs) with 95% confidence intervals (CIs) were calculated by using random-effects models.
Results: Twenty-two publications covering seven IRDs containing data from 833,004 patients were identified for quantitative analysis. The pooled results indicated that ankylosing spondylitis (RR = 1.55, 95% CI: 1.31–1.83, I2 = 32.1%, P < 0.001), Sjögren's syndrome (RR = 1.34, 95% CI: 1.22–1.47, I2 = 58.5%, P < 0.001), and Behcet's disease (RR = 1.93, 95% CI: 1.07–3.49, I2 = 57.6%, P = 0.030) were associated with an increased PD risk. However, no significant associations were observed between gout, rheumatoid arthritis, systemic lupus erythematosus, as well as polymyalgia rheumatica and the subsequent development of PD.
Conclusion: Ankylosing spondylitis, Sjögren's syndrome, and Behcet's disease may increase PD risk.
Parkinson's disease (PD) is a progressive neurodegenerative disorder that causes substantial motor impairments such as resting tremor, bradykinesia, rigidity, and postural instability, as well as a series of non-motor symptoms (1, 2). The main pathological changes of PD are the progressive loss of dopaminergic neurons in the substantia nigra, along with the deposition of synuclein, also known as Lewy bodies (3). Aging, environmental, genetic, and lifestyle factors seem to be involved in the formation of underlying etiologies (4, 5). However, the exact mechanisms leading to programmed dopamine death in PD are still unknown (6). It has been suggested that chronic inflammation may play a crucial role in the pathogenesis of PD (7).
Inflammatory rheumatic diseases (IRDs) encompass a wide range of conditions, including chronic inflammatory arthritis such as rheumatoid arthritis (RA), gout, and ankylosing spondylitis (AS). It also contains vasculitis and connective tissue disorders, like Sjögren's syndrome (SS) and systemic lupus erythematosus (SLE). This highly heterogeneous group of disorders was characterized by persistent systemic inflammation mainly affecting the musculoskeletal system and connective tissue (8–10). Previous studies have demonstrated that IRDs were associated with an increased risk of dementia, depressive disorders, and stroke (11–13). Furthermore, several articles have attempted to explore the correlation between IRDs and PD risk. However, these findings are inconsistent (14–18). Therefore, a meta-analysis is warranted to synthesize these results and further elucidate the association between IRDs and PD risk.
This analysis study was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-analysis guidelines (PRISMA) (19). Two researchers (LLH and FLW) independently searched relevant articles published in the PubMed, Embase, Cochrane Library, and Web of Science databases up to June 2022. The following search terms were used with restriction to English: “inflammatory rheumatic disease”, “rheumatoid arthritis”, “systemic lupus erythematosus”, “ankylosing spondylitis”, “Sjögren's syndrome”, “systemic sclerosis”, “myositis”, “dermatomyositis”, “polymyositis”, “axial spondyloarthritis”, “psoriatic arthritis”, “arthritis, reactive”, “systemic vasculitis”, “giant cell arteritis”, “temporal arteritis”, “Takayasu's arteritis”, “granulomatosis with polyangiitis”, “Churg Strauss syndrome”, “Behcet Syndrome”, “gout”, and “Parkinson disease”. In addition, we manually screened the references of articles to identify additional eligible studies.
The inclusion criteria were as follows: (1) Studies reporting relationships between PD risk and IRDs as abovementioned; (2) case–control, cross-sectional, or cohort designs; (3) studies presenting a measure of association (such as an odds ratio [OR], relative risk [RR], hazard ratio [HR]), standardized incidence ratio [SIR], or incidence rate ratio [IRR]) for the association between IRDs and PD risk, with 95% confidence interval (CI).
Exclusion criteria included (1) case reports, letters, reviews, conference abstracts, and editorials; (2) animal and in vitro studies.
For the cohort and case–control studies, we adopted the Newcastle–Ottawa Quality Assessment Scale (NOS) to assess the quality of the studies (20). For cross-sectional studies, we used the Agency for Healthcare Research and Quality (AHRQ) to detect the bias in the studies (21). Two investigators (LLH and HCZ) independently evaluated the included studies and extracted relevant information such as first author, publication year, different types of IRDs, study populations, study designs, duration of the study, diagnosis criteria of PD and IRDs, effect estimates with 95% CIs, and adjusted variables (e.g., age, comorbidities, sex, region, medication, chronic obstructive, tobacco consumption, socioeconomic status, and body mass index). Any discrepancies were resolved by reaching a consensus or rechecking the original literature data.
Stata 15.0 software was used to analyze the data. Adjusted effect estimates with corresponding 95% CIs for the association between different IRDs and PD risk were chosen as the primary endpoints of the interest of pooling. The risk estimate measures involved (OR, RR, HR, IRR, and SIR) were considered equivalent (22). Heterogeneity was estimated by using I2 statistic. We used the fixed-effects model for pooled analysis when I2 < 50% and P ≥ 0.1, whereas the random-effects model was chosen when I2 ≥ 50% or P < 0.1 due to the relatively significant heterogeneity. Subgroup analyses were performed to investigate the potential heterogeneity. Sensitivity analyses were conducted to check the stability of outcomes by eliminating each study in turn. Finally, publication bias was conducted through Begg's test. A P-value < 0.05 showed the existence of publication bias (23).
A comprehensive search yielded a total of 5,150 articles. We removed 5,042 articles due to duplicated documents, unmatched titles, and unmatched abstracts. Following a review of the remaining 108 full-text articles, twenty-two articles were found to meet the inclusion criteria and were included in the current meta-analysis (14–18, 24–40). The process of selection is described in Figure 1.
The qualitative analysis included twenty-two observational studies (fourteen cohort designs, one cross-sectional design, and seven case–control designs) reporting associations between seven types of IRDs and the subsequent development of PD. In total, 833,004 patients from three continents (Europe, Asia, and North America) and eight countries are involved in the study. The characteristics of the eligible studies are summarized in Table 1.
Among twenty-two studies, one study showed higher quality (nine stars at the NOS), twenty were moderate quality (nineteen studies ranked at seven–eight stars at the NOS, one study ranked at eight scores by the AHRQ checklist), and one study was low quality (less than seven stars at the NOS). Studies above six scores were considered to have a low risk of bias (Supplementary Tables S1a,b).
Higher risk of PD was observed in cases with ankylosing spondylitis (RR = 1.55, 95% CI: 1.31–1.83, I2 = 32.1%, P < 0.001), Sjögren's syndrome (RR = 1.34, 95% CI: 1.22–1.47, I2 = 58.5%, P < 0.001), and Behcet's disease (RR = 1.93, 95% CI: 1.07–3.49, I2 = 57.6%, P = 0.030) (Table 2, Figure 2).
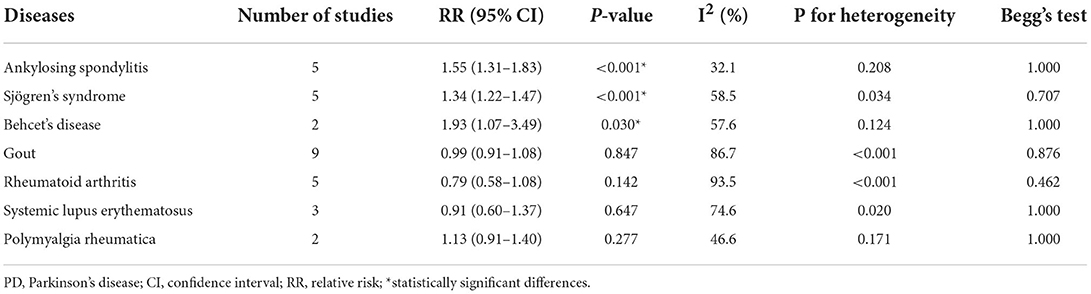
Table 2. Overall meta-analysis results on associations between different types of inflammatory rheumatic diseases and PD risk.
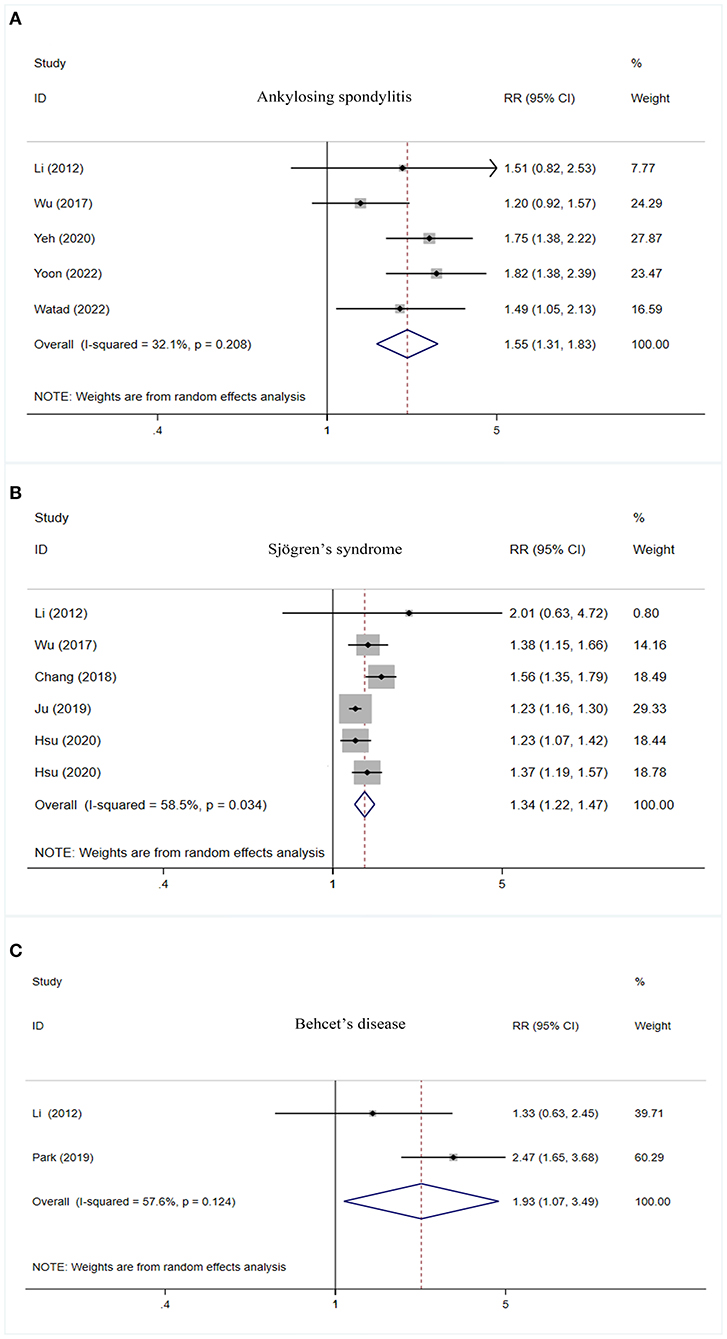
Figure 2. Forest plot of PD risk in patients with ankylosing spondylitis (A), Sjögren's syndrome (B), and Behcet's disease (C). PD, Parkinson's disease; CI, confidence interval; RR, relative risk.
No significant association was observed between gout, rheumatoid arthritis, systemic lupus erythematosus, as well as polymyalgia rheumatica and PD risk (Table 2, Figure 3).
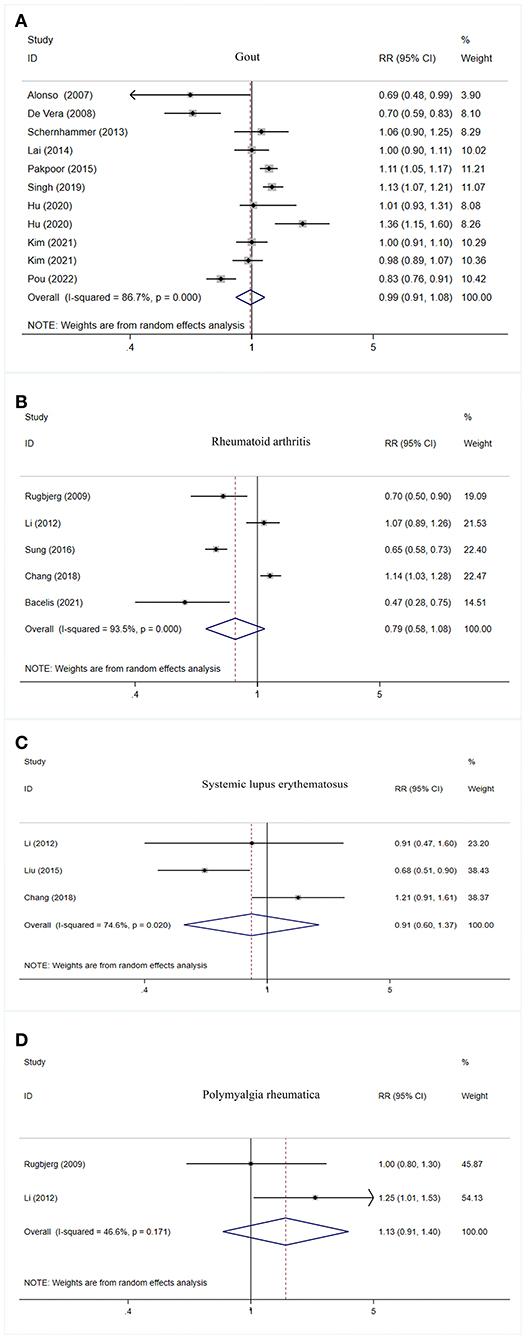
Figure 3. Forest plot of PD risk in patients with gout (A), rheumatoid arthritis (B), systemic lupus erythematosus (C), and polymyalgia rheumatic (D). PD, Parkinson's disease; CI, confidence interval; RR, relative risk.
Significant heterogeneities were found, so we pooled results of ankylosing spondylitis, Sjögren's syndrome, rheumatoid arthritis, and gout by using random-effects models and proceed to explore the potential discrepancies in terms of genders, study designs, effect sizes, and regions. We did not conduct subgroup analysis for systemic lupus erythematosus, Behcet's disease, and polymyalgia rheumatica due to the limited number.
Ankylosing spondylitis increased PD risk in both male patients (RR = 1.76, 95% CI: 1.39–2.22, P < 0.001) and female patients (RR = 1.80, 95% CI: 1.35–2.39, P < 0.001). Female patients (RR = 1.28, 95% CI: 1.21–1.35, P < 0.001) with Sjögren's syndrome had a higher risk of PD. There was a trend of decreased risk of PD in patients with rheumatoid arthritis in both male patients (RR = 0.61, 95% CI: 0.49–0.76, P < 0.001) and female patients (RR = 0.58, 95% CI: 0.38–0.89, P = 0.013). The detailed results of the gender subgroup analyses are listed in Supplementary Table S2 and Supplementary Figures S1–S4.
Ankylosing spondylitis patients had a higher risk of PD, as shown in cohort studies (RR = 1.75, 95% CI: 1.48–2.08, P < 0.001) and cross-sectional designs (RR = 1.49, 95% CI: 1.05–2.12, P = 0.027). Sjögren's syndrome showed an increased risk of PD in both cohort studies (RR = 1.33, 95% CI: 1.20–1.48, P < 0.001) and case–control studies (RR = 1.38, 95% CI: 1.15–1.66, P = 0.001). Rheumatoid arthritis was associated with a decreased risk of PD in case–control designs (RR = 0.6, 95% CI: 0.41–0.88, P = 0.009). The results of the subgroup analyses based on the study design are presented in Supplementary Table S3 and Supplementary Figures S5–S8.
Ankylosing spondylitis had an increased PD risk by using both “HR” (RR = 1.78, 95% CI: 1.49–2.13, P < 0.001) and “OR” (RR = 1.30, 95% CI: 1.05–1.61, P = 0.016) as effect sizes. Patients with Sjögren's syndrome had a higher PD incidence when the effect size was estimated by “OR” (RR = 1.38, 95% CI: 1.15–1.66, P = 0.010), “HR” (RR = 1.32, 95% CI: 1.15–1.52, P < 0.001), and “IRR” (RR = 1.37, 95% CI: 1.19–1.57, P < 0.001). Rheumatoid arthritis was associated with a decreased risk of PD when using “OR” as the assessment criterion (RR = 0.60, 95% CI: 0.41–0.88, P = 0.009). The results of the subgroup analyses based on effect size are listed in Supplementary Table S4 and Supplementary Figures S9–S12.
Patients in Asia with ankylosing spondylitis (RR = 1.55, 95% CI: 1.28–1.89, P = 0.001) and Sjögren's syndrome (RR = 1.33, 95% CI: 1.22–1.46, P < 0.001) had a higher risk of PD. Patients with gout in North America had a lower risk of PD (RR = 0.70, 95% CI: 0.59–0.83, P < 0.001). Detailed results are shown in Supplementary Table S5 and Supplementary Figures S13–S16.
Sensitivity analyses were carried out by eliminating one study in turn to evaluate the stability and reliability of the individual outcome on the overall analysis. Sensitivity analyses demonstrated that the pooled RRs with 95% CIs were not affected by any individual study. It confirmed the consistency and dependability of our findings (Supplementary Figures S17–S23). Potential publication bias was assessed by Begg's test (PAS = 1.000; PSS = 0.707; PBD = 1.000; PGout = 0.876; PRA = 0.462; PSLE = 1.000; PPMR = 1.000), and no significant publication bias was detected (Supplementary Figures S24–S30).
This meta-analysis is the first comprehensive review to investigate the associations between IRDs and PD risk. The results suggest that ankylosing spondylitis, Sjögren's syndrome, and Behcet's disease may increase the risk of PD.
The exact mechanisms are unclear. There are some possible explanations. First, systemic inflammation involving in neuroinflammation may contribute to the pathogenesis of PD through cytokine-induced inflammatory responses or abnormal immune responses (41). Accumulating evidence showed that the major products of IRDs-peripheral cytokine may cross the blood–brain barrier directly through the leaky areas of blood–brain barrier (such as damaged tight junctions or circumventricular organs) or through the pathway of receptor-mediated transcytosis (42). Peripheral cytokine reaching the brain can activate the microglia and upregulate inflammatory response, thus leading to loss of dopaminergic neurons (42–44). Peripheral cytokine can also have an indirect impact on brain signals by stimulating peripheral afferent nerves, which can trigger strong responses of neurodegenerative processes (45). Furthermore, peripheral cytokine may activate the inflammasomes, such as nucleotide-binding oligomerization domain-like receptor protein 3, which can promote the maturation of interleukin IL-1β and IL-18, thus accelerating neurodegeneration (46). Meanwhile, reactive oxygen species and oxidative stress generated by inflammation may also contribute to the damage of dopamine neurons in PD (43).
Second, several types of research have manifested a connection between physical inactivity and the subsequent development of PD (47). Patients with IRDs may have less physical activities due to the symptoms of IRDs, such as arthritis, pain, and fatigue, which may play a role in the susceptibility to PD.
In agreement with Ungprasert's findings (48), we found that gout showed no correlation with PD risk. We speculate that the gout-related inflammation may be offset by the neuroprotective effect of hyperuricemia due to its antioxidant property in gout (48). However, no significant association between rheumatoid arthritis and PD risk was observed, which was not consistent with Li's results (49). We included more comprehensive studies and sample sizes in the current analysis may explain the inconsistent findings.
Gender subgroup analysis indicated rheumatoid arthritis might decrease PD risk. Previous studies showed that high levels of lysosomal cathepsin D released by rheumatoid arthritis may reduce aggregation of α-synuclein (50). Therefore, it may play a neuroprotective role in the development of PD through the lysosome pathway (51–53). Moreover, higher frequent using non-steroidal anti-inflammatory drugs, particularly ibuprofen (54) to relieve arthritis in patients with rheumatoid arthritis, may play a part role in decreasing PD risk.
The current meta-analysis has the following strengths. First, the majority of included studies have relatively high quality and large sample sizes, which provide more reliable sources of evidence. Second, subgroup analyses based on the stratification factors are carried out.
Nevertheless, there are some limitations. First, most of the studies relied on diagnosis codes from medical record databases. Inconsistencies of diagnostic criteria of IRDs and PD may create ascertainment bias. Second, language bias should be considered because only studies published in English were included. Third, for Behcet's disease, there were only two studies included in the current meta-analysis; therefore, the statistical power for the results of Behcet's disease may be not sufficient enough. More studies are needed to verify the conclusion that Behcet's disease may increase PD risk. Fourth, unconsidered or unmeasured variables influencing the findings of the included original studies may give rise to some bias. Thus, our results may be explained with cautions.
In summary, this systematic review and meta-analysis indicate that patients with ankylosing spondylitis, Sjögren's syndrome, and Behcet's disease may have a higher risk of PD. More prospective studies are needed to verify our findings.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
LH and XG participated in design. LH and FW have independently screened the literature. LH and HZ were involved in collecting and analyzing data. This article was written by LH, with revisions by XG. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.999820/full#supplementary-material
1. Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. (2008) 79:368–76. doi: 10.1136/jnnp.2007.131045
2. Obeso JA, Stamelou M, Goetz CG, Poewe W, Lang AE, Weintraub D, et al. Past, present, and future of Parkinson's disease: A special essay on the 200th Anniversary of the Shaking Palsy. Mov Disord. (2017) 32:1264–310. doi: 10.1002/mds.27115
3. Kalia LV, Lang AE. Parkinson's disease. Lancet. (2015) 386:896–912. doi: 10.1016/S0140-6736(14)61393-3
4. Davie CA. A review of Parkinson's disease. Br Med Bull. (2008) 86:109–27. doi: 10.1093/bmb/ldn013
5. Paul KC, Chuang YH, Shih IF, Keener A, Bordelon Y, Bronstein JM, et al. The association between lifestyle factors and Parkinson's disease progression and mortality. Mov Disord. (2019) 34:58–66. doi: 10.1002/mds.27577
6. Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. (2003) 24:197–211. doi: 10.1016/S0197-4580(02)00065-9
7. Tan EK, Chao YX, West A, Chan LL, Poewe W, Jankovic J. Parkinson disease and the immune system — associations, mechanisms and therapeutics. Nat Rev Neurol. (2020) 16:303–18. doi: 10.1038/s41582-020-0344-4
8. Saas P, Toussirot E, Bogunia-Kubik K. Editorial: Recent advances in potential biomarkers for rheumatic diseases and in cell-based therapies in the management of inflammatory rheumatic diseases. Front Immunol. (2021) 12:836119. doi: 10.3389/fimmu.2021.836119
9. Fernandez-Gutierrez B, Leon L, Madrid A, Rodriguez-Rodriguez L, Freites D, Font J, et al. Hospital admissions in inflammatory rheumatic diseases during the peak of COVID-19 pandemic: incidence and role of disease-modifying agents. Ther Adv Musculoskelet Dis. (2021) 13:1759720x20962692. doi: 10.1177/1759720X20962692
10. Selmi C, Generali E, Massarotti M, Bianchi G, Sciré CA. New treatments for inflammatory rheumatic disease. Immunol Res. (2014) 60:277–88. doi: 10.1007/s12026-014-8565-5
11. Atzeni F, Pipitone N, Iaccarino L, Masala IF, Weiss R, Alciati A, et al. Rheumatic diseases and autoimmune vascular dementia. Autoimmun Rev. (2017) 16:1265–9. doi: 10.1016/j.autrev.2017.10.011
12. Varan Ö, Babaoglu H, Göker B. Associations between depressive disorders and inflammatory rheumatic diseases. Curr Top Med Chem. (2018) 18:1395–401. doi: 10.2174/1568026618666180516100805
13. Wiseman SJ, Ralston SH, Wardlaw JM. Cerebrovascular disease in rheumatic diseases: a systematic review and meta-analysis. Stroke. (2016) 47:943–50. doi: 10.1161/STROKEAHA.115.012052
14. Sung YF, Liu FC, Lin CC, Lee JT, Yang FC, Chou YC, et al. Reduced risk of parkinson disease in patients with rheumatoid arthritis: a nationwide population-based study. Mayo Clin Proc. (2016) 91:1346–53. doi: 10.1016/j.mayocp.2016.06.023
15. Li X, Sundquist J, Sundquist K. Subsequent risks of Parkinson disease in patients with autoimmune and related disorders: a nationwide epidemiological study from Sweden. Neurodegenerative Dis. (2012) 10:277–84. doi: 10.1159/000333222
16. Bacelis J, Compagno M, George S, Pospisilik JA, Brundin P, Naluai T, et al. Decreased risk of parkinson's disease after rheumatoid arthritis diagnosis: a nested case-control study with matched cases and controls. J Parkinson's Dis. (2021) 11:821–32. doi: 10.3233/JPD-202418
17. Schernhammer E, Qiu J, Wermuth L, Lassen CF, Friis S, Ritz B. Gout and the risk of Parkinson's disease in Denmark. Eur J Epidemiol. (2013) 28:359–60. doi: 10.1007/s10654-013-9791-1
18. Hu L-Y, Yang AC, Lee S-C, You Z-H, Tsai S-J, Hu C-K, et al. Risk of Parkinson's disease following gout: a population-based retrospective cohort study in Taiwan. Bmc Neurol. (2020) 20:1–7. doi: 10.1186/s12883-020-01916-9
19. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
20. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
21. AHRQ Methods for Effective Health Care. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality (US). (2008).
22. Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. (1987) 9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298
23. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
24. Chang C-C, Lin T-M, Chang Y-S, Chen W-S, Sheu J-J, Chen Y-H, et al. Autoimmune rheumatic diseases and the risk of Parkinson disease: a nationwide population-based cohort study in Taiwan. Ann Med. (2018) 50:83–90. doi: 10.1080/07853890.2017.1412088
25. Rugbjerg K, Friis S, Ritz B, Schernhammer ES, Korbo L, Olsen JH. Autoimmune disease and risk for Parkinson disease: A population-based case-control study. Neurology. (2009) 73:1462–8. doi: 10.1212/WNL.0b013e3181c06635
26. Alonso A, Garcia Rodriguez LA, Logroscino G, Hernan MA. Gout and risk of Parkinson disease - A prospective study. Neurology. (2007) 69:1696–700. doi: 10.1212/01.wnl.0000279518.10072.df
27. De Vera M, Rahman MM, Rankin J, Kopec J, Gao X, Choi H. Gout and the risk of Parkinson's disease: a cohort study. Arthritis Rheum. (2008) 59:1549–54. doi: 10.1002/art.24193
28. Lai S-W, Lin C-H, Lin C-L, Liao K-F. Gout and Parkinson's disease in older people: an observation in Taiwan. Int J Gerontol. (2014) 8:166–7. doi: 10.1016/j.ijge.2013.01.006
29. Liu FC, Huang WY, Lin TY, Shen CH, Chou YC, Lin CL, et al. Inverse association of Parkinson disease with systemic lupus erythematosus a nationwide population-based study. Medicine (United States). (2015) 94:e2097. doi: 10.1097/MD.0000000000002097
30. Pakpoor J, Seminog OO, Ramagopalan SV, Goldacre MJ. Clinical associations between gout and multiple sclerosis, Parkinson's disease and motor neuron disease: record-linkage studies. BMC Neurol. (2015) 15:16. doi: 10.1186/s12883-015-0273-9
31. Wu MC, Xu X, Chen SM, Tyan YS, Chiou JY, Wang YH, et al. Impact of Sjogren's syndrome on Parkinson's disease: A nationwide case-control study. PLoS One. (2017) 12:e0175836. doi: 10.1371/journal.pone.0175836
32. Ju UH, Liu FC, Lin CS, Huang WY, Lin TY, Shen CH, et al. Risk of Parkinson disease in Sjögren syndrome administered ineffective immunosuppressant therapies: A nationwide population-based study. Medicine. (2019) 98:e14984. doi: 10.1097/MD.0000000000014984
33. Singh JA, Cleveland JD. Gout and the risk of Parkinson's disease in older adults: a study of U.S. Medicare data. BMC Neurol. (2019) 19:4. doi: 10.1186/s12883-018-1234-x
34. Hsu HC, Hou TY, Lin TM, Chang YS, Chen WS, Kuo PI, et al. Higher risk of Parkinson disease in patients with primary Sjögren's syndrome. Clin Rheumatol. (2020) 39:2999–3007. doi: 10.1007/s10067-020-05053-z
35. Yeh FC, Chen HC, Chou YC, Lin CL, Kao CH, Lo HY, et al. Positive association of Parkinson's disease with ankylosing spondylitis: a nationwide population-based study. J Transl Med. (2020) 18:1–8. doi: 10.1186/s12967-020-02629-w
36. Kim JH, Choi IA, Kim A, Kang G. Clinical Association between Gout and Parkinson's Disease: A Nationwide Population-Based Cohort Study in Korea. Medicina. (2021) 57:1292. doi: 10.3390/medicina57121292
37. Pou MA, Orfila F, Pagonabarraga J, Ferrer-Moret S, Corominas H, Diaz-Torne C. Risk of Parkinson's disease in a gout Mediterranean population: A case-control study. Joint Bone Spine. (2022) 89:105402. doi: 10.1016/j.jbspin.2022.105402
38. Watad A, McGonagle D, Anis S, Carmeli R, Cohen AD, Tsur AM, et al. TNF inhibitors have a protective role in the risk of dementia in patients with ankylosing spondylitis: Results from a nationwide study. Pharmacol Res. (2022) 182:106325. doi: 10.1016/j.phrs.2022.106325
39. Yoon SY, Heo SJ, Kim YW, Yang SN, Moon HI. Ankylosing Spondylitis: A Risk Factor for Parkinsonism-A Nationwide Population-Based Study. J Parkinsons Dis. (2022) 12:353–60. doi: 10.3233/JPD-212878
40. Park HY, Lee JH, Lee SY Yu DS, Han K-D, Park YG, et al. Risk for Parkinson's disease in patients with Behcet's disease: a nationwide population-based dynamic cohort study in Korea. J Parkinsons Dis. (2019) 9:583–9. doi: 10.3233/JPD-191622
41. Caggiu E, Arru G, Hosseini S, Niegowska M, Sechi G, Zarbo IR, et al. Inflammation, infectious triggers, and Parkinson's disease. Front Neurol. (2019) 10:122. doi: 10.3389/fneur.2019.00122
42. Kaplan C, Minc A, Basu N, Schrepf A. Inflammation and the central nervous system in inflammatory rheumatic disease. Curr Rheumatol Rep. (2019) 21:67. doi: 10.1007/s11926-019-0870-5
43. Pajares M, A IR, Manda G, Boscá L, Cuadrado A. Inflammation in Parkinson's Disease: Mechanisms and Therapeutic Implications. Cells. (2020) 9:1687. doi: 10.3390/cells9071687
44. Wang Q, Liu Y, Zhou J. Neuroinflammation in Parkinson's disease and its potential as therapeutic target. Transl Neurodegener. (2015) 4:19. doi: 10.1186/s40035-015-0042-0
45. Ferrari CC, Tarelli R. Parkinson's disease and systemic inflammation. Parkinsons Dis. (2011) 2011:436813. doi: 10.4061/2011/436813
46. Yan YQ, Fang Y, Zheng R, Pu JL, Zhang BR. NLRP3 Inflammasomes in Parkinson's disease and their Regulation by Parkin. Neuroscience. (2020) 446:323–34. doi: 10.1016/j.neuroscience.2020.08.004
47. Fan B, Jabeen R, Bo B, Guo C, Han M, Zhang H, et al. What and How Can Physical Activity Prevention Function on Parkinson's Disease? Oxid Med Cell Longev. (2020) 2020:4293071. doi: 10.1155/2020/4293071
48. Ungprasert P, Srivali N, Thongprayoon C. Gout is not associated with a lower risk of Parkinson's disease: A systematic review and meta-analysis. Parkinsonism Relat Disord. (2015) 21:1238–42. doi: 10.1016/j.parkreldis.2015.08.030
49. Li D, Hong X, Chen T. association between rheumatoid arthritis and risk of Parkinson's disease: a meta-analysis and systematic review. Front Neurol. (2022) 13:885179. doi: 10.3389/fneur.2022.885179
50. Cullen V, Lindfors M, Ng J, Paetau A, Swinton E, Kolodziej P, et al. Cathepsin D expression level affects alpha-synuclein processing, aggregation, and toxicity in vivo. Mol Brain. (2009) 2:5. doi: 10.1186/1756-6606-2-5
51. Settembre C, Fraldi A, Medina DL, Ballabio A. Signals from the lysosome: a control centre for cellular clearance and energy metabolism. Nat Rev Mol Cell Biol. (2013) 14:283–96. doi: 10.1038/nrm3565
52. Bonam SR, Wang F, Muller S. Lysosomes as a therapeutic target. Nat Rev Drug Discov. (2019) 18:923–48. doi: 10.1038/s41573-019-0036-1
53. Li C, Ou R, Shang H. Rheumatoid arthritis decreases risk for Parkinson's disease: a Mendelian randomization study. NPJ Parkinsons Dis. (2021) 7:17. doi: 10.1038/s41531-021-00166-x
Keywords: Parkinson's disease, inflammatory rheumatic diseases, risk, systematic review, meta-analysis
Citation: He L, Zhao H, Wang F and Guo X (2022) Inflammatory rheumatic diseases and the risk of Parkinson's disease: A systematic review and meta-analysis. Front. Neurol. 13:999820. doi: 10.3389/fneur.2022.999820
Received: 21 July 2022; Accepted: 24 October 2022;
Published: 10 November 2022.
Edited by:
Xintong Ge, Tianjin Medical University General Hospital, ChinaReviewed by:
Konstantin Senkevich, McGill University, CanadaCopyright © 2022 He, Zhao, Wang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoyan Guo, Z3h5MTk4MDAzMTJAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.