
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 29 September 2022
Sec. Dementia and Neurodegenerative Diseases
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.996093
 Bei Li1†
Bei Li1† Dejun Liu2†
Dejun Liu2† Qiaoqin Wan3
Qiaoqin Wan3 Can Sheng4
Can Sheng4 Xiting Wang4
Xiting Wang4 Fangda Leng1
Fangda Leng1 Qing Peng1
Qing Peng1 Ting Wang1
Ting Wang1 Ailian Du5
Ailian Du5 Feiqi Zhu6
Feiqi Zhu6 Dunzhu Mima7
Dunzhu Mima7 Huali Wang8
Huali Wang8 Hengge Xie9
Hengge Xie9 Zhaoxia Wang1*
Zhaoxia Wang1* Haiqiang Jin1*
Haiqiang Jin1* Yongan Sun1*
Yongan Sun1*Introduction: In China, the increasing number of people with Alzheimer's disease (AD) poses a great challenge to families and the country. Economic and cultural differences cause a urban-rural gap in medical resources. This multicenter survey aimed to investigate the real-world practice of disease treatment among people with AD.
Methods: People with AD and their caregivers from 30 provincial regions in mainland China were enrolled from October 2020 to December 2020 to be surveyed for their treatment experience. Logistic regression was used to explore the factors that influence medication adherence in all areas, urban areas, and rural areas.
Results: In this survey, 1,427 participants came from urban areas, and 539 participants came from rural areas. Patients in urban areas were older (mean age 74 vs. 70, p = 0.001), less frequently had mild AD (36.0 vs. 52.1%, p < 0.001), and more often were cared for at professional institutions (8.8 vs. 3.2%, p < 0.001). In terms of pharmacotherapy, 77.8% of people accepted taking lifelong medication, whereas 61.3% of patients insisted on taking medications. Although 72.0% of rural people believed in taking lifelong medication, only 30.0% adhered to drug use. The major factors that influenced medication adherence for all patients with AD were regional distribution (p < 0.001, OR = 6.18, 95% CI: 4.93–7.74) and family earnings (p = 0.003, OR = 1.22, 95% CI: 1.07–1.38). In rural areas, family earnings (p = 0.008, OR = 1.44, 95% CI: 1.10–1.89) and severity of AD (p = 0.033, OR = 1.31, 95% CI: 1.02–1.68) were the main factors. Family earnings (p = 0.038, OR = 1.16, 95% CI: 1.01–1.34) was the only factor among urban areas. Among all non-pharmaceutical activities except for cognitive intervention, the participation rates of rural patients were significantly higher than those of urban patients (p < 0.05).
Conclusion: Although national progress has been made in the public awareness of disease treatment, adequate diagnosis and medication adherence need to be prompted, especially in rural areas. Furthermore, lifelong treatment should be improved based on regional characteristics through the joint efforts of the government, health workers, and social volunteers.
Alzheimer's disease (AD), a major type of dementia, has become a great public health challenge (1). Globally, nearly 50 million people live with dementia (2). The results of one worldwide survey organized by the WHO showed that AD and other forms of dementia have become the 7th leading cause of death (3). In China, a surge in the elderly population has resulted in 9.83 million people aged 60 years or older suffering from AD (4). AD rose to the fifth leading cause of death in China (5). In the US, the annual total costs of AD were estimated to be $167.74 billion in 2015 and were predicted to reach $507.49 billion in 2030 and $1.89 trillion in 2050 (6). This projected growth of care and economic burden of AD arouses national attention to the management of AD.
In response to the Global Action Plan on Dementia 2017–2025 published by the WHO and ADI, the Chinese Government and dementia organizations have made great efforts in the development of diagnosis and treatment criteria, reinforcement of training dementia doctors, and cultivation of public awareness about this disease (7, 8). In 2020, the national guidelines for the diagnosis and treatment of mental disorders pointed to the therapeutic principles of AD, which included early diagnosis, timely treatment, and lifelong management (9). Regarding the treatment of AD, this guideline emphasized pharmaceutical and non-pharmaceutical therapy. Adherence to pharmacotherapy was the key to delaying the deterioration of cognitive ability. In addition, supplementary non-pharmacotherapy played a momentous role in alleviating behavioral and psychiatric symptoms. However, the urban-rural gap in the medical field in China presents challenges for national promotion. Compared to people living in urban areas, people in rural areas often have lower levels of income and education and more difficulties accessing advanced medicine and technology (7, 10). Patients with AD in urban and rural areas in China also have different experiences in the treatment of dementia (7). Thus, examining the real-world practice of disease treatment in different regions of China is essential to provide a basis to formulate regulations and minimize the regional gap.
In this study, we aimed to investigate the demographic and clinical characteristics as well as the treatment experience of patients with AD in urban and rural areas. According to the data of this national survey, various guidelines should be considered and adopted by policy-makers, health workers, and researchers in China and worldwide to improve patients' access and adherence to the treatment of AD.
This large-sample, multicenter, cross-sectional study surveyed participants from 30 provincial, municipal, and autonomous regions in mainland China between October 2020 and December 2020, including Zhejiang, Beijing, Shaanxi, Shanxi, Hunan, Hebei, Henan, Heilongjiang and other provinces. Patients who were diagnosed with probable AD dementia by cognitive experts from comprehensive psychiatric hospitals according to the criteria of the National Institute of Aging and Alzheimer's Association (NIA-AA) in 2011 were included (11). The tools of neuropsychological assessment for diagnosis of AD were based on the national guidelines for the diagnosis and treatment of mental disorders, such as MoCA, MMSE, ADAS-cog, CDR and ADL (9, 12). If patients had other neurological diseases that were related to cognitive impairment and major psychiatric disorders, they were excluded. After obtaining patients' or their caregivers' consent, they were enrolled in the completion of questionnaires.
The demographic and clinical characteristics and treatment experiences of patients with AD were assessed in this study. The questionnaire was derived from the results of a previous survey (13, 14) and had been modified many times by several specialists according to the aim of this study.
The demographic and clinical characteristics of patients were assessed with a self-designed questionnaire, including gender, age, residential area, length of education, family earnings, medical insurance, severity of AD, duration since AD dementia diagnosis, and form of care. The urban and rural typologies for the Chinese geographical regions were defined based on the “Provisions on the Statistical Division of Urban and Rural Areas” produced by the National Bureau of Statistics, which considered the differences in demographic, social and economic development (15). This survey classified urban and rural areas according to the residential area of patients and the national standards for regional division. Family earnings referred to per capita monthly household income, which included salary, bonuses, and allowances. The severity of AD was estimated by neurologists based on patients' symptoms and neuroimaging data. The criterion of MTA, which ranks the degree of hippocampal atrophy, is considered a major indicator of disease severity (16). Unlike home care, where family members or paid caregivers played the primary caregiving roles, professional care was consisted of day care services provided by community health services, omnidirectional hosting offered by nursing homes, and respite care provided by professional caregivers.
The treatment experiences of patients with AD were assessed with a self-designed questionnaire. The status of treatment was assessed by several items, including attitude toward lifelong medication, behavior when taking AD medications, views on AD medications, and participation in non-pharmaceutical therapy. In addition, one item was augmented based on the pharmaceutical behavior of patients. If patients were currently taking medications, they completed the item about the duration of drug adherence. On the condition that patients had never started or withdrawn from pharmacotherapy, the participants would answer the questions about the reasons for their abstinence or withdrawal. AD medications in this study included cholinesterase inhibitors, memantine, and/or traditional Chinese medicines. Participation in non-pharmaceutical therapy was related to whether patients ever participated in adjunctive activities, such as physical activity, cognitive intervention, mental activity and musical therapy.
If the patients and their caregivers were willing to participate in this survey after the introduction of study purposes, they completed a questionnaire under the guidance of the diagnostic doctors who received comprehensive training. When patients were unable to cooperate, their caregivers were asked for assisting in completing questionnaires.
This study was approved by the Ethics Committee of Peking University First Hospital. The right to informed consent, voluntary participation and confidentiality of responses were assured.
First, all variables were subjected to descriptive analyses with the SPSS 23.0 software. For categorical variables, data are presented as the number of cases and percentages. Continuous variables are summarized as the means and standard deviations. Then, the t-sample test and chi-square test were used to calculate p-values for differences between urban and rural areas. Finally, binary logistic regression was used to explore the factors that influence medication adherence. Based on the pharmacotherapy behaviors, people who did not adhere to medication regimens were classified as never having taken or having withdrawn from taking medication. All the demographic and clinical variables that showed statistically significant difference in univariate analysis were filtered by backward method with likelihood ratio test.
Finally, a total of 2,813 questionnaires (1,674 from urban areas and 1,139 from rural areas) were collected, and 1,966 valid questionnaires (1,427 from urban areas and 539 from rural areas) remained for analysis. Questionnaires were eliminated for the incorrect and leaked filling of items. The demographic and clinical characteristics of the urban and rural patients are presented in Table 1.
Their average age was 73.1 ± 10.7 years. In addition, urban patients were, on average, older than rural patients (74.3 ± 10.0 vs. 70.0 ± 11.7; t = −8.132, p = 0.001). Moreover, 40.4% of patients had mild AD, whereas 33.1% of patients were classified as having severe AD. However, fewer people suffered from mild AD in urban areas than in rural areas (36.0 vs. 52.1%, p < 0.001). Among rural patients with low education (length of education: 0–9 years), 49.5% (n = 213) of them had mild AD, while 21.2% (n = 91) of them were sever. In urban areas, 35.0% (n = 245) of patients with low education were categorized as mild AD and 35.7% (n = 250) of them had sever AD. Moreover, the form of care significantly differed between urban and rural areas, and more patients obtained care from professional institutions in urban areas than in rural areas (8.8 vs. 3.2%, p < 0.001). A total of 92.7% of patients lived at home and were cared for by family members (n = 1,561, 1,072 from urban areas and 489 from rural areas) or paid caregivers (n = 262, 229 from urban areas and 33 from rural areas).
The characteristics of the treatment experiences of the urban and rural patients are shown in Table 2.
In terms of pharmacotherapy, 77.8% of people agreed with lifelong medication, while 61.3% of patients insisted on taking medications. Moreover, 65.4% of people insisted on taking medications for more than 1 year. Their attitudes toward current medications were that the treatments were expensive (65.2%) and poorly effective (64.7%). In rural areas, 72.0% of people believed in lifelong medication, but only 30.0% of patients adhered to drug treatment. The most frequent reasons for medication withdrawal among rural people were high price (40.5%) and poor curative effect (40.5%). In addition, 42.6% of patients considered the severity of their cognitive impairment to be too mild to require medications. In urban areas, patients generally withdrew from or denied medication treatment because of a poor curative effect (49.8%) and a fear of adverse effects (35.7%). Moreover, the two most common non-pharmaceutical therapies were regular physical activity (urban areas: 62.2% vs. rural areas: 67.7%, p = 0.022) and cognitive intervention (urban areas: 56.9% vs. rural areas: 61.4%, p = 0.071). The participation rates of rural patients in all non-pharmaceutical activities except cognitive intervention were significantly higher than those of urban patients (p < 0.05).
Table 3 shows the screening results of binary logistic regression models, which were built using the demographic and clinical characteristics from Table 1 and behavior of pharmacotherapy from Table 2 (people with medication adherence vs. people without medication adherence). The major factors that influence medication adherence for patients with AD were regional distribution (p < 0.001, OR = 6.18, 95% CI: 4.93–7.74) and family earnings (p = 0.003, OR = 1.22, 95% CI: 1.07–1.38). The results of further analyses with factors that influenced medication adherence in different areas showed that family earnings (p = 0.038, OR = 1.16, 95% CI: 1.01–1.34) was the only factor among urban areas, whereas family earnings (p = 0.008, OR = 1.44, 95% CI: 1.10–1.89) and the severity of AD (p = 0.033, OR = 1.31, 95% CI: 1.02–1.68) were the main factors for rural people.
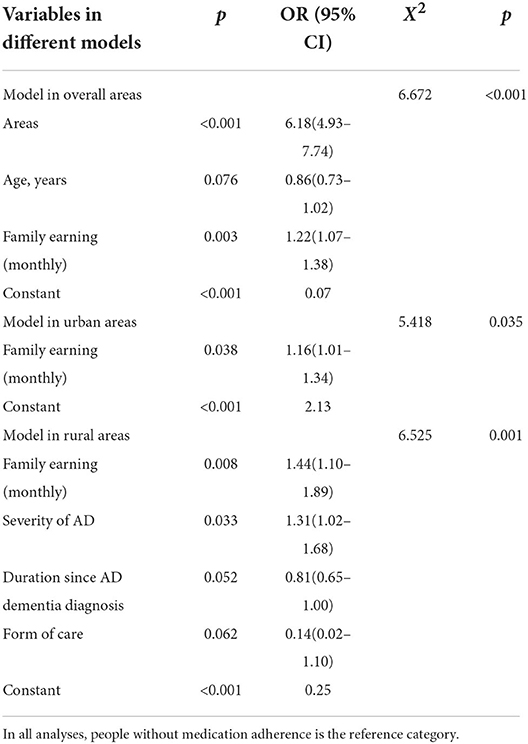
Table 3. Binary logistic regressions of medication adherence among the urban and rural people with AD.
The increasing incidence of AD among the aging population is imposing a significant public health burden in China. The national government and related organizations have made great efforts in the development of practice guidelines, professional training and public education in the last decade (4, 7). However, this study is, to the authors' best knowledge, the first national cross-sectional survey that explored the characteristics and influencing factors of treatment among people with AD from urban and rural areas in China. The results identified differences in the pharmacological and non-pharmacological therapies employed between urban and rural areas, especially in terms of medication compliance.
In our survey, more than 70% of people agreed with lifelong medication, irrespective of their origin (rural or urban areas). Over 80% of all patients had used at least one antidementia drug. The treatment rate was appreciably higher than that reported by a previous study conducted in China 10 years ago, which showed that 30.1% of people with AD were treated with medication (17). This achievement would not be possible without mutual efforts by the government, hospitals, institutions and media to raise public awareness (18). In addition, ~60% of patients in our survey insisted on drug treatment, 65% of whom had been taking medications for more than 1 year. However, only 30% of rural patients in our research adhered to a medication regimen, and more than 60% of patients consistently used medication for less than a year. A previous survey indicated that the rates of medication compliance ranged from 17 to 100% among people with AD, which might be attributed to cognitive deficits in various domains, unique sociocultural backgrounds, and different reporters (19–21). Given that the prompt initiation of and adherence to medication regimens after diagnosis are crucial to cognitive preservation and achieving maximal treatment efficacy (9, 22), pharmacotherapy compliance needs to be further improved in China, especially in rural areas, as it is determined by specific individual characteristics.
The logistic results of this study found that regional factors and family earnings primarily influenced medication adherence. The survey also showed regional differences in the reasons for non-compliance with medication regimens. In terms of reasons for not starting pharmacotherapy, nearly half of rural participants thought their illness was not sufficiently serious to require medication, while most urban participants were afraid of the adverse effects. Unlike in urban areas, 40% of patients in rural areas cannot afford to adhere to a medication regimen. Imbalances in economic and cultural development in different regions of China might cause discrepancies in access to medical resources (10). Conversely, a shortage of dementia doctors and memory clinics in rural areas could prevent some patients with mild AD from accessing care, resulting in a low number of prescriptions for treating AD (7, 17). Consequently, patients did not realize the importance of taking medication. Similar to the results of other studies, awareness of the disease and an emphasis on medication were positively related to treatment adherence (23, 24). Moreover, challenges in obtaining financial reimbursement for drug costs may have influenced access to AD treatment, which also corroborated previous studies (19, 25). Hence, financial support, professional training, the implementation of memory clinics and popularization of knowledge about disease among rural areas may alleviate regional gaps in pharmacotherapy adherence.
Because most rural participants were younger and at an earlier stage of AD than urban participants, pharmacotherapy might be more effective for this population in preventing cognitive decline and maintaining the ability to complete daily activities (9). The logistic analysis of this study concentrating on rural patients illustrated that family earnings and the severity of AD influenced medication adherence. Economic factors for non-compliance to AD medications were coincident with the classification by the WHO (19, 26). In addition, AD with memory loss and impaired executive function was closely related to medication adherence (21). A previous study explored whether the differential outcome procedure that attempts to improve discriminative learning and memory might simulate adherence to medical prescriptions (27). Because of these symptoms of AD, social support from caregivers is important to ensure adherence to a medication regimen. Family members, especially spouses, can supervise patients' medications and even expand their role to provide emotional support and manage disruptive behaviors (23, 28). Overall, not only financial support but also cognitive training programs and caregiver support should be improved to increase medication adherence in rural areas.
In accordance with rural people, family earnings was a factor that influenced medication adherence for urban people. But apart from that, this study identified reasons for non-compliance among these patients that could not be neglected. Most urban participants did not start pharmacotherapy due to a fear of adverse effects and suspended AD medications because of poor curative effects. In accordance with other studies, side effects from medications, including diarrhea, nausea, sleep disturbances and ineffectiveness, contributed to non-compliance (9, 19, 29). Older people with comorbidities usually took multiple medications and consequently worried about health outcomes, such as longer survival, prevention of disease-specific events, and tolerable risk of adverse drug reactions. Therefore, they preferentially adhered to treatment for chronic deases other than AD, such as hypertension, diabetes mellitus and dyslipidemia (19, 21). Moreover, negative therapeutic experiences due to adverse drug effects and the ineffectiveness of medication might be due to the mode of use and inadequate pharmaceutical form for the clinical condition, such as dysphagia (29, 30). Combination medication with augmented therapeutic benefits and replacement with transdermal rivastigmine might be useful to ease the worries of patients and their caregivers (31, 32). Therefore, appropriate medication guidance by a multidisciplinary team and a suitable medication form might be useful to facilitate adherence to a drug regimen among urban patients.
In addition to pharmacotherapy, non-pharmacological therapy is essential to postpone cognitive decline and manage neuropsychiatric symptoms. In our survey, more than half of respondents participated in non-pharmacological activities, such as cognitive intervention and regular physical activity. Cognitive training and exercise were recognized as common interventions once AD was diagnosed, despite a lack of evidence for the optimal mode of these activities to maximize benefit (22). Nevertheless, the findings indicated that a majority of interventions involved fewer urban patients than rural patients, which might be attributed to the characteristics of urban patients, who were older and had more serious AD characterized by impaired execution of daily activities and neuropsychiatric symptoms. Furthermore, the heavy traffic in urban areas hinders patients from leaving their homes to participate in various activities. Caregivers in previous Chinese studies also expressed the urgent need for formal support for caregiving (14, 33). In brief, the primary health service requires further improvement in terms of professional advice and convenient access to non-pharmacological therapy to ease the burden on caregivers.
The present study had several limitations. First, this study adopted a convenience sampling method, and 27.4% of participants came from rural areas, which might restrict its generalization in rural areas; however, this result may suggest that AD is underdiagnosed in rural areas. Second, this study was based on patient self-reports and interviews with their caregivers, and recall bias may have influenced the reported experiences of treatment. Third, this study defined rural or urban areas according to resident population, which might restrict direct comparisons with studies from other countries that rely on different definitions of living areas. Fourth, except for neuroimaging examination, patients with various demographic and clinical characteristics were assessed by different neuropsychological tools. In order to enhance professional training with diagnosis and management of dementia, future research could develop a national uniform system of neuropsychological evaluation for AD or other types of dementia. Finally, this survey adopted self-designed questionnaires which originated from the results of a previous survey and were modified by several specialists. Even though the results had astriction in comparability with studies from other countries, the characteristics of treatment among people with AD from urban and rural areas in China were revealed. Furthermore, people with mild cognitive impairment (MCI) were not included in this survey. People with MCI were encouraged to improve healthy lifestyle and prevent the occurrence of dementia (22). The management status of MCI could be investigated in the future. There is a window of intervention for delaying the progression of cognitive impairment.
This study is the first to feature a large sample to examine differences in the characteristics of disease treatment in patients with AD in rural and urban areas and reflects the epidemiological reality in China. To achieve the Healthy China Initiative (2019–2030) (8), and there is still a pressing need to promote adequate diagnosis and treatment, especially in rural areas. Furthermore, lifelong treatment, including pharmacotherapy and non-pharmacological therapy, should be improved based on the characteristics of urban and rural areas through the joint efforts of government, health workers, and social volunteers.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of Peking University First Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
BL and DL contributed to the analysis and interpretation of the data and drafting and revision of the manuscript. CS, XW, FL, QP, TW, AD, FZ, and DM contributed to collection of data, quality control, and establishing the database. QW, HW, and HX contributed to advertising and coordination of the study. ZW, HJ, and YS contributed to the conceptualization of the study, formulation of study protocol, and intellectual revision of the manuscript. All authors contributed to the article and approved the submitted version.
The study was supported by Science and Technology Innovation 2030- Major Project (2021ZD0201800, 2021ZD0201805).
We thank all the patients and their caregivers who participated selflessly and all investigators in sampled hospitals for their collaboration in the contact with participants.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Alzheimer's Disease International (ADI). Alzheimer's Disease Facts and Figures (2021). Available online at: https://www.alzint.org/about/dementia-facts-figures/ (accessed June 15, 2021).
2. Alzheimer's Disease International (ADI). World Alzheimer Report 2018. (2018). Available online at: https://www.alz.co.uk/research/world-report-2018 (accessed March 29, 2019).
3. World Health Organization (WHO). The top 10 causes of death. (2018). Available online at: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed January 29, 2019).
4. Jia L, Du Y, Chu L, Zhang Z, Li F, Lyu D, et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health. (2020) 5:e661–71. doi: 10.1016/S2468-2667(20)30185-7
5. Zeng X, Qi J, Yin P, Wang, Li J, Liu Y, Liu J, et al. Report on the burden of disease in China and provincial regions, 1990–2016. Chin Circ J. (2018) 33:1147–58. doi: 10.3969/j.issn.1000-3614.2018.12.002
6. Jia J, Wei C, Chen S, Li F, Tang Y, Qin W, et al. The cost of Alzheimer's disease in China and re-estimation of costs worldwide. Alzheimers Dement. (2018) 14:483–91. doi: 10.1016/j.jalz.2017.12.006
7. Jia L, Quan M, Fu Y, Zhao T, Li Y, Wei C, et al. Dementia in China: epidemiology, clinical management, and research advances. Lancet Neurol. (2020) 19:81–92. doi: 10.1016/S1474-4422(19)30290-X
8. National Health Commission of China. Notice on the exploration and development of depression, senile dementia prevention and treatment feature services. (2020). Available online at: http://www.nhc.gov.cn/jkj/s7914/202009/a63d8f82eb53451f97217bef0962b98f.shtml (accessed June 15, 2021).
9. National Health Commission of China. Guidelines for the diagnosis and treatment of mental disorders. (2020). Available online at: http://www.nhc.gov.cn/yzygj/s7653p/202012/a1c4397dbf504e1393b3d2f6c263d782.shtml (accessed June 15, 2021).
10. Gu H, Ma C, Gi L. Disparities and inequity in urban-rural health care: illustrated by the example of outpatients. J Nanjing Agri Univ. (2015) 15:53–61.
11. McKhann GM, Knopman DS. Chertkow H, Hyman BT, Jack CR, Kawas CH, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the national institute on aging-Alzheimer's association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's Demen. (2011) 7:263–9. doi: 10.1016/j.jalz.2011.03.005
12. Peng DT, Zhang ZJ. Operational Guidance of Neuropsychological Cognitive Scale. Beijing: People's Medical Publishing House. (2015).
13. Fukuda M, Lyu X, Li T, Xie T, Wang H, Yu X. Caregiver burden and related factors among caregivers of people with diagnosis of Alzheimer disease within one year. Chin Mental Health J. (2020) 34:572–7. doi: 10.3969/j.issn.1000-6729.2020.7.004
14. Li Y, Leng F, Xiong Q, Zhou J, Ailian D, Zhu F, et al. Factors associated with caregiving status and caregiver burden for patients with Alzheimer's disease in China. Front Aging Neurosci. (2022) 14:1–9. doi: 10.3389/fnagi.2022.865933
15. National Bureau of Statistics. Provisions on the Statistical Division of Urban and Rural Areas. (2008). Available online at: http://www.stats.gov.cn/tjsj/tjbz/200610/t20061018_8666.html (accessed September 17, 2021).
16. MR Group of Chinese Society of Radiology. Chinese experts consensus on standard of MRI technology of Alzheimer disease. Chin J Radiol. (2019) 53:665–71. doi: 10.3760/cma.j.issn.1005-1201.2019.08.002
17. Jia J, Zuo X, Jia X, Chu C, Wu L, Zhou A, et al. Diagnosis and treatment of dementia in neurology outpatient departments of general hospitals in China. Alzheimers Dement. (2016) 12:446–53. doi: 10.1016/j.jalz.2015.06.1892
18. Alzheimer S Disease Chinese. The sodality of the family with AD: the 20th anniversary of the popular science propaganda | help older people with memory decline in a protracted war. (2020). Available online at: https://www.adc.org.cn/index.php/article/509.html (accessed July 9, 2021).
19. Lum ZK, Suministrado M, Venketasubramanian N, Ikram MK, Chen C. Medication compliance in Singaporean patients with Alzheimer's disease. Singapore Med J. (2019) 60:154–60. doi: 10.11622/smedj.2018076
20. El-Saifi N, Moyle W, Jones C, Tuffaha H. Medication adherence in older patients with dementia: a systematic literature review. J Pharm Pract. (2018) 31:322–34. doi: 10.1177/0897190017710524
21. Smith D, Lovell J, Weller C, Kennedy B, Winbolt M, Young C, et al. A systematic review of medication non-adherence in persons with dementia or cognitive impairment. PLoS ONE. (2017) 12:e170651. doi: 10.1371/journal.pone.0170651
22. Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. (2020) 396:413–46. doi: 10.1016/S0140-6736(20)30367-6
23. Lima S, Gago M, Garrett C, Pereira MG. Medication adherence in Alzheimer's disease: the mediator role of mindfulness. Arch Gerontol Geriatr. (2016) 67:92–7. doi: 10.1016/j.archger.2016.06.021
24. Brady R, Weinman J. Adherence to cholinesterase inhibitors in Alzheimer's disease: a review. Dement Geriatr Cogn Disord. (2013) 35:351–63. doi: 10.1159/000347140
25. Kongpakwattana K, Dilokthornsakul P, Dejthevaporn C, Pattanaprateep O, Chaiyakunapruk N. Compliance and persistence with Alzheimer's disease treatment: a retrospective analysis of multiregional hospital databases in Thailand. J Med Econ. (2019) 22:26–34. doi: 10.1080/13696998.2018.1534739
26. World Health Organization. Adherence to long-term therapies: evidence for action. (2003). Available online at: http://apps.who.int/iris/bitstream/10665/42682/1/9241545992.pdf (accessed April 28, 2022).
27. Molina M, Carmona I, Fuentes LJ, Plaza V, Estevez AF. Enhanced learning and retention of medical information in Alzheimer's disease after differential outcomes training. PLoS ONE. (2020) 15:e231578. doi: 10.1371/journal.pone.0231578
28. Berry B, Apesoa-Varano EC. Medication takeovers: covert druggings and behavioral control in Alzheimer's. Soc Sci Med. (2017) 188:51–9. doi: 10.1016/j.socscimed.2017.07.003
29. Mastroianni PC, Forgerini M. Compliance and drug-related problems in probable Alzheimer's disease elderly. Int Psychogeriatr. (2019) 31:1677–8. doi: 10.1017/S104161021800234X
30. Borah B, Sacco P, Zarotsky V. Predictors of adherence among Alzheimer's disease patients receiving oral therapy. Curr Med Res Opin. (2010) 26:1957–65. doi: 10.1185/03007995.2010.493788
31. Calhoun A, King C, Khoury R, Grossberg GT. An evaluation of memantine ER + donepezil for the treatment of Alzheimer's disease. Expert Opin Pharmacother. (2018) 19:1711–7. doi: 10.1080/14656566.2018.1519022
32. Pai M, Aref H, Bassil N, Kandiah N, Lee J, Srinivasan A, et al. Real-world evaluation of compliance and preference in Alzheimer's disease treatment. Clin Interv Aging. (2015) 10:1779–88. doi: 10.2147/CIA.S85319
Keywords: dementia, Alzheimer's, treatment, medication adherence, regional difference
Citation: Li B, Liu D, Wan Q, Sheng C, Wang X, Leng F, Peng Q, Wang T, Du A, Zhu F, Mima D, Wang H, Xie H, Wang Z, Jin H and Sun Y (2022) Differences in treatment for Alzheimer's disease between urban and rural areas in China. Front. Neurol. 13:996093. doi: 10.3389/fneur.2022.996093
Received: 17 July 2022; Accepted: 12 September 2022;
Published: 29 September 2022.
Edited by:
Jian-Quan Shi, Nanjing Medical University, ChinaReviewed by:
Fabián Román, Costa University Corporation, ColombiaCopyright © 2022 Li, Liu, Wan, Sheng, Wang, Leng, Peng, Wang, Du, Zhu, Mima, Wang, Xie, Wang, Jin and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongan Sun, c3lhQGJqbXUuZWR1LmNu; Haiqiang Jin, amhxOTExQGJqbXUuZWR1LmNu; Zhaoxia Wang, ZHJ3YW5nenhAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.