- 1Department of Neonatology, Women and Children's Hospital, School of Medicine, Xiamen University, Xiamen, Fujian, China
- 2Xiamen Key Laboratory of Perinatal-Neonatal Infection, Women and Children's Hospital, Xiamen, Fujian, China
- 3Department of Neonatology, Children's Hospital of Fudan University, Shanghai, China
- 4Department of Neonatology, Children' Hospital of Nanjing Medical University, Nanjing, Jiangsu, China
- 5Department of Neonatology, Suzhou Municipal Hospital, Suzhou, Jiangsu, China
- 6Department of Neonatology, Quanzhou Maternity and Children's Hospital, Quanzhou, Fujian, China
Objective: To investigate the protective effect of high-proportion breast milk feeding (>50%) on intraventricular hemorrhage (IVH) in very preterm infants (VPIs).
Methods: This was a retrospective secondary analysis of a prospective multi-center study, which included 604 VPIs from six hospitals in eastern China between September 2019 and December 2020. The 604 VPIs were divided into two groups according to whether IVH occurred. High-proportion breast milk feeding was defined as breast milk accounting for 51–100% of the total feeding amount both within 7 days and throughout the hospitalization. The IVH grades and the rate of high-proportion breast milk feeding were analyzed. Furthermore, to explore the relationship between high-proportion breast milk feeding and IVH grading, the VPIs' general information, perinatal factors, growth, and nutritional status during hospitalization, and related complications were compared between the two groups.
Results: High-proportion breast milk feeding was reported in 63.41% of the VPIs. Furthermore, IVH grades I–II and III–IV were noted in 39.73% (240/604) and 1.66% (10/604) of the VPIs, respectively. Univariate analysis revealed that IVH occurrence in VPIs is influenced by perinatal factors, invasive respiratory therapy, high-proportion breast milk feeding, start feeding with breast milk, the cumulative amount of early parenteral nutrition, postnatal complications, physical growth, and other factors (P < 0.05). After adjustments for gestational age, birth weight, and possible influencing factors through binary logistic regression analysis, the results revealed that high-proportion breast milk feeding and and start feeding with breast milk were associated with a lower total incidence of IVH. Further stratification showed that high-proportion breast milk feeding was associated with a lower incidence of grade I–II IVH. Similarly, after adjusting for the same factors, breast milk feeding >50% in the 1st week was associated with a decreased incidence of total IVH and further stratification showed that it was associated with a lower incidence of grade I–II IVH.
Conclusion: High-proportion breast milk feeding and breast milk feeding more than 50% of total intake during the 1st week might be protective factors for IVH grade I–II in VPIs, which further verified the neuroprotective effect of breast milk. In clinical practice, the construction of breast milk banks should be strengthened, breast milk feeding should be encouraged in neonatal intensive care units, and efforts should be made to increase breast milk feeding rates to improve the outcomes of VPIs.
Introduction
Very preterm infants (VPIs) have immature nervous systems and are susceptible to brain injury due to hypoxia, ischemia, infection, and other factors. Intraventricular hemorrhage (IVH) is the most common type of brain injury and is associated with death, neurodevelopmental delay, and cerebral palsy in VPIs (1–5). Studies published in 2019–2022 reported that in China, the rates of IVH grades I–II and III–IV in preterm infants with gestational age 28–31+6 weeks were 14.3% and 2.7–5.2%, respectively. Among preterm infants with gestational age < 28 weeks, the rates of IVH grades I–II and III–IV were 18.7–21.8% and 10.8–14.5%, respectively (6–8). The incidence of IVH grade III–IV in preterm infants with gestational age < 28 weeks has been significantly higher in China than in developed countries (5.2–7%) (9, 10). Multiple studies have demonstrated that breast milk can reduce the incidence of necrotizing enterocolitis (NEC), bronchopulmonary dysplasia (BPD), retinopathy of prematurity, and late-onset sepsis and improve neurodevelopmental outcomes at a corrected gestational age of 24 months (11–16). Long-chain polyunsaturated fatty acids and oligosaccharides in breast milk can promote brain development and myelination, improve white matter microstructure, and increase cortical thickness through inflammation reduction (17, 18). Various bioactive factors contained in breast milk, particularly colostrum, may also have protective effects for IVH in preterm infants. Exclusive breast milk feeding in preterm infants is challenging in China, but a certain percentage of breast milk feeding is achievable in the neonatal intensive care unit (NICU). Hence, this study was conducted to explore the relationship between high-proportion breast milk feeding and IVH occurrence in VPIs.
Objectives and methods
Research objectives
Patient source and grouping
This study is a retrospective secondary analysis of a multi-center prospective observational study, including 604 VPIs from a neonatology department of six tertiary hospitals in East China from September 2019 to December 2020, namely four children's hospitals, one tertiary general hospital, and one maternal and child health care hospital. For the analysis of influencing factors, the infants were divided into two groups based on whether IVH occurred.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (1) gestational age at birth < 32 weeks, (2) hospitalization time >2 weeks, and (3) admission within 24 h after birth.
The exclusion criteria were as follows: (1) congenital malformations or genetic metabolic diseases; (2) underwent various surgical operations during the neonatal period, except for NEC; (3) hospital stay ≤ 2 weeks; (4) death during hospitalization, treatment interruption, or automatic discharge; and (5) incomplete information.
Ethics and clinical registration
This study was initiated and organized by the Nutrition Professional Committee of the Neonatologist Branch of the Chinese Medical Doctor Association and was registered in the Chinese Clinical Trials Registry with the registration number ChiCTR1900023418. The research protocol was approved by the Ethics Committee of Xiamen University Affiliated Women and Children's Hospital/Xiamen Maternal and Child Health Hospital (batch number KY-2019–016).
Research methods
Data collection
The VPIs' general information, perinatal factors, nutritional status during hospitalization, growth, and complications were collected according to a unified questionnaire. Perinatal data (gestational age at birth, birth weight, sex, delivery mode, multiple births, small-for-gestational-age infants, prenatal hormone use, 5-min Apgar score ≤ 7, and the duration of invasive mechanical ventilation use), maternal comorbidities (gestational hypertension and gestational diabetes mellitus), nutrition and growth-related indicators during hospitalization (time to start feeding; start feeding with breast milk; the proportion of breast milk feeding >50%; accumulative doses of amino acid, fat emulsions, and calorie intake during the first week of hospitalization; maximum body weight loss; days to regain birth weight; growth velocity after regaining birth weight; and head circumference [change in Z score, ΔZ] value during hospitalization), and primary complications during hospitalization (neonatal respiratory distress syndrome [NRDS], BPD, early-onset sepsis [EOS], NEC, IVH, periventricular leukomalacia [PVL], hemodynamically significant patent ductus arteriosus [hsPDA], and extrauterine growth restriction [EUGR]) were compared, and the related influencing factors of IVH were analyzed.
Data quality control
The data entry personnel of each participating unit were uniformly trained, and the entered data were in strict accordance with the requirements of the research program. EpiData database was established in this study, and the data of the case report form were recorded by two persons. Each participating unit collected and uploaded the relevant data of VPIs in time and locked the database after verification and confirmation. The team leader maintained close contact with all participating units, checked the case records, and solved the possible problems in time. Considering the relatively uniform treatment regimens, this study selected data from six tertiary hospitals in the economically developed East China region for analysis.
Definitions, diagnostic criteria, and calculation methods of related diseases
Small for gestational age was defined as newborns whose birth weight is lower than the 10th percentile of the average birth weight of the same sex and gestational age. EUGR was defined according to the same-sex Fenton growth curve in 2013 (19) as body weight below the 10th percentile at the corrected gestational age of 36 weeks or discharge. For the clinical diagnosis and diagnostic criteria of EOS, we referred to the expert consensus published in 2019 (20). Furthermore, hsPDA was defined as PDA catheter diameter >1.5 mm, left atrial diameter/aortic diameter ≥1.4 mm, or left ventricular end-diastolic diameter/aortic diameter ≥2.1 mm, accompanied by one of the following clinical manifestations: heart murmur, tachycardia (≥160 beats/min), increased breathing, increased pulse pressure (>25 mmHg), hypotension, flushing, or cardiac dilation. For IVH diagnostic and grading criteria, we referred to Volpe's Neurology of the Newborn (21). Growth velocity after regaining birth weight (g/kg·d) was calculated as follows: GV = [1,000 × ln(Wn/W1)]/(Dn-D1), where GV is growth velocity, Wn is weight at discharge (g), W1 is the birth weight (g), Dn is the length of hospital stay (days), and D1 is days to regain birth weight (days). High-proportion breast milk feeding was defined as breast milk (consisting of the mother's breast milk and donated human milk) accounting for 51–100% of the total feeding amount both within 7 days and throughout the hospitalization. Contrarily, low-proportion breast milk feeding was defined as breast milk accounting for 0–50% of the total feeding amount both within 7 days and throughout the hospitalization, including those infants fed with formula exclusively. For the diagnosis of NRDS, BPD, NEC, and PVL, we referred to Practical Neonatology (fifth edition) (22).
Nutrition program of VPIs during hospitalization
The nutrition program of VPIs during hospitalization was based on the published guidelines and expert consensus (23–25) with appropriate adjustments. VPIs were initiated with breast milk feeding as early as possible. Maternal breast milk feeding was the first choice, and if mother's milk was unavailable, donated human milk was the second choice; the third choice was formula milk. Feeding was started within 12 h after birth for infants without congenital gastrointestinal malformations, suspected or diagnosed NEC, hemodynamic instability, or multiple organ dysfunction. Tube feeding was started after birth and was gradually transitioned to oral feeding at the corrected gestational age of 32–34 weeks. When the total breast milk feeding amount reached 50–80 mL/kg/d, breast milk was added as a fortifier. Total enteral feeding was defined as the enteral feeding amount of 150 mL/kg/d. Parenteral nutrition was started as soon as possible (12–24 h after birth) and administered through a central vein (umbilical venous catheter or peripherally inserted central catheter). The initial amino acid dose was 1.5–2 g/kg/d, and the maximum dose was 3.5–4.0 g/kg/d. The initial fat emulsion dose was 1.0 g/kg/d, which increased by 0.5–1.0 g/kg/d, and the maximum dose was 3.0 g/kg/d. The recommended initial glucose infusion dose was 4–8 mg/kg/min and was increased by 1–2 mg/kg/min per day, with a maximum dose of 11–14 mg/kg/min. When the enteral feeding amount reached 120 mL/kg/d, intravenous nutrition was stopped.
Protocols of monitoring IVH and PVL in VPIs
All VPIs were monitored through cranial ultrasonography at 1–3 days after birth, 7 days after birth, and every 1–2 weeks thereafter. For preterm infants with positive results of cranial ultrasonography, magnetic resonance imaging was recommended before discharge or at a corrected gestational age of 36 weeks.
Statistical analysis
SPSS 23.0 software was used for the statistical analysis of the data. The measurement data that conform to the normal distribution are expressed as mean±standard deviation, the measurement data that do not conform to the normal distribution are expressed as the median (P25, P75), and the count data are expressed as the frequency (percentage). Continuous variables were compared between the two groups by using the t-test or Mann–Whitney U test, and categorical variables were compared using the chi-square test or Fisher's exact test. Collinearity diagnostic analysis was performed because of the possibility of multicollinearity in >50% breastfeeding during the 1st week and a high proportion of breastfeeding. Binary Logistic regression model was used to correct the possible influencing factors. Odds ratio (OR) and 95% confidence interval (CI) were used to compare the association between high proportion of breastfeeding, breastfeeding >50% in the 1st week, breastfeeding initiation with the risk of IVH and IVH with different stratification (shown in Tables 4, 5). P < 0.05 was considered statistically significant.
Outcomes
General information
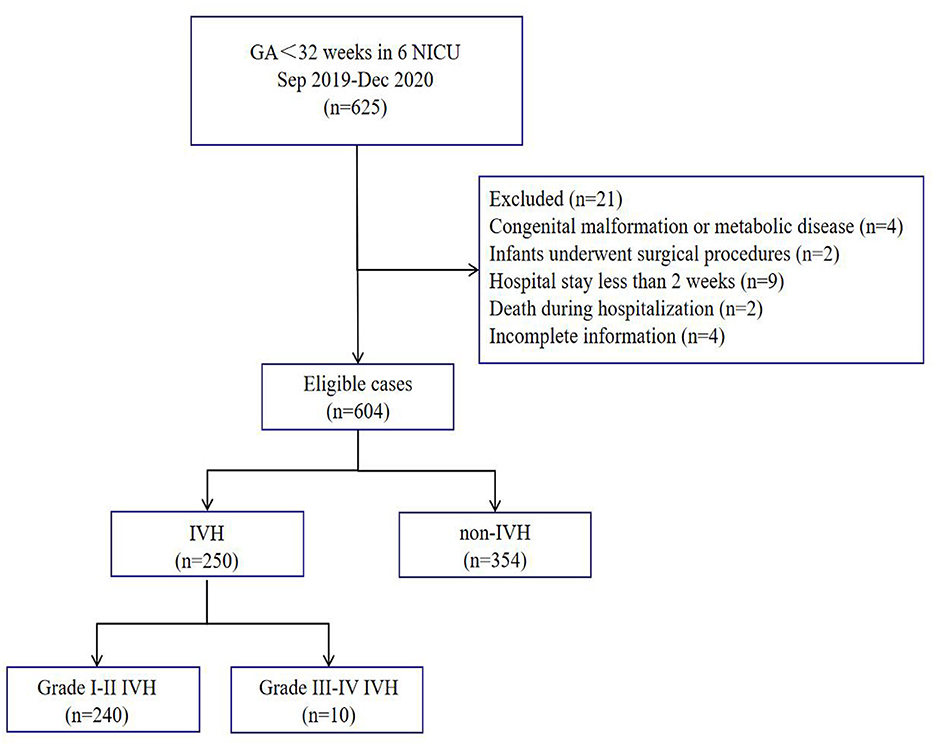
During the study period, 625 VPIs met the inclusion criteria, of whom 21 were excluded according to the exclusion criteria, and 604 VPIs were finally included for the statistical analysis (Figure 1). The average gestational age at birth was 29.93 ± 1.52 weeks, and the average birth weight was 1395.00 ± 298.97 g. The gestational age at birth was < 26 weeks in 9 VPIs, 26–27+6 weeks in 66 VPIs, 28–29+6 weeks in 1,176 VPIs, and 30–31+6 weeks in 353 VPIs. The birth weight was < 750 g in 4 VPIs, 750–999 g in 58 VPIs, 1,000–1,249 g in 131 VPIs, 1,250–1,499 g in 173 VPIs, and >1,500 g in 238 VPIs.
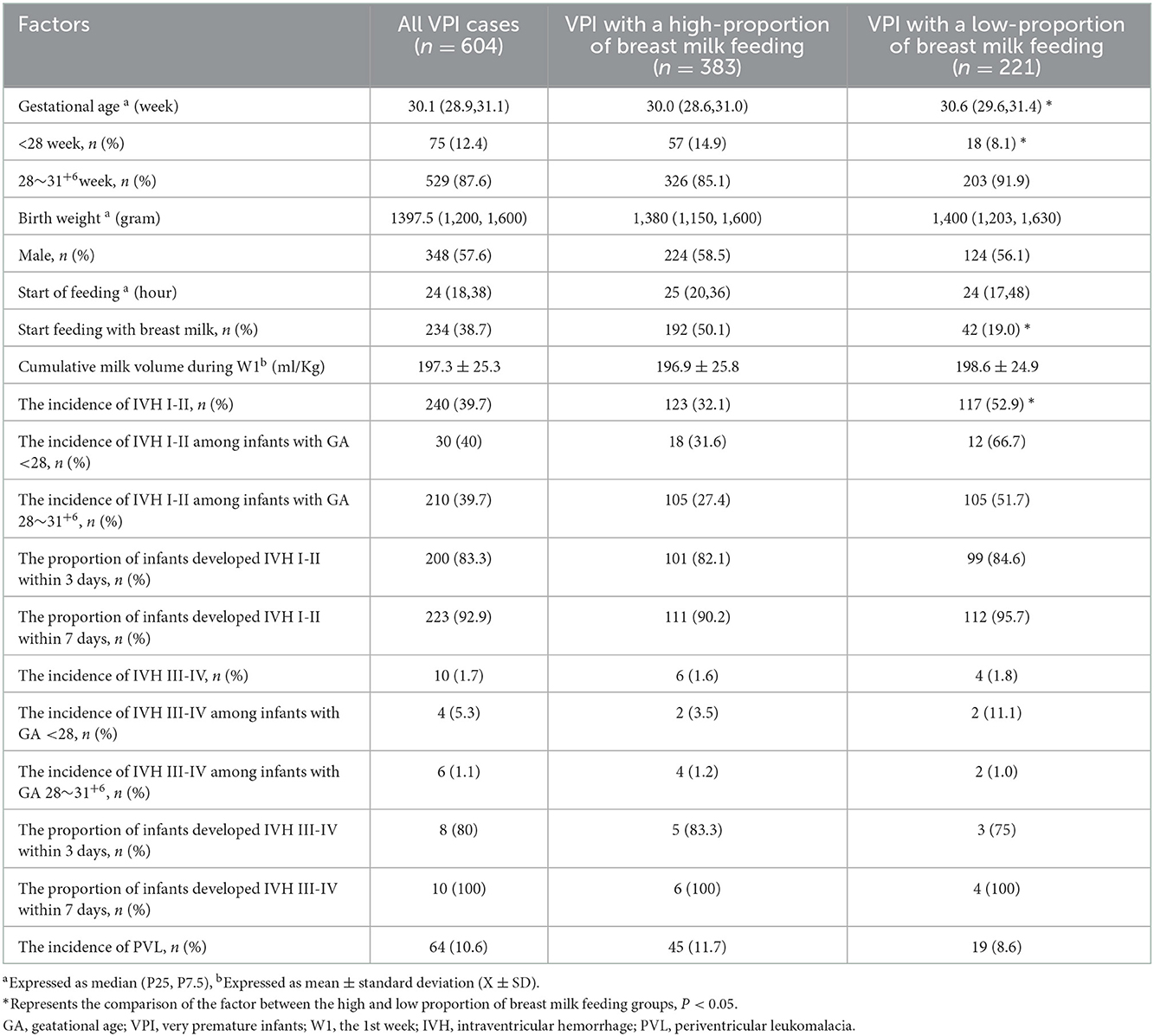
The characteristics of all and high-proportion breast milk feeding VPIs are presented in Table 1. The results showed that 63.41% (383/604) VPIs received high-proportion breast milk feeding, and the rates of IVH grades I–II and III–IV among the VPIs were 39.73% (240/604) and 1.66% (10/604), respectively. Among preterm infants with a gestational age of < 28 weeks, the rates of IVH grades I–II and III–IV were 40.00% (30/75) and 5.33% (4/75), respectively. Among preterm infants with a gestational age of 28–31+6 weeks, the incidences of IVH grades I–II and III–IV were 39.70% (210/529) and 1.13% (6/529), respectively. The incidence of IVH grades I–II was lower in the high-proportion breast milk feeding group (χ2 = 25.38, P < 0.001), while the incidence of IVH grades III–IV and PVL was not associated with high-proportion breast milk feeding (P > 0.05). The high-proportion breast milk feeding group had a higher proportion of start feeding with breast milk (χ2 = 57.21, P < 0.001).
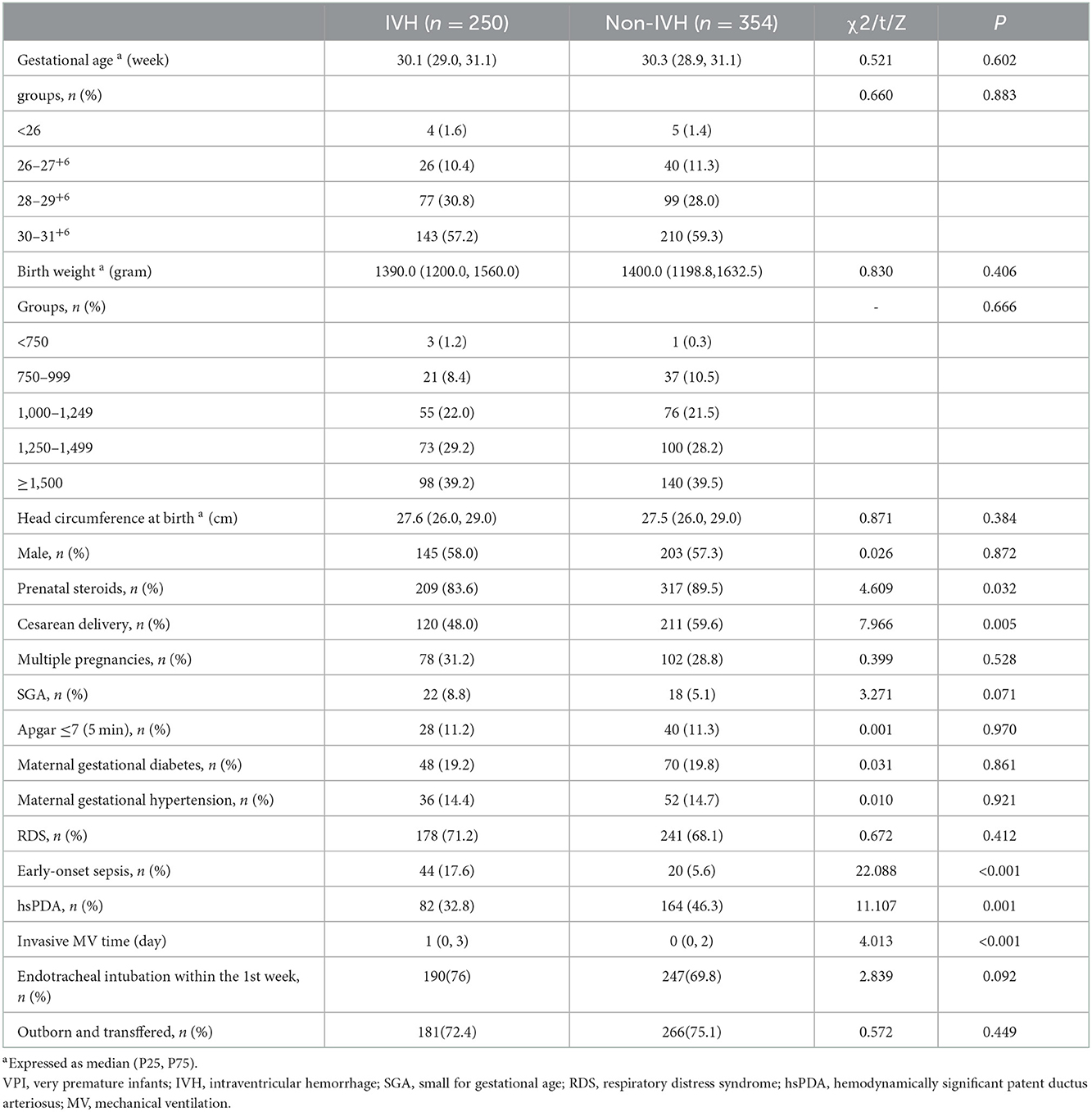
The infants were divided into two groups according to IVH occurrence, and the perinatal characteristics of the VPIs between the two groups were compared (Table 2). The IVH group had lower rates of prenatal hormone use, cesarean section, and hsPDA incidence and higher incidence of EOS and longer invasive respiratory treatment time; the differences were statistically significant (P < 0.05).
Analysis of the influencing factors of IVH in VPIs
Analysis of nutritional status, growth, and complications of preterm infants between the two groups
The patients were divided into two groups according to whether IVH occurred. The nutritional status, growth, and complications of premature infants were compared between the two groups. The results are presented in Table 3. The results suggested that VPIs with IVH had a lower probability of receiving high-proportion breast milk feeding, a lower incidence of start feeding with breast milk, less infants receiving breast milk feeding more than 50% of total intake during the 1st week, lower amino acid accumulation and calorie accumulation in the 1st week, and lower head circumference (ΔZ value) during hospitalization. Furthermore, in the 1st week, the cumulative amount of fat was higher, the weight loss was larger, and the incidence of NEC and EUGR at discharge was higher in the IVH group than in the non-IVH group. The difference was statistically significant (P < 0.05).
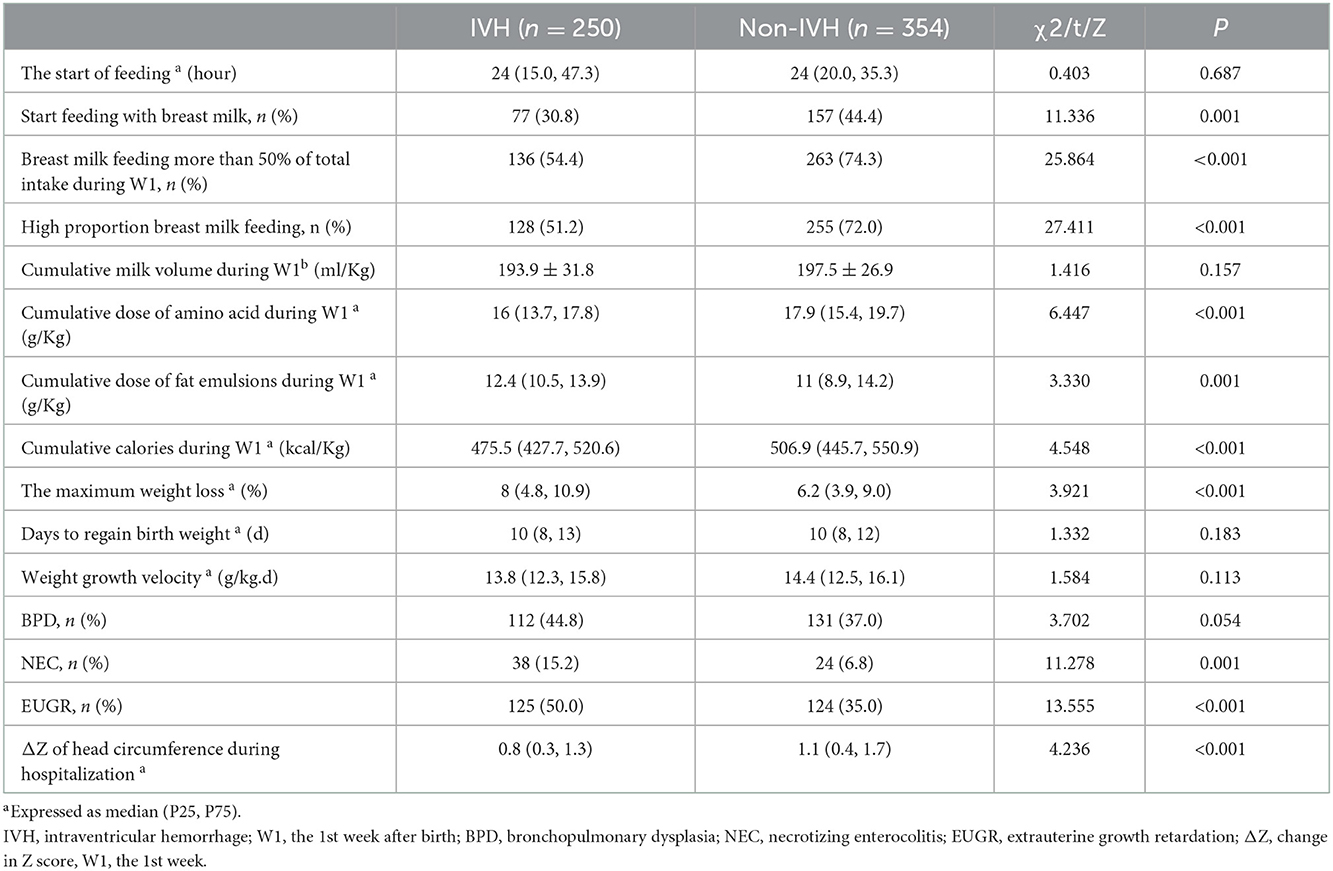
Table 3. Comparison of nutritional status, growth, and complications of preterm infants between the two groups.
Analysis of high proportion-breast milk feeding, start feeding with breast milk, and IVH risk in VPIs
Since the data of breast milk feeding >50% in the 1st week and high proportion breast milk feeding were similar, we carried out collinearity diagnostic analysis, and the results showed that Tolerance was 0.171, < 0.2; The variance inflation factor was 5.858, >5, indicating collinearity among variables. Hence, binary logistic regression analysis was performed to compare breast milk feeding >50% in the 1st week and high proportion breast milk feeding with other significant univariate factors.
After adjustments for gestational age, birth weight, and possible influencing factors by using binary logistic regression analysis, the results suggested that high-proportion breast milk feeding and and start feeding with breast milk were associated with a lower total incidence of IVH, with the adjusted OR (95% CI) values being 0.492 (0.327, 0.739) and 0.513 (0.340, 0.773), respectively. Further stratification showed that high-proportion breast milk feeding was associated with a lower incidence of grade I–II IVH, with adjusted OR (95% CI) value of 0.547 (0.359, 0.832). Similarly, after adjusting for the same factors, breast milk feeding >50% in the 1st week was associated with a decreased incidence of total IVH, with an aOR and 95% CI of 0.547 (0.367, 0.815). Further stratification showed that breastfeeding >50% in the 1st week was associated with a lower incidence of grade I–II IVH, with an aOR and 95% CI of 0.562 (0.379, 0.835) (shown in Tables 4, 5). Adjusted factors included gestational age at birth, birth weight, cesarean section, prenatal steroid, SGA, start of feeding, early onset sepsis, hsPDA, endotracheal intubation within the 1st week, outborn and transfferd, duration of invasive ventilator, cumulative amount of amino acids, fat emulsion and calories in the 1st week, and weight loss.
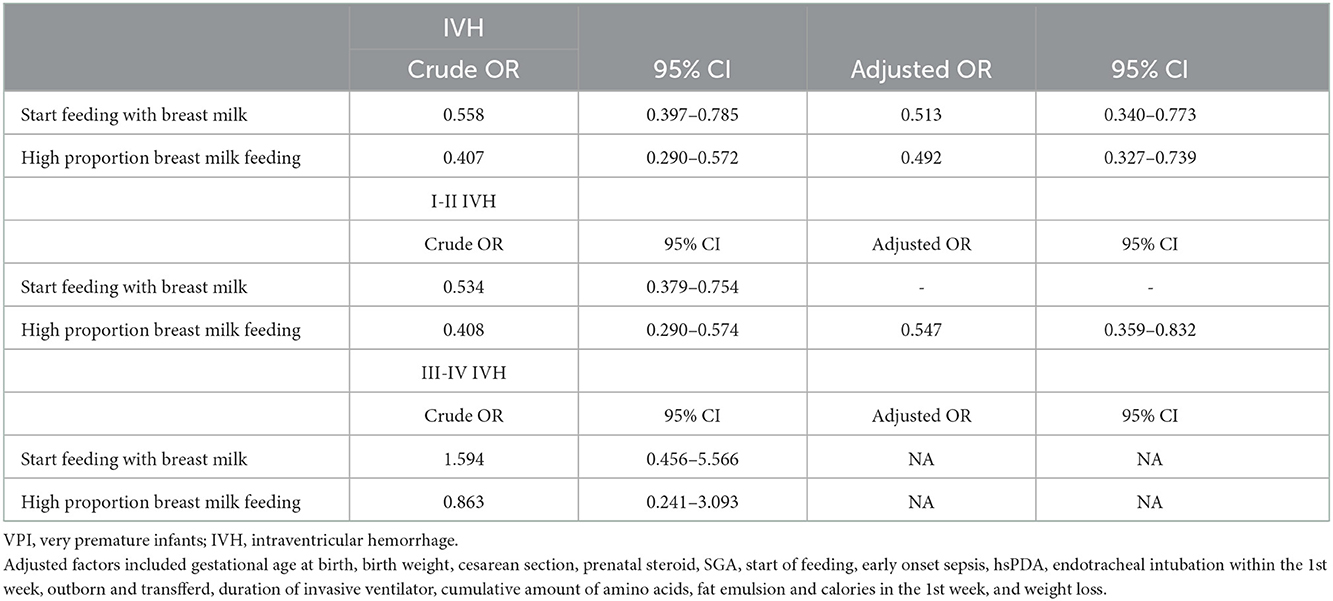
Table 4. Analysis of the association of high-proportion breast milk feeding and start breast milk feeding with IVH (include IVH, I-II IVH, III-IV IVH) risk in VPIs.
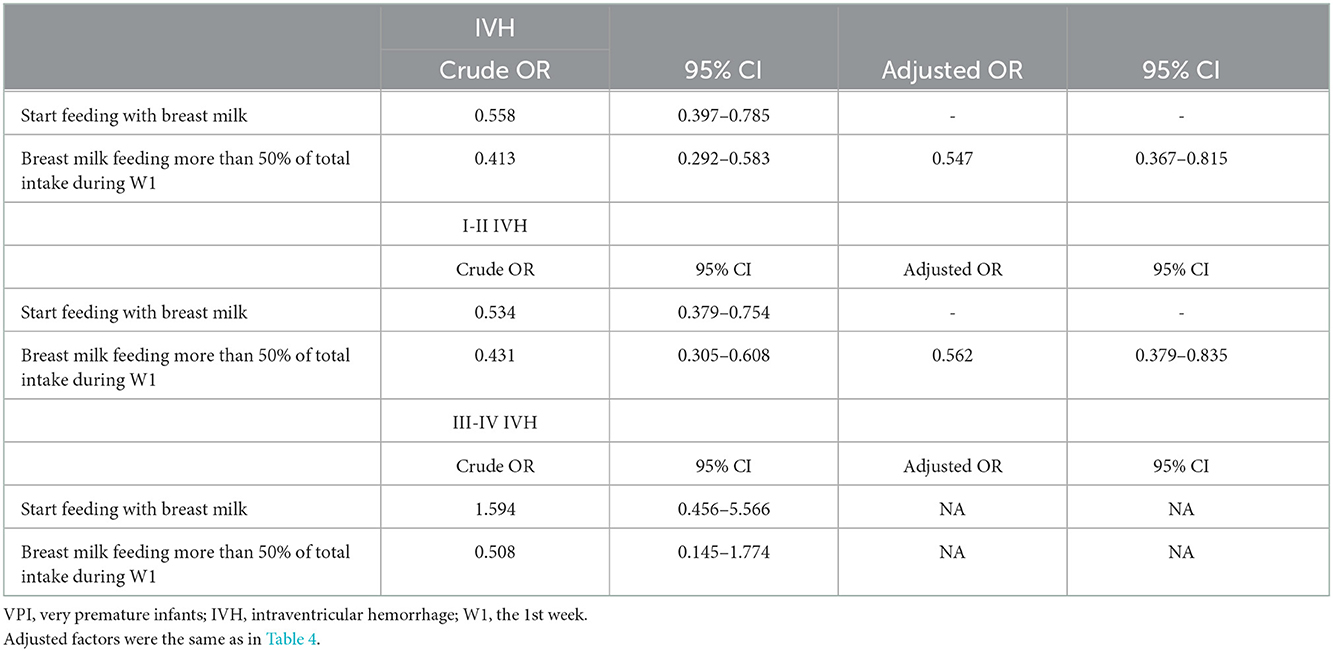
Table 5. Analysis of the association of breast milk feeding more than 50% of total intake during the 1st week and start breast milk feeding with IVH (include IVH, I-II IVH, III-IV IVH) risk in VPIs.
Discussion
The subependymal germinal stroma contains a rich capillary bed that lacks tight junctions and is irregular in shape. These capillary beds are susceptible to ischemia and hypoxia, reperfusion, infection, and other factors, leading to hemodynamic disturbances. This results in the hemorrhage of the germinal matrix and rupture into the ventricular system, eventually leading to IVH occurrence. IVH is the most common type of brain injury and is closely associated with long-term adverse neurological outcomes (1–5). In our study, the rates of IVH grades I–II and III–IV were 39.73 and 1.66%, respectively. In this study, the incidence of grade I-II IVH was generally high, mainly because most of the VPI in this study came from children's hospital and neonatal rescue center. 74.01% of the children experienced extrauterine transport within 24 h after birth, and the rate of endotracheal intubation within the 1st week was 72.35%, which may lead to the increased incidence of IVH. The sample size of preterm infants < 28 weeks was small in this study, if the sample size is further expanded, the differences in the incidence of grade I-II IVH among different gestational age groups may be more significant and the data results will be more representative.
IVH is currently considered a multifactorial disease, where gestational age at birth, prenatal hormone use, cesarean section, early-onset infection, invasive respiratory therapy, and resuscitation of neonatal asphyxia are the main factors influencing IVH (10, 26, 27). A study by Carome et al. (28) demonstrated that for very low birth weight infants, early initiation and continuation of exclusive breast milk feeding until the corrected gestational age of 34 weeks was associated with a low incidence of IVH grade III–IV and PVL. Torres-Muñoz et al. (29) found that preterm infants who achieved total enteral nutrition with exclusive breast milk feeding within 7 days of life had an 85% lower incidence of IVH than those who reached the same target after 8 days of life. Exclusive breast milk feeding has been speculated to exert a protective effect on IVH in preterm infants, and therefore, this study further hypothesized and explored this speculation.
Not only have clinical studies reported on the protective effect of exclusive breast milk feeding on IVH in preterm infants (28–30) but also animal studies have reported that feeding colostrum to fetal mice early after birth can reduce apoptosis after IVH (31). Therefore, bioactive components in colostrum may cross the intestinal barrier and enter the bloodstream to exert important immune-regulatory and anti-inflammatory effects. Breast milk can help reduce the incidence of IVH, and the possible mechanisms are as follows. One, transforming growth factor β (TGF-β) is the most abundant cytokine family in breast milk, and its content in colostrum is much higher than that in mature milk (32, 33). In animal models, TGF-β plays an important role in the stabilization of the blood-brain barrier, and the disruption of the TGF-β pathway could induce IVH occurrence (34). In preterm infants, TGF-β is hypothesized to exert protective effects by reducing anticoagulant factor levels in the brain and stimulating pericyte production, providing structural support for the germinal matrix vasculature and promoting maturation (35). Two, epidermal growth factor (EGF) is a growth factor contained in breast milk that promotes cell proliferation and maturation (32, 36). EGF binds to the EGF receptor in the brain, promotes astrocyte differentiation, oligodendrocyte progenitor cell proliferation, and neuron development, which further improves the blood–brain barrier and promotes gray and white matter maturation (37–39). Therefore, EGF is considered to play an important role in IVH prevention and recovery. Three microRNAs (miRNAs) are small non-coding RNAs that are present in breast milk and can be absorbed through intestinal wall endocytosis and play a role in posttranscriptional gene regulation and immune protection (40–42). Furthermore, miRNAs can promote the recovery of hypoxic-ischemic encephalopathy and maintain the stability of the blood–brain barrier after intracerebral hemorrhage (43, 44), which may be another potential mechanism by which breast milk prevents IVH occurrence.
The exclusive breast milk feeding rate of VPIs in NICUs in developed countries is high (>90%), but it is still difficult to implement exclusive breast milk feeding for VPIs in China. Insufficient breast milk secretion at the time of mother–infant separation, contraindications to breast milk feeding in infants, and the general lack of breast milk banks, donated breast milk, and human milk fortifiers all affect the rate of exclusive breast milk feeding in China. China currently lacks large-sample-size multi-center studies on breast milk feeding rates in VPIs. The Pediatric Hospital of Fudan University reported that the breast milk feeding rate of very low birth weight infants during hospitalization reached 84% after continuous quality improvement. Our study observed high-proportion breast milk feeding in 63.41% of the VPIs, which indicates that high-proportion breast milk feeding in China is achievable. However, whether high-proportion breast milk feeding has neuroprotective effects on VPIs is unclear.
This study analyzed the relationship between high-proportion breast milk feeding and the occurrence of different grades of IVH and PVL in VPI and found that the rate of IVH grade I–II was decreased in the high-proportion breast milk feeding group (32.1 vs. 52.9%, P < 0.001), whereas no significant difference was observed in the rate of IVH grade III–IV and PVL between the high-/low-ratio breast milk feeding groups. After adjustment for gestational age, birth weight, and possible influencing factors through a binary logistic regression analysis, the results showed that high-proportion breast milk feeding and breast milk feeding more than 50% of total intake during the 1st week were associated with a lower incidence of IVH, and further stratification showed that these two factors were also associated with a lower incidence of IVH grade I–II. That is, high-proportion breast milk feeding in VPIs can reduce the occurrence of IVH grade I–II by 45.3%. Therefore, this study showed that high-proportion breast milk feeding and breast milk feeding more than 50% of total intake during the 1st week might be protective factors for IVH occurrence in VPIs and mainly has a protective effect on IVH grade I–II, whereas no statistical difference was observed in IVH grade III–IV, which is inconsistent with a study reported by Torres (29). The possible reasons are as follows. First, IVH is a multifactor disease. The main influencing factors are gestational age at birth, use of prenatal hormones, and invasive respiratory therapy. Breast milk may be a neuroprotective component. In this study, the aforementioned risk factors were included in the binary logistic regression analysis. Therefore, we believe that for IVH grades III–IV, the high-risk factors may be more complex and severe, and the neuroprotective effect of breast milk feeding may be diluted among many influencing factors. Conversely, for IVH grades I–II, the high-risk factors were few and less complex and severe, and high-proportion breast milk feeding had a neuroprotective effect. In addition, as breast milk feeding has a protective effect on both prevention and repair of IVH, some mild cases of IVH have effectively improved. Thus, high-proportion breast milk feeding may reduce the likelihood of IVH progression from grade I–II to grade III–IV among some infants. Second, the neuroprotective effect of breast milk is time-and dose-dependent. This study considered data of only high-proportion breast milk feeding, which may not completely reflect the neuroprotective effect of breast milk. Third, this study had few patients with IVH grade III–IV, and effective statistical analysis could not be carried out. It is necessary to include a larger sample size for further research.
The data for this study were obtained from a multi-center study and had good representativeness. This study has several limitations. First, this study was not a prospective randomized trial, and certain differences in baseline characteristics exist between the two groups; therefore, our results must be interpreted with caution. Second, this study lacks data records on hypothermia in delivery room, hypotension and vasoactive drug use in preterm infants, which have clear effects on IVH; this may cause bias in the results. Lastly, this study lacks follow-up assessments of neurodevelopmental outcomes or cognitive function after VPI discharge. Therefore, prospective randomized controlled studies are needed with larger sample sizes and follow-up data analysis.
Conclusions
This study concludes that high-proportion breast milk feeding and breast milk feeding more than 50% of total intake during the 1st week might be protective factors for IVH grade I–II in VPIs, which further verifies the protective effect of breast milk. Follow-up studies are needed to confirm and further clarify the protective mechanism. Therefore, the construction of breast milk banks should be strengthened, breast milk feeding should be encouraged in NICUs, and efforts should be made to increase breast milk feeding rates to improve the outcomes of VPIs.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Women and Children's Hospital, School of Medicine, Xiamen University (KY-2019–016). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
Conceptualization, visualization, and supervision: X-ZL and CC. Methodology, software, and validation: ZZ and WS. Formal analysis: L-XT. Investigation and resources: RZ, RC, S-NW, and D-MC. Data curation: ZZ, WS, and L-XT. Writing—original draft preparation: ZZ. Writing—review and editing: WS. Project administration: ZZ, WS, X-ZL, and CC. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Guidance Project of Xiamen Science and Technology Plan (Grant Numbers: 3502Z20209198, 3502Z20214ZD1225, and 3502Z20199139) and Natural Science Foundation of Fujian Province (Grant Number: 2022D004).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of sub-ependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 g. J Pediatr. (1978) 92:529–34. doi: 10.1016/S0022-3476(78)80282-0
2. Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD neonatal research network. Pediatrics. (2010) 126: 443–56. doi: 10.1542/peds.2009-2959
3. Mercier CE, Dunn MS, Ferrelli KR, Howard DB, Soll RF, Group VONEIF-US. Neurodevelopmental outcome of extremely low birth weight infants from the Vermont Oxford network: 1998–2003. Neonatology. (2010) 97:329–38. doi: 10.1159/000260136
4. Vohr BR, Wright LL, Poole WK, McDonald SA. Neurodevelopmental outcomes of extremely low birth weight infants < 32 weeks' gestation between 1993 and 1998. Pediatrics. (2005) 116:635–43. doi: 10.1542/peds.2004-2247
5. Schindler T, Koller-Smith L, Lui K, Bajuk B, Bolisetty S, Collection NSWaACTNICUD. Causes of death in very preterm infants cared for in neonatal intensive care units: a population-based retrospective cohort study. BMC Pediatr. (2017) 17:59. doi: 10.1186/s12887-017-0810-3
6. Zhao Y, Tian XY, Zhang WX. Analysis of related factors of intraventricular hemorrhage in very low birth weight premature infants. Tianjin Med J. (2021) 49:608–12.
7. Wu J, Lu AD, Zhang LP, Zuo YX, Jia YP. Study of clinical outcome and prognosis in pediatric core binding factor-acute myeloid leukemia. Chin J Neonatol. (2019) 40:52–7. doi: 10.3760/cma.j.issn.0253-2727.2019.01.010
8. Reduction of Infection in Chinese Neonatal Intensive Care Units Using the Evidence-based Practice for Improving Quality Study Group. Incidence and risk factors of severe intraventricular hemorrhage in very low and extremely low birth weight infants: a multi-center study. Chin J Ped. (2019) 57:258–64. doi: 10.3760/cma.j.issn.0578-1310.2019.04.006
9. Shah PS, Lui K, Sjörs G, Mirea L, Reichman B, Adams M, et al. Neonatal outcomes of very low birth weight and very preterm neonates: an international comparison. J Pediatr. (2016) 177. doi: 10.1016/j.jpeds.2016.04.083
10. Yeo KT, Thomas R, Chow SS, Bolisetty S, Haslam R, Tarnow-Mordi W, et al. Improving incidence trends of severe intraventricular hemorrhages in preterm infants < 32 weeks gestation: a cohort study. Arch Dis Child Fetal Neonatal Ed. (2020) 105:145–50. doi: 10.1136/archdischild-2018-316664
11. Quigley M, Embleton ND, McGuire W. Formula versus donor breast milk for feeding preterm or low birth weight infants. Cochrane Database Syst Rev. (2019) 7:CD002971. doi: 10.1002/14651858.CD002971.pub5
12. Hylander MA, Strobino DM, Pezzullo JC, Dhanireddy R. Association of human milk feedings with a reduction in retinopathy of prematurity among very low birthweight infants. J Perinatol. (2001) 21:356–62. doi: 10.1038/sj.jp.7210548
13. Patel AL, Johnson TJ, Robin B, Bigger HR, Buchanan A, Christian E, et al. Influence of own mother's milk on bronchopulmonary dysplasia and costs. Arch Dis Child Fetal Neonatal Ed. (2017) 102:F256–F61. doi: 10.1136/archdischild-2016-310898
14. Lechner BE, Vohr BR. Neurodevelopmental outcomes of preterm infants fed human milk: a systematic review. Clin Perinatol. (2017) 44:69–83. doi: 10.1016/j.clp.2016.11.004
15. Belfort MB, Inder TE. Human milk and preterm infant brain development: a narrative review. Clin Ther. (2022) 44:612–21. doi: 10.1016/j.clinthera.2022.02.011
16. Gibertoni D, Corvaglia L, Vandini S, Rucci P, Savini S, Alessandroni R, et al. Positive effect of human milk feeding during NICU hospitalization on 24 month neurodevelopment of very low birth weight infants: an Italian cohort study. PLoS One. (2015) 10:e0116552. doi: 10.1371/journal.pone.0116552
17. Ottolini KM, Andescavage N, Kapse K, Jacobs M, Limperopoulos C. Improved brain growth and microstructural development in breast milk-fed very low birth weight premature infants. Acta Paediatr. (2020) 109:1580–7. doi: 10.1111/apa.15168
18. Blesa M, Sullivan G, Anblagan D, Telford EJ, Quigley AJ, Sparrow SA, et al. Early breast milk exposure modifies brain connectivity in preterm infants. Neuroimage. (2019) 184:431–9. doi: 10.1016/j.neuroimage.2018.09.045
19. Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. (2013) 13:59. doi: 10.1186/1471-2431-13-59
20. Subspecialty Subspecialty Group of Neonatology the the Society of Pediatric Chinese Chinese Medical Association; Professional Committee of Infectious Diseases Neonatology Society Chinese Medical Doctor Association. Expert consensus on the diagnosis and management of neonatal sepsis (version 2019). Chin J Ped. (2019) 57:252–7. doi: 10.3760/cma.j.issn.0578-1310.2019.04.005
21. Inder TE, Perlman JM, Volpe JJ: Chapter 24 - Preterm Intraventricular Hemorrhage/Posthemorrhagic Hydrocephalus. In Volpe's Neurology of the Newborn (Sixth Edition). Volpe JJ, Inder TE, Darras BT, de Vries LS, du Plessis AJ, Neil JJ (Eds), Perlman JM: Elsevier (2018). 637-698.e621 doi: 10.1016/B978-0-323-42876-7.00024-7
22. Qiu XS, Ye HM, Shao XM. Practice of Neonatology. 5th ed Beijing: People's Medical Publishing House (2018).
23. The The Editorial Board of Chinese Journal of Pediatrics; the Subspecialty Groups of Neonatology and Child Health Care the the Society of Pediatrics; Chinese Medical Association. Feeding recommendations for preterm infants and low birth weight infants. Chin J Pediat. (2009) 47:508–10.
24. Experts Experts Working Group on Consensus on the Use of Human Milk Fortifier in Preterm Infants Editorial Editorial Board Committee of Chinese Journal of Neonatology. Consensus on the use of human milk fortifier for preterm infants. Chin J Neonatol. (2019) 34:321–8. doi: 10.3760/cma.j.issn.2096-2932.2019.05.001
25. Neonatology P Chinese Medical Association The The Group of Neonatology Pediatric Surgical Society Chinese Medical Association. Chinese guideline for newborn nutrition support in neonates. Chin J Pediatr Surg. (2013) 34:782–7.
26. Handley SC, Passarella M, Lee HC, Lorch SA. Incidence trends and risk factor variation in severe intraventricular hemorrhage across a population based cohort. J Pediatr. (2018) 200:20. doi: 10.1016/j.jpeds.2018.04.020
27. Soraisham AS, Singhal N, McMillan DD. A multi-center study on the clinical outcome of chorioamnionitis in preterm infants. Am J Obstet Gynecol. (2009) 200:372. doi: 10.1016/j.ajog.2008.11.034
28. Carome K, Rahman A, Parvez B. Exclusive human milk diet reduces incidence of severe intraventricular hemorrhage in extremely low birth weight infants. J Perinatol. (2021) 41:535–43. doi: 10.1038/s41372-020-00834-5
29. Torres-Muñoz J, Jimenez-Fernandez CA, Murillo-Alvarado J, Torres-Figueroa S, Castro JP. Clinical results of the implementation of a breast milk bank in premature infants (under 37 Weeks) at the Hospital Universitario del Valle 2018–2020. Nutrients. (2021) 13:2187. doi: 10.3390/nu13072187
30. Keller T, Körber F, Oberthuer A, Schafmeyer L, Mehler K, Kuhr K, et al. Intranasal breast milk for premature infants with severe intraventricular hemorrhage-an observation. Eur J Pediatr. (2019) 178:199–206. doi: 10.1007/s00431-018-3279-7
31. Kim SE, Ko IG, Shin MS, Kim CJ, Ko YG, Cho H. Neuroprotective effects of bovine colostrum on intracerebral hemorrhage-induced apoptotic neuronal cell death in rats. Neural Regen Res. (2012) 7:1715–21. doi: 10.3969/j.issn.1673-5374.2012.22.006
32. Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin North Am. (2013) 60:49–74. doi: 10.1016/j.pcl.2012.10.002
33. Gila-Diaz A, Arribas SM, Algara A, Martín-Cabrejas MA, López de Pablo ÁL, Sáenz de Pipaón M, et al. A review of bioactive factors in human breastmilk: a focus on prematurity. Nutrients. (2019) 11:1307. doi: 10.3390/nu11061307
34. Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. (2004) 16:16. doi: 10.1016/j.nbd.2003.12.016
35. Sweeney MD, Ayyadurai S, Zlokovic B V. Pericytes of the neurovascular unit: key functions and signaling pathways. Nat Neurosci. (2016) 19:771–83. doi: 10.1038/nn.4288
36. Gale SM, Read LC, George-Nascimento C, Wallace JC, Ballard FJ. Is dietary epidermal growth factor absorbed by premature human infants? Biol Neonate. (1989) 55:104–10. doi: 10.1159/000242903
37. Liu B, Neufeld AH. Activation of epidermal growth factor receptors in astrocytes: from development to neural injury. J Neurosci Res. (2007) 85:3523–9. doi: 10.1002/jnr.21364
38. Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. (2010) 67:1–8. doi: 10.1203/PDR.0b013e3181c1b176
39. Kornblum HI, Hussain R, Wiesen J, Miettinen P, Zurcher SD, Chow K, et al. Abnormal astrocyte development and neuronal death in mice lacking the epidermal growth factor receptor. J Neurosci Res. (1998) 53:697–717.3. doi: 10.1002/(SICI)1097-4547(19980915)53:6<697::AID-JNR8>3.0.CO;2-0
40. Zhou Q, Li M, Wang X, Li Q, Wang T, Zhu Q, et al. Immune-related microRNAs are abundant in breast milk exosomes. Int J Biol Sci. (2012) 8:118–23. doi: 10.7150/ijbs.8.118
41. Alsaweed M, Lai CT, Hartmann PE, Geddes DT, Kakulas F. Human milk miRNAs primarily originate from the mammary gland resulting in unique miRNA profiles of fractionated milk. Sci Rep. (2016) 6:20680. doi: 10.1038/srep20680
42. Golan-Gerstl R, Shiff YE, Moshayoff V, Schecter D, Leshkowitz D, Reif S. Characterization and biological function of milk-derived miRNAs. Mol Nutr Food Res. (2017) 61:9. doi: 10.1002/mnfr.201700009
43. Qiu J, Zhou X, Zhou X, Cheng R, Liu H, Li Y. Neuroprotective effects of microRNA-210 on hypoxic-ischemic encephalopathy. Biomed Res Int. (2013) 2013:350419. doi: 10.1155/2013/350419
Keywords: very preterm infants, intraventricular hemorrhage, breast milk feeding, high-proportion breast milk feeding, risk factors
Citation: Zheng Z, Shen W, Tang L-X, Zhang R, Cheng R, Wang S-N, Chen D-M, Chen C and Lin X-Z (2023) High-proportion breast milk feeding is associated with a reduction in the incidence of IVH in very preterm infants. Front. Neurol. 13:993985. doi: 10.3389/fneur.2022.993985
Received: 14 July 2022; Accepted: 19 December 2022;
Published: 18 January 2023.
Edited by:
Carl E. Stafstrom, Johns Hopkins Medicine, United StatesReviewed by:
Wen-Hao Yu, National Cheng Kung University Hospital, TaiwanDongli Song, Santa Clara Valley Medical Center, United States
Copyright © 2023 Zheng, Shen, Tang, Zhang, Cheng, Wang, Chen, Chen and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Chen,  Y2hlbjYwMTBAMTYzLmNvbQ==; Xin-Zhu Lin,
Y2hlbjYwMTBAMTYzLmNvbQ==; Xin-Zhu Lin,  eGluemh1ZmpAMTYzLmNvbQ==
eGluemh1ZmpAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Zhi Zheng1,2†
Zhi Zheng1,2† Wei Shen
Wei Shen Rong Zhang
Rong Zhang Rui Cheng
Rui Cheng Dong-Mei Chen
Dong-Mei Chen Chao Chen
Chao Chen