- 1Department of Neurosurgery, West China School of Medicine/West China Hospital, Sichuan University, Chengdu, China
- 2Chengdu Seventh People's Hospital, Chengdu, China
The γ-aminobutyric acid type A receptors (GABAAR) have been reported to contribute to the pathogenesis of epilepsy and the recurrence of chronic seizures. Genetic polymorphisms in GABRA1 and GABRA6 may confer a high risk of epilepsy and multiple drug resistance, but with conflicting results. We aimed to assess the association of GABRA1 rs2279020 and GABRA6 rs3219151 with epilepsy risk using a meta-analysis. The databases of Pubmed, Ovid, Web of Science, and China National Knowledge Infrastructure were searched. Summary odds ratios (ORs) and 95% confidence intervals (CIs) were computed to evaluate the association between the polymorphisms and epilepsy risk using a fixed- or random-effect model. Trial sequential analysis (TSA) was performed to assess the results of the meta-analysis. No significant association between the GABRA1 rs2279020 and GABRA6 rs3219151 and the risk of epilepsy was found in the Asian and Arabic populations. The negative results were also observed when comparing the GABRA1 rs2279020 and GABRA6 rs3219151 polymorphism to antiepileptic drug responsiveness. The trial sequential analysis confirmed the results of the meta-analysis. This meta-analysis suggests that GABRA1 rs2279020 and GABRA6 rs3219151 are not risk factors for the etiology of epilepsy and antiepileptic drug responsiveness in the Asian and Arabic populations.
Introduction
Among all neurological diseases, epilepsy is the most common one with over 50 million people diagnosed worldwide (1, 2). It is estimated that about 80% of the patients reside in resource-poor countries, causing an enormous medical, social, and economic burden (2). Although the etiology of epilepsy remains unclear, the amount of evidence has shown that genetic factors may contribute to the occurrence of epilepsy (3–5). Matuja et al. reported that family history was an independent factor for the risk of developing epilepsy, with an odd ratio of 3.52 (3). Kjeldsen et al. reported that monozygotic twins had a 5.4-fold higher risk of febrile seizures compared with dizygotic twins (4).
Antiepileptic drugs (AEDs) were commonly used to prevent epileptic seizures. Almost one-third of patients, however, continue to show recurrent seizures despite optimal AEDs treatment (6). Genetic abnormalities may disrupt pharmacokinetics and pharmacodynamics of AEDs, and thus affect treatment efficacy. As major targets of AEDs, γ-aminobutyric acid type A receptors (GABAAR) play pivotal roles in the suppression of epileptogenesis by maintaining homeostasis over brain excitation (7–10). In a rat model of temporal lobe epilepsy, the alteration in the structure and function of GABAAR subtypes may be involved in drug resistance (9, 10).
GABAARs are heteropentamers encoded by 19 subunits of α (1–6), β (1–3), γ (1–3), δ, ε, θ, π, and ρ (1–3) (11). The α1β2γ2 subunit is most abundant in almost all regions of the brain and usually underlies excitability disorders, such as epilepsy (7, 8). In human and animal studies, the differential expression and composition of GABAARs subunits may contribute to the pathogenesis of epilepsy and the recurrence of chronic seizures (12). Single nucleotide polymorphisms (SNP) in any of the subunits may alter the expression of GABA, and ultimately influence epilepsy seizures and responsiveness to AEDs (13–15). For example, Kumari et al. reported that the rs2279020 G allele in GABRA1 conferred a high risk of epilepsy and multiple drug resistance (16), whereas Al-Eitan et al. reported that the rs2279020 polymorphism did not show any linkage with the occurrence of epilepsy and treatment responsiveness (17). Given the conflicting results, a meta-analysis is of great importance to be performed to obtain the real effect of the rs2279020 on epilepsy risk. Moreover, the relationship between rs3219151 polymorphism in GABRA6 and the susceptibility of epilepsy is also evaluated in this meta-analysis. We found that neither the GABRA1 rs2279020 nor the GABRA6 rs3219151 polymorphism was a risk factor for the etiology of epilepsy and antiepileptic drug responsiveness.
Materials and methods
Search strategy
The methodology of this meta-analysis followed the PRISMA 2020 statement guidelines. We identified records from the databases of Pubmed, Ovid, Web of Science, and the China National Knowledge Infrastructure using the following searching strategies: (gamma-aminobutyric acid type A receptor subunit alpha1 or GABRA1 or gamma-aminobutyric acid type A receptor subunit alpha6 or GABRA6) and (polymorphis* or variant* or SNP*) and (epilepsy). The literature screening was performed until 18 May 2022 without language and ethnic restrictions. If the language of publication was non-English, we translated the required information into English.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (a) investigated the association of GABRA1 rs2279020 and GABRA6 rs3219151 polymorphisms with the risk of epilepsy or outcome of AED treatment; (b) contained sufficient original data to compute summary odds ratios (ORs) and 95% confidence intervals (CIs). The exclusion criteria were as follows: (a) review articles; (b) absence of available data; and (c) overlapping data reported by the same research group.
Data extraction
ZT and YY independently extracted the following data from the included studies: the name of the first author, year of publication, ethnicity of study population, number of cases and controls, criteria used to define drug-responsiveness and drug-resistance, and genotyping technique and genotype distributions of GABRA1 rs2279020 and GABRA6 rs3219151 polymorphisms.
Assessment of study quality
ZT and YY independently assessed the eligible studies using the modified Newcastle-Ottawa Scale (18). Studies were graded 0–8 stars based on the following items: the selection of the study population, the comparability of cases and controls, and the ascertainment of the outcome. Studies with equal to or more than four stars were considered high quality and studies with <4 stars were considered low quality. Any disagreement during the assessment was resolved by discussion with SX.
Trial sequential analysis
During the comparison of the GABRA1 rs2279020 and GABRA6 rs3219151 polymorphisms with the risk of epilepsy and drug resistance, TSA software (version 0.9.5.10 beta) was performed to evaluate the reliability of the meta-analysis under a recessive model. The O'Brien-Fleming boundary, sequential monitoring boundary, futility boundary, and cumulative test statistic (Z-curve) were calculated with an alpha of 5%, power level of 80%, and relative risk reduction of 20% (19).
Statistical analysis
Data analysis was carried out using the STATA software version 10 (STATA Corporation, College Station, TX). The pooled odds ratio (OR) and its 95% confidence interval (CI) were calculated to assess the association of the GABRA1 rs2279020 and GABRA6 rs3219151 polymorphisms with the risk of epilepsy and AEDs responsiveness. Stratified analyses were also performed if two or more studies were available based on ethnicity (Asian and Arabian), the Hardy–Weinberg equilibrium (HWE), and study quality (high and low). Cochran's Q-test and I2 statistics were used to assess the inter-study heterogeneity (20). A significant Q statistic (P < 0.10) indicated high heterogeneity. Therefore, a random-effects model was used for the meta-analysis (21) and meta-regression was used to explain the origin of the heterogeneity. Otherwise, a P-value of more than 0.10 indicated low heterogeneity, and then a fixed-effect model was applied (22). Sensitivity analysis was performed to estimate the effect when an individual study was sequentially excluded from the summary analysis. According to the guideline of meta-analysis, a publication bias may be done if eligible studies were more than nine. In this meta-analysis, the highest number of studies in all comparisons was eight. Publication bias, therefore, was not performed.
Results
Flow diagram of selection studies
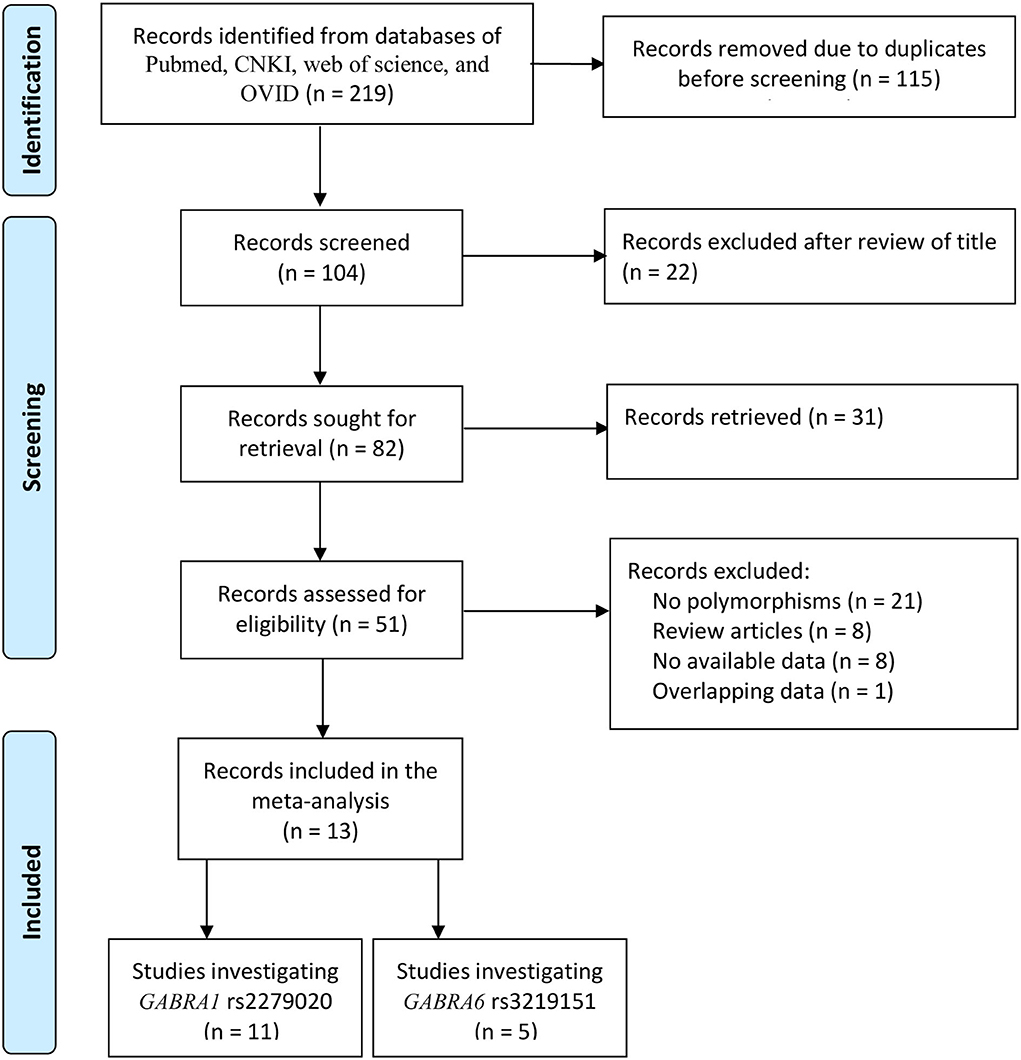
In total, 219 records were identified after database searching. Before the screening, 115 records were removed due to duplicates. The remaining 104 records were sought for retrieval, and 53 were excluded after reviewing the title and the abstract. Full texts of the remaining 51 records were assessed for eligibility, and 38 were excluded due to no GABRA1 rs2279020 and GABRA6 rs3219151 polymorphisms (n = 21), lack of available data (n = 8), review articles (n = 8), and overlapping data (n = 1). Finally, 13 studies were included in this meta-analysis, including 11 investigating GABRA1 rs2279020 polymorphism and five investigating GABRA6 rs3219151 polymorphism (Figure 1).
Characteristics of included studies
The characteristics of the included studies are summarized in Table 1. Of the 13 studies, 11 were carried out in Asians and 2 in Arabians. Various genotyping assays for analyzing the GABRA1 rs2279020 and GABRA6 rs3219151 polymorphisms were reported, namely, polymerase chain reaction-restriction fragment length polymorphism, matrix-assisted laser desorption/ionization time-of-flight mass spectrometry, TaqMan, Illumina VeraCode GoldenGate genotyping assay, SnapShot, and Sanger sequencing. Approximately half of the included studies described matched case-control factors, such as age, gender, ethnicity, and residence. However, <30% (4/13) of the studies used the second genotyping method for quality control in this study.
Meta-analysis of the association of the GABRA1 rs2279020 and GABRA6 rs3219151 polymorphisms with risk of epilepsy in Asian and Arabic populations
For the GABRA1 rs2279020 polymorphism, eight studies were included involving 1,688 patients with epilepsy and 1,233 controls. Among them, 75% (6/8) were performed in Asians and 25% (2/8) were performed in Arabians; 62.5% (5/8) were high-quality studies and did not derive from HWE in controls. In an overall analysis, no significant association between the GABRA1 rs2279020 and the risk of epilepsy was found. Similarly, the negative results were also observed in subgroup analyses based on ethnicity and study quality. Even though the GABRA1 rs2279020 was associated with an increased risk of epilepsy among studies without HWE under heterozygous comparison (OR = 1.83, 95% CI, 1.30–2.58) and dominant genetic model (OR = 1.52, 95% CI, 1.13–2.03), the polymorphism was not associated with the risk of epilepsy among studies with HWE in controls (Table 2, Figure 2A). Therefore, we may conclude that the GABRA1 rs2279020 was not a risk factor for the occurrence of epilepsy.
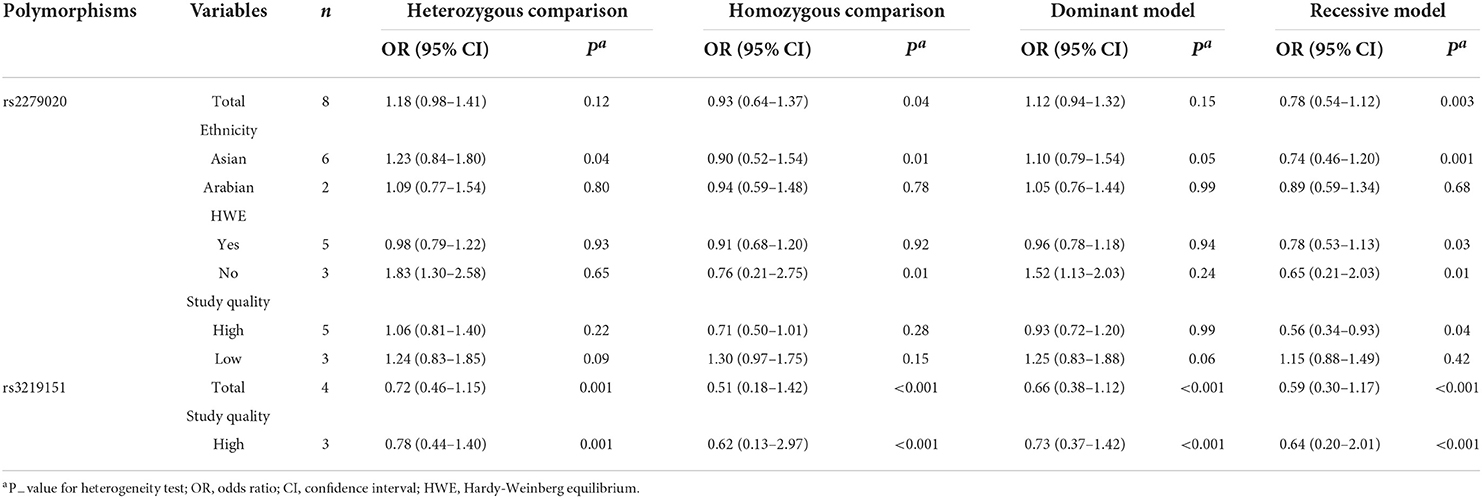
Table 2. Meta-analysis of GABRA1 rs2279020 and GABRA6 rs3219151 polymorphisms with the risk of epilepsy.
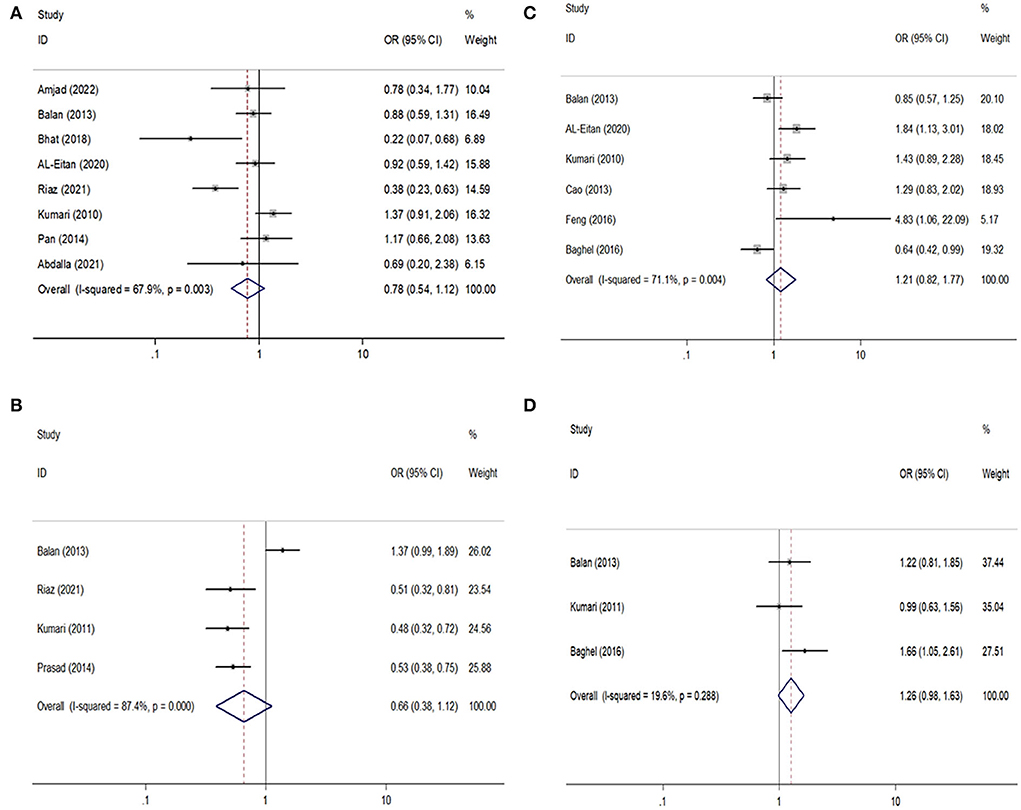
Figure 2. Forest plots of GABRA1 rs2279020 and GABRA6 rs3219151 polymorphisms with the risk of epilepsy and drug resistance. (A,B) Comparison of rs2279020 and rs3219151 with the risk of epilepsy; (C,D) comparison of rs2279020 and rs3219151 with the risk of drug resistance.
For the GABRA6 rs3219151 polymorphism, four studies were included involving 1,299 patients with epilepsy and 929 controls. All the studies were performed in Asians and were in HWE except for one (32). No significant association between the GABRA6 rs3219151 and the risk of epilepsy was found in both overall comparisons and subgroup analyses based on study quality (Table 2,Figure 2B).
Meta-analysis of the association of the GABRA1 rs2279020 and GABRA6 rs3219151 polymorphisms with risk of drug resistance in Asian and Arabic populations
For the GABRA1 rs2279020 polymorphism, six studies were included involving 1,860 patients. High heterogeneity among studies was observed in overall analysis rather than in subgroup analysis based on study quality. The meta-regression analysis also showed that study quality can explain all the heterogeneity under heterozygous comparison and dominant genetic model. There was no significant association of the GABRA1 rs2279020 polymorphism with resistance to AEDs in the overall pooled populations and Asians. As a matter of interest, a significant association of the GABRA1 rs2279020 polymorphism with resistance to AEDs was observed in low-quality studies (heterozygous comparison: OR = 1.49, 95% CI, 1.12–1.98; dominant model: OR = 1.57, 95% CI, 1.20–2.04; recessive model: OR = 1.31, 95% CI, 1.01–1.71) but not in the high-quality studies (Table 3, Figure 2C), indicating that the negative results may be more reliable.
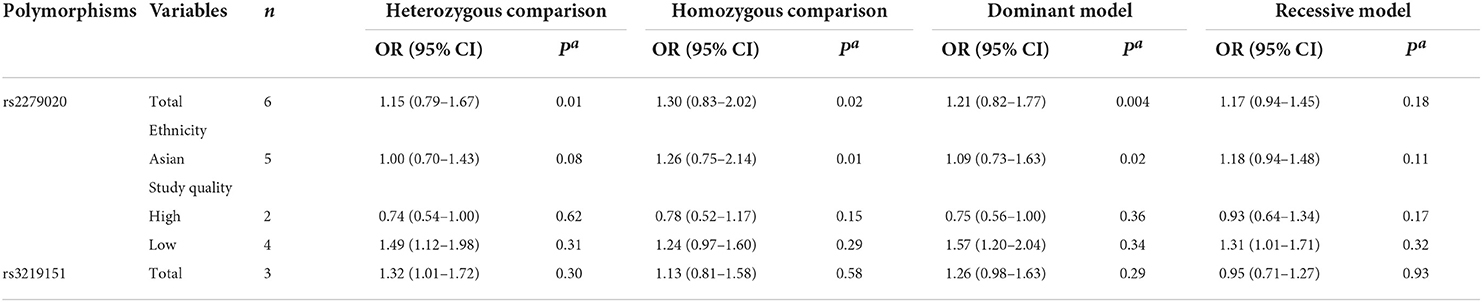
Table 3. Meta-analysis of GABRA1 rs2279020 and GABRA6 rs3219151polymorphisms with drug-resistance epilepsy.
For the GABRA6 rs3219151 polymorphism, three studies were included involving 1,202 patients. A borderline significant association of the GABRA6 rs3219151 polymorphism with resistance to AEDs was detected under a heterozygous comparison (OR = 1.32, 95% CI, 1.01–1.72) (Table 3, Figure 2D).
Sensitivity analysis
A reverse outcome of the GABRA1 rs2279020 polymorphism with epilepsy risk was observed when the study by Balan et al. was excluded from the heterozygous comparison (25) and the study by Kumari et al. from the recessive genetic model (16). The reverse outcome of the GABRA1 rs2279020 polymorphism with resistance to AEDs was also observed when the study by Baghel et al. was excluded (24).
TSA analysis
The TSA analysis was used to evaluate the reliability of the meta-analysis. As shown in Figures 3A,C,D, the cumulative Z-curve crossed the futility boundary, supporting the negative results in the meta-analysis. However, the cumulative Z-curve in Figure 3B did not cross any boundary, indicating that additional studies investigating the association between the GABRA6 rs3219151 polymorphism and epilepsy risk are required.
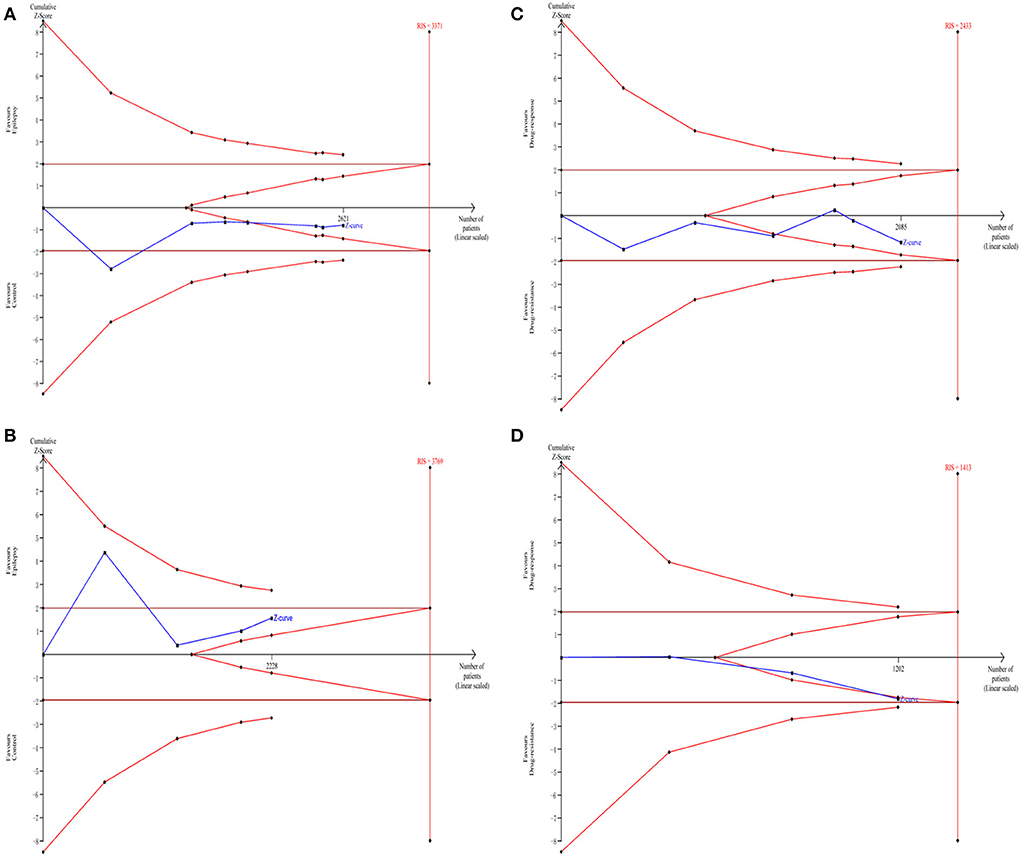
Figure 3. Trial sequential analysis of the association between rs2279020 and rs3219151 and the risk of epilepsy (A,B). Trial sequential analysis of the association between rs2279020 and rs3219151 and the risk of drug resistance (C,D). RIS, required information size.
Discussion
The GABA, a crucial inhibitory neurotransmitter, has a role in both pre- and post-synaptic neuronal processes by binding to GABAARs. Genetic mutations in GABAAR subunits have been demonstrated to be the risk factors for the development of epilepsy through multiple mechanisms, including reduction of mRNA stability, impairment of subunit folding, and aberrant glycosylation causing inhibition of receptor assembly and trafficking (33). In addition, GABAARs constitute the targets for many AEDs such as benzodiazepines, phenobarbital, gabapentin, and topiramate (34). Therefore, the SNPs in GABAAR subunits may influence the susceptibility of epilepsy and different responsiveness to AEDs.
Previously, an SNP located in intron 10 of GABRA1 (rs2279020) was reported to be potentially functional, with the A → G shift changing the shape of the protein that led to alternative splicing (32). A significant association of the rs2279020 with epilepsy risk was reported by some research groups (16, 26, 32), whereas no significant association was reported by other research groups (13, 17, 23, 25). The controversial results were also observed in comparison to the rs2279020 with AED resistance (16, 17, 24, 25). For example, some authors reported that the rs2279020 was a risk factor for affecting treatment efficacy (16, 17). However, some authors reported that the rs2279020 was not associated with AED resistance (24, 25). One reason for the inclusive results may be the insufficient power due to small samples in each individual study. The meta-analysis was then used to overcome the drawback by pooling all the published data together. The findings from the current meta-analysis showed no correlation of the rs2279020 to the susceptibility of epilepsy and AED responsiveness. Furthermore, the TSA revealed that the cumulative Z-curve crossed the futility boundary, indicating that the negative results may be robust.
The rs3219151, located in the 3′ untranslated region of GABRA6 was predicted to affect the microRNA-binding site activity (35). The association of the rs3219151 with epilepsy susceptibility and AED resistance was also examined in a series of studies, but with uncertain findings. Balan et al. reported that the rs3219151 was not related to epilepsy susceptibility and AED resistance (25). However, Riaz et al. (32), Kumari et al. (29), and Prasad et al. (31) reported that the rs3219151 was a significant risk factor for developing epilepsy. By pooling all the original data together, this meta-analysis found no association of the rs3219151 with epilepsy susceptibility but a borderline significant association with AED resistance. The results seem to be unreliable for the following reasons. First, subsequent TSA showed that the current evidence was insufficient. Second, each technique has an advantage and a risk for genotyping error. To make the genotyping results robust, it is necessary to perform quality control by using a Sanger sequencing confirmation, which is a gold standard for genotyping (36). However, <30% of the studies used the second genotyping method for quality control in this study. Finally, there were only four studies investigating the distribution of the rs3219151 among cases and controls and only three studies investigating the difference of the rs3219151 among patients with AED responsiveness and resistance, which may make the findings uncertain. Further association studies, therefore, should be performed to confirm the results in the meta-analysis.
Heterogeneity analysis in this study showed that strong heterogeneity was observed in some overall comparisons. The meta-regression analysis was used to explore the possible reason, and we found that study quality can explain all the heterogeneity, suggesting that high-quality studies were so important to obtain precise observational data. The sensitivity analysis revealed that the outcome altered if we excluded a single study each time (16, 24, 25). Only studies carried out with the Asian and Arabic populations were included in this study. As it is necessary for association studies performed in diverse ethnic groups, additional studies involving subjects of different races are required. There are different types of epilepsy, and thus it is of great value to perform subgroup analysis based on the subtype of epilepsy. However, insufficient data in this study prevented our further subgroup analysis. Although the limitations existed in this meta-analysis, the evidence of null association of the rs2279020 with epilepsy risk and AEDs resistance seems to be reliable based on supporting data from TSA. However, further studies evaluating the effect of rs3219151 on the predisposition of epilepsy are of great importance, especially in Africans and Caucasians.
In conclusion, the present accumulated evidence revealed that neither the GABRA1 rs2279020 nor the GABRA6 rs3219151 polymorphism was a risk factor for the etiology of epilepsy and antiepileptic drug responsiveness in the Asian and Arabic populations. Larger population-based case-cohort studies are warranted to verify the quantitative association in diverse ethnicities, especially in Africans and Caucasians.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
XS designed and wrote the manuscript. TZ and YY collected and analyzed the data. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the 1.3.5 project for disciplines of excellence, the West China Hospital, Sichuan University (Grant Nos. TJZ202006 and 20HXJS007), the Key Project for Science and Technology Department of Sichuan Province (Grant No. 2018SZ0216), and the Sichuan University (Grant No. 2017scu11038).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Birbeck GL. Revising and refining the epilepsy classification system: priorities from a developing world perspective. Epilepsia. (2012) 53 (Suppl. 2):18–21. doi: 10.1111/j.1528-1167.2012.03554.x
2. Radhakrishnan K. Challenges in the management of epilepsy in resource-poor countries. Nat Rev Neurol. (2009) 5:323–30. doi: 10.1038/nrneurol.2009.53
3. Matuja WB, Kilonzo G, Mbena P, Mwango'mbola RL, Wong P, Goodfellow P, et al. Risk factors for epilepsy in a rural area in Tanzania. A community-based case-control study. Neuroepidemiology. (2001) 20:242–7. doi: 10.1159/000054797
4. Kjeldsen MJ, Kyvik KO, Friis ML, Christensen K. Genetic and environmental factors in febrile seizures: a Danish population-based twin study. Epilepsy Res. (2002) 51:167–77. doi: 10.1016/S0920-1211(02)00121-3
5. Kjeldsen MJ, Corey LA, Solaas MH, Friis ML, Harris JR, Kyvik KO, et al. Genetic factors in seizures: a population-based study of 47,626 US, Norwegian and Danish twin pairs. Twin Res Hum Genet. (2005) 8:138–47. doi: 10.1375/twin.8.2.138
6. Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. (2000) 342:314–9. doi: 10.1056/NEJM200002033420503
7. Macdonald RL, Gallagher MJ, Feng HJ, Kang J. GABA(A) receptor epilepsy mutations. Biochem Pharmacol. (2004) 68:1497–506. doi: 10.1016/j.bcp.2004.07.029
8. Maljevic S, Moller RS, Reid CA, Perez-Palma E, Lal D, May P, et al. Spectrum of GABAA receptor variants in epilepsy. Curr Opin Neurol. (2019) 32:183–90. doi: 10.1097/WCO.0000000000000657
9. Bethmann K, Fritschy JM, Brandt C, Loscher W. Antiepileptic drug resistant rats differ from drug responsive rats in GABA A receptor subunit expression in a model of temporal lobe epilepsy. Neurobiol Dis. (2008) 31:169–87. doi: 10.1016/j.nbd.2008.01.005
10. Minty AJ, Caravatti M, Robert B, Cohen A, Daubas P, Weydert A, et al. Mouse actin messenger RNAs. Construction and characterization of a recombinant plasmid molecule containing a complementary DNA transcript of mouse alpha-actin mRNA. J Biol Chem. (1981) 256:1008–14. doi: 10.1016/S0021-9258(19)70080-5
11. Albery WJ, Cass AE, Shu ZX. Inhibited enzyme electrodes. Part 1: theoretical model. Biosens Bioelectron. (1990) 5:367–78. doi: 10.1016/0956-5663(90)80016-7
12. Fritschy JM. Epilepsy, E/I balance and GABA(A) receptor plasticity. Front Mol Neurosci. (2008) 1:5. doi: 10.3389/neuro.02.005.2008
13. Amjad M, Tabassum A, Sher K, Kumar S, Zehra S, Fatima S. Impact of GABA(A) receptor gene variants (rs2279020 and rs211037) on the risk of predisposition to epilepsy: a case-control study. Neurol Sci. (2022) 43:4431–8. doi: 10.1007/s10072-022-05947-7
14. Srivastava S, Cohen J, Pevsner J, Aradhya S, McKnight D, Butler E, et al. A novel variant in GABRB2 associated with intellectual disability and epilepsy. Am J Med Genet Part A. (2014) 164a:2914–21. doi: 10.1002/ajmg.a.36714
15. Bai YF, Chiu M, Chan ES, Axerio-Cilies P, Lu J, Huh L, et al. Pathophysiology of and therapeutic options for a GABRA1 variant linked to epileptic encephalopathy. Mol Brain. (2019) 12:92. doi: 10.1186/s13041-019-0513-9
16. Kumari R, Lakhan R, Kalita J, Misra UK, Mittal B. Association of alpha subunit of GABAA receptor subtype gene polymorphisms with epilepsy susceptibility and drug resistance in north Indian population. Seizure. (2010) 19:237–41. doi: 10.1016/j.seizure.2010.02.009
17. Al-Eitan LN, Al-Dalala IM, Elshammari AK, Khreisat WH, Nimiri AF, Alnaamneh AH, et al. Genetic association of epilepsy and anti-epileptic drugs treatment in jordanian patients. Pharmacogenomics Pers Med. (2020) 13:503–10. doi: 10.2147/PGPM.S273125
18. Norris JM, Simpson BS, Ball R, Freeman A, Kirkham A, Parry MA, et al. A modified Newcastle-Ottawa scale for assessment of study quality in genetic urological research. Eur Urol. (2021) 79:325–6. doi: 10.1016/j.eururo.2020.12.017
19. Kang H. Trial sequential analysis: novel approach for meta-analysis. Anesth Pain Med. (2021) 16:138–50. doi: 10.17085/apm.21038
20. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21:1539–58. doi: 10.1002/sim.1186
21. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
22. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. (1959) 22:719–48.
23. Abdalla SF, Eltahir HB, El-Magzoub SM. Assessment of gene polymorphism in GABAA1 receptor among sudanese patients with juvenile myoclonic epilepsy (JME). J Pharm Res Int. (2021) 33:13–9. doi: 10.9734/jpri/2021/v33i33B31793
24. Baghel R, Grover S, Kaur H, Jajodia A, Rawat C, Srivastava A, et al. Evaluating the role of genetic variants on first-line antiepileptic drug response in North India: significance of SCN1A and GABRA1 gene variants in phenytoin monotherapy and its serum drug levels. CNS Neurosci Ther. (2016) 22:740–57. eng. doi: 10.1111/cns.12570
25. Balan S, Sathyan S, Radha SK, Joseph V, Radhakrishnan K, Banerjee M. GABRG2, rs211037 is associated with epilepsy susceptibility, but not with antiepileptic drug resistance and febrile seizures. Pharmacogenet Genomics. (2013) 23:605–10. doi: 10.1097/FPC.0000000000000000
26. Bhat MA, Guru SA, Mir R, Waza AA, Zuberi M, Sumi MP, et al. Association of GABAA receptor gene with epilepsy syndromes. J Mol Neurosci. (2018) 65:141–153. doi: 10.1007/s12031-018-1081-7
27. Cao Y, Xiao B. Association study between the polymorphisms of ABCC2, ABCB1, SCN1A, SCN2A, GABRA1 and drug-resistant epilepsy in Han Chinese (dissertation/master's thesis). Central South University, Changsha, China (2013).
28. Feng W, Mei S, Zhu L, Yu Y, Yang W, Gao B, et al. Effects of UGT1A6 and GABRA1 on standardized valproic acid plasma concentrations and treatment effect in children with epilepsy in China. Ther Drug Monit. (2016) 38:738–43. doi: 10.1097/FTD.0000000000000337
29. Kumari R, Lakhan R, Kalita J, Garg RK, Misra UK, Mittal B. Potential role of GABAA receptor subunit; GABRA6, GABRB2 and GABRR2 gene polymorphisms in epilepsy susceptibility and pharmacotherapy in North Indian population. Clin Chim Acta. (2011) 412:1244–8. doi: 10.1016/j.cca.2011.03.018
30. Pan X, Wang X. A primary study on the association of GABA(A) receptor subunit gene single nucleotide polymorphisms and epilepsy (dissertation/doctor's thesis). Fudan University, Shanghai, China (2014).
31. Prasad DK, Shaheen U, Satyanarayana U, Prabha TS, Jyothy A, Munshi A. Association of GABRA6 1519 T>C (rs3219151) and synapsin II (rs37733634) gene polymorphisms with the development of idiopathic generalized epilepsy. Epilepsy Res. (2014) 108:1267–73. doi: 10.1016/j.eplepsyres.2014.07.001
32. Riaz M, Abbasi MH, Sheikh N, Saleem T, Virk AO. GABRA1 and GABRA6 gene mutations in idiopathic generalized epilepsy patients. Seizure. (2021) 93:88–94. doi: 10.1016/j.seizure.2021.10.013
33. Macdonald RL, Kang JQ, Gallagher MJ. Mutations in GABAA receptor subunits associated with genetic epilepsies. J Physiol. (2010) 588 (Pt. 11):1861–9. doi: 10.1113/jphysiol.2010.186999
34. Rogawski MA, Loscher W. The neurobiology of antiepileptic drugs. Nat Rev Neurosci. (2004) 5:553–64. doi: 10.1038/nrn1430
35. Li D, Sulovari A, Cheng C, Zhao H, Kranzler HR, Gelernter J. Association of gamma-aminobutyric acid A receptor alpha2 gene (GABRA2) with alcohol use disorder. Neuropsychopharmacology. (2014) 39:907–18. doi: 10.1038/npp.2013.291
Keywords: epilepsy, GABRA1, GABRA6, polymorphism, meta-analysis, trial sequential analysis (TSA)
Citation: Zhang T, Yang Y and Sima X (2022) No association of GABRA1 rs2279020 and GABRA6 rs3219151 polymorphisms with risk of epilepsy and antiepileptic drug responsiveness in Asian and Arabic populations: Evidence from a meta-analysis with trial sequential analysis. Front. Neurol. 13:996631. doi: 10.3389/fneur.2022.996631
Received: 18 July 2022; Accepted: 22 August 2022;
Published: 14 September 2022.
Edited by:
Kette D. Valente, University of São Paulo, BrazilReviewed by:
Yahya Wahba, Mansoura University, EgyptJuliana Alcantara, University of São Paulo, Brazil
Copyright © 2022 Zhang, Yang and Sima. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiutian Sima, c2ltYXhpdXRpYW5Ac2N1LmVkdS5jbg==
†These authors have contributed equally to this work
 Tiejun Zhang
Tiejun Zhang Yi Yang2†
Yi Yang2† Xiutian Sima
Xiutian Sima