
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Neurol., 26 September 2022
Sec. Neurocritical and Neurohospitalist Care
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.992885
This article is part of the Research TopicNeurocritical and Neurohospitalist Care - Case Report Collection 2022View all 9 articles
Background: The effect of head position on stroke is not clear. The current study aimed to observe the effect of head-down tilt on acute ischemic stroke (AIS) patients with large vessel occlusion.
Methods: We observed the influence of head-down tilt position on clinical outcomes, myocardial enzymogram and N-terminal pro b-type Natriuretic Peptide in 4 AIS patients who suffered early neurological deterioration (END). Cerebral perfusion imaging was performed in 3 patients using arterial spin labeling.
Results: In series of AIS patients with END, head down tilt (-20°) prevented further neurological deterioration and improved clinical outcomes. An increase in cerebral blood flow was observed by arterial spin labeling after head down tilt treatment. No obvious adverse events occurred.
Conclusion: The case series suggest that head-down tilt may improve clinical outcome in AIS patients through increasing the cerebral perfusion with no obvious adverse events. The finding needs to be confirmed in future clinical trials.
The prognosis in patients with acute ischemic stroke (AIS) depend on the location and size of the occluded cerebral vessel (1), the extent of collateral blood flow (2, 3) and the time to reperfusion therapy (4). Head position after ischemic stroke affects cerebral blood flow (CBF), intracranial pressure, blood flow velocity, cerebral perfusion pressure, oxygenation, as well as other factors which are closely related to the prognosis after ischemic stroke (5, 6). Promoting blood flow during the acute phase of ischemic stroke may directly influence the development of brain infarct and clinical deficit (7). Until now, for the acute ischemic stroke patients, the question of optimal body position has not been fully addressed (4).
The previous studies about the influence of lying-flat vs. sitting-up position on clinical outcome after stroke were controversial. Comparing with an upright position, lying flat induces a significant increase in intracranial pressure in patients with brain injury (8, 9). Moderate head elevation is a standard practice in the management of intracranial pressure (10). Conversely, in AIS patients, some studies indicated that an increase in blood flow velocity and cerebral perfusion pressure could be achieved after changing the upright position to lying flat (11–14), which will promote residual blood flow to ischemic brain tissue (15). However, the HeadPoST (Head Position in Acute Stroke Trial) study did not find the difference in disability outcomes between AIS patients assigned to a lying-flat position and sitting-up position with the head elevated to at least 30° for 24 h (6). We contend that the aggressive head position such as head-down tilt can exert neuroprotective effect in particular AIS patients such as those with large vessel occlusion (LVO). To the best of knowledge, there was no study to investigate the effect of head down tilt position on neurological outcome of ischemic stroke patients.
Head-down tilt may improve clinical outcome of AIS patients through increasing collateral blood flow and CBF. CBF refers to perfusion per unit of tissue, and is optimally measured with a diffusible tracer that can exchange between the blood and the brain. In arterial spin labeling (ASL), the diffusible tracer is a magnetic label applied to blood water molecules, produced by saturating or inverting the longitudinal component of the magnetic resonance signal (16). ASL perfusion magnetic resonance imaging (MRI) sequences are increasingly being used to provide non-invasive MR-based CBF quantification. Clinically, ASL has mainly been used in cerebrovascular disease including stroke (17), steno-occlusive disease (18), arteriovenous malformation (19), and Moyamoya disease (20). Comparing with transcranial Doppler, which only provided indirect information of CBF, ASL perfusion imaging provides quantification of CBF. Additionally, ASL can be performed routinely and repeatedly without contrast administration or ionizing radiation (21).
In this study, we observed the influence of head down position on clinical outcomes and CBF in a small group of AIS patients who suffered neurological deterioration.
We performed a pilot trial in AIS patients. This study was approved by the Ethics Committee of former General Hospital of Shenyang Military Region (No. k2017-10). Informed consent was obtained from all study subjects, and the study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Main inclusion Criteria: (1) age ≥18 years; (2) AIS with large vessel occlusion; (3) the patients occurred neurological deterioration within 7 days of onset; (4) 6–16 NIHSS after deterioration; (5) first stroke onset or past stroke without neurological deficit (modified Rankin Scale, mRS = 0); (6) fully understand and cooperate with the doctor's instructions; (7) the availability of informed consent. Exclusion Criteria: (1) Hemorrhagic stroke or mixed stroke; (2) combined with severe organ dysfunction; (3) a history of hemorrhagic stroke; (4) a history of stroke with severe sequelae; (5) planning revascularization in 3 months; (6) ischemic stroke due to surgical intervention; (7) participating in other clinical trials within 3 months; (8) pregnant or lactating women. Early neurological deterioration (END) was defined as an increase of 2 points or more on the NIHSS, except for cerebral hemorrhage. Schematic for head down tilt treatment of AIS patients is shown in Figure 1.
After the informed consent was signed, the patient was treated with head down tilt as follows. The patients lay supine on tilt platform bed, which was adjusted to −20° for about 60 min for one time. During the treatment, a 3–5 min break to lay flat was allowed for patients who cannot tolerate head down tilt position. Head down tilt treatment was performed 3 times daily for 7 days. During the treatments, the patients were continuously monitored by ECG and blood oxygen saturation, and the side-lying head down position with −20° was allowed if high risk of aspiration was suspected.
After finishing the first treatment of head down tilt, ASL was performed to determine the influence of head down tilt on cerebral blood flow. In the ASL examination, nodes can be ideally described as regions which have coherent patterns of connections in anatomical structure or function in brain. Different parcellation methods of brain regions caused distinct brain network constructions, usually the entire brain surface would be covered completely by the parcellation method. The procedure to determine nodes in the study is as follows. First, the PdWI image of every subject was linearly registered to T1-weighted image and the result matrix was obtained. The T1-weighted image was normalized into MNI space, using both linear registration and non-linear registration. The transformation field derived from T1 to MNI normalization warped the co-registered PdWI image in structural space. Both of the warp-fields and transformation matrix were inverted by the command of convert_xfm and invwarp respectively, and then they were applied to the Human Brainnetome Atlas which is based on connectional architecture. At last, the atlas registered to each subject was resampled to CBF pcolor image, then 246 regions were obtained by using this procedure and each region represents a node in the brain network. Formula for the calculation is as follow.
Results were presented as mean values ± standard deviation (SD). The comparisons of the changes in myocardial enzymogram and NT-proBNP before and after head down tilt position were analyzed by Paired t- test. A value of p < 0.05 was considered statistically significant.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
In the present study, 4 eligible AIS patients who occurred END within 7 days after onset were treated with head down tilt position (ages 48–74, average 60 years). Baseline characteristics of patients, symptoms, culprit arteries, etiology, presence of cervical artery atherosclerosis, onset to head down tilt time, prior intravenous thrombosis, prior mechanical thrombectomy, NIHSS score at different stages, and adverse events were listed in Table 1. The results showed that neurological function did not further deteriorate after head down tilt, and neurological deficit at discharge and neurological outcome 90 days after onset were significantly improved. No patients reported obvious uncomfortable events. The electrocardiographic monitoring did not show visible change during head down tilt. There were no changes in myocardial enzymogram and N-terminal pro b-type Natriuretic Peptide (NT-proBNP) before and after head down tilt in all patients (Table 2).
To explore the differences in CBF changes between head down tilt position and lying flat position, 3 anterior circulation ischemic stroke patients (Case 1, 3 and 4) underwent arterial spin labeling examination. The CBF value under lying flat and head down tilt positions were subtracted and ranked. In most regions of Case 1 and Case 3, the CBF value under head down tilt position increased significantly compared to that under lying flat position (Figure 2). The CBF value in Case 4 also increased although not significant. The top 20 brain regions changed the most in each subject were shown in Figures 2C–E.
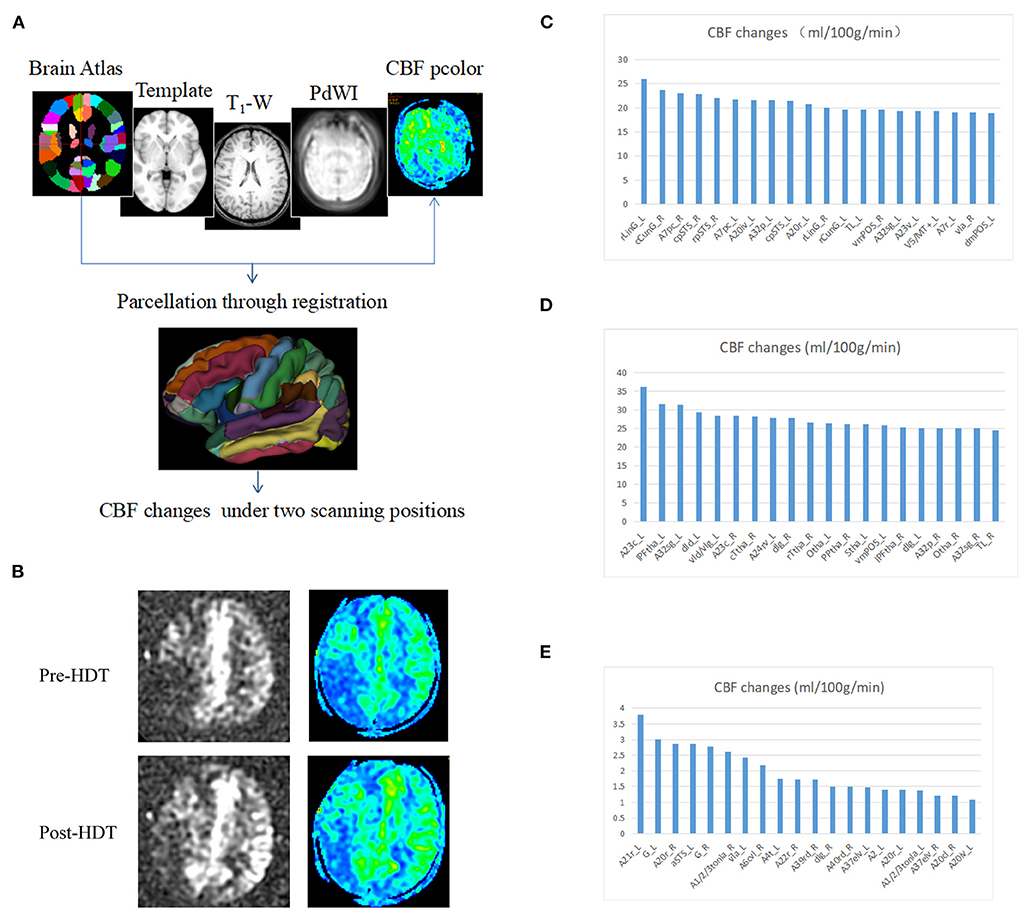
Figure 2. Changes in cerebral blood flow after head down tilt position. (A) Pipeline for the registration and determination of regions. The Brainnetome Atlas, template, T1-weighted, PdWI and CBF pcolor were used to co-registration and 246 regions of interest were obtained in every subject. In addition, the CBF changes were calculated under two scanning positions (head down tilt position and lying flat position). (B) Representative ASL and CBF pcolor images from Case 1. (C) CBF changes in Case 1. (D) CBF changes in Case 3. (E) CBF changes in Case 4. ASL, arterial spin labeling; CBF, cerebral blood flow; HDT, head down tilt.
In AIS patients, various interventions were performed to improve CBF through collateral arteries, leptomeningeal recruitment, and increasing residual blood flow, aiming to improve cerebral perfusion and decrease injury within the ischemic penumbra region. But none so far has been demonstrated effective except reperfusion strategy. HeadPoST study demonstrated no difference in the functional recovery of patients assigned to flatting or sitting up position (6), which may be due to the broad inclusion of the patients (14). Head positioning trials should be performed in discrete patient cohorts with endpoints supported by pilot data (15). The present study first showed that the head down tilt position improved neurological function in acute ischemic stroke patients by possibly increasing the CBF of affected side.
We found that head down tilt treatment could prevent the further deterioration of clinical symptoms in AIS patients with END, because symptoms in all patients stop progression after the treatment. Meanwhile, improvements in NIHSS scoring were detected after head down tilt treatment and an increase in CBF of the ischemic area was induced in patients with anterior circulation infarction by head down tilt position. As we know, ischemic stroke is a potentially reversible process that is dependent on restoration of CBF within a time window of cellular viability that varies according to the severity and duration of the flow deficit. The prognosis of acute ischemic stroke was determined according to the location and size of the occluded cerebral vessel (1), the extent of collateral blood flow (1, 2) and the time to reperfusion therapy (22). Previous studies indicated that changes in head position could influence the prognosis of AIS patients through regulating cerebral perfusion pressure, intracranial pressure, cortical oxygenation (23), systolic and diastolic blood pressure, residual CBF to the ischemic brain tissue (24), collateral blood flow (25, 26), and mean velocity of the residual arterial blood flow (7). A study on “collateral therapeutics” in a rat stroke model of transient MCAO evaluated the effect of different strategies including induced hypertension, intravascular volume load, cerebral arteriolar vasodilation, and head down tilt on stroke outcome, and indicated that treatment with collateral therapeutics was associated with lower infarct volumes and higher chance of good functional outcome. Notably, the highest efficacy and safety profile was observed for head down tilt treatment (27). In addition, cerebral autoregulation ability was impaired in stroke patients and could lead to a decrease in CBF velocity during orthostatic stress with head-up tilt. Therefore, we argue that positioning AIS patients with head down tilt may exert neuroprotective effect through increasing CBF due to collateral circulation improvement by gravitational force (28). Furthermore, we argue that this benefit may be more obvious in the moderate stroke patients with LVO in the acute phase (within 24 h). For acute mild stroke, intensive antithrombosis should be best strategy to prevent the deterioration or reoccurrence of stroke (29). For severe ischemic stroke patients, recanalization as soon as possible may be the best way to acquire good outcome due to limited ischemic penumbra that can be salvaged. In addition, head down tilt treatment may further increase intracranial pressure for severe stroke. In the present study, 2 of the 4 patients had a posterior circulation stroke, which is expected to have a smaller response to collateral therapeutics due to less developed collaterals, compared to anterior circulation stroke. Taken together, we argue that the best target population should be acute moderate stroke patients with LVO, which is being determined by our ongoing trial (NCT03744533).
In previous studies, transcranial laser Doppler-recorded mean flow velocity (MFV) was usually used to reflect the changes in CBF after head positioning treatment. However, Transcranial Doppler only provided indirect information of CBF, due to measuring the flow velocity and not the volume flow. Therefore, changes of blood flow velocity could correspond to the change of CBF only if the vessel diameter was constant, which cannot be assumed a priori. Moreover, even very small changes in the position of the transcranial Doppler probe could alter the blood flow velocity readings. Furthermore, the relevance of changes in MFV to any improvement in clinical outcomes after AIS is uncertain (30). In our study, CBF was evaluated by arterial spin labeling, which steadily reflect the volume flow.
It was reported that serious adverse events of head down tilt treatment included visual field loss, cognitive aberrations, potentially life-threatening complications of the respiratory and cardiovascular systems (31). In our study, no serious adverse events, including aspiration pneumonia, were observed in patients treated with head down tilt position, which may be due to short-term and moderate tilt of head down position. Another important concern was about its effect on CBF, because decreased CBF may occur due to increased intracranial pressure (ICP) induced by head down tilt. The mechanism appeared to be related to the changes in venous outflow through the valueless jugular veins and vertebral venous plexuses. Cerebral venous and jugular venous pressures increase with head position lowering, leading to an increase in the cerebral venous blood volume and a subsequent increase in ICP. The cerebral perfusion pressure (CPP) was calculated as the difference between mean arterial pressure and ICP. The CPP may increase when head position was under the level of heart since the mean arterial pressure increased in this head down tilt position. We argued that in certain angle and duration time, head down tilt position exerted neuroprotective effect through increasing CPP. However, ICP increased due to the impairment of venous outflow in a greater minus angle, leading to the decrease in CPP and the deterioration of neurological function. The main limitation of the clinical study is the small sample, no control design.
For the first time, the case series study suggests that head down tilt may exert neuroprotective effect in AIS patients possibly through increasing the cerebral perfusion. Due to its low cost and easy operation, the efficacy and safety of head down tilt position in acute ischemic patients are urgently needed to be confirmed in further clinical trials.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the former General Hospital of Shenyang Military Region (No. k2017-10). The patients/participants provided their written informed consent to participate in this study.
Z-AZ, N-NZ, and LT prospectively enrolled patients and acquired the data. ML, YC, and Z-AZ did statistical analysis. Z-AZ, N-NZ, and H-SC wrote the draft. S-LQ critically revised the manuscript. H-SC designed the study and critically edited the manuscript.
This work was supported by grants from National Key R&D Program of China (2017YFC1308200) and the Science and Technology Project Plan of Liaoning Province (2018225023 and 2019JH2/10300027).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.992885/full#supplementary-material
1. Menon BK, Goyal M. Clots, collaterals, and the intracranial arterial tree. Stroke. (2016) 47:1972–3. doi: 10.1161/STROKEAHA.116.013829
2. Shuaib A, Butcher K, Mohammad AA, Saqqur M, Liebeskind DS. Collateral blood vessels in acute ischaemic stroke: a potential therapeutic target. Lancet Neurol. (2011) 10:909–21. doi: 10.1016/S1474-4422(11)70195-8
3. Bang OY, Saver JL, Kim SJ, Kim GM, Chung CS, Ovbiagele B, et al. Collateral flow predicts response to endovascular therapy for acute ischemic stroke. Stroke. (2011) 42:693–9. doi: 10.1161/STROKEAHA.110.595256
4. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
5. Anderson CS, Olavarria VV. Head positioning in acute stroke. Stroke. (2018) 50:224–8. doi: 10.1161/STROKEAHA.118.020087
6. Anderson CS, Arima H, Lavados P, Billot L, Hackett ML, Olavarria VV, et al. Cluster-randomized, crossover trial of head positioning in acute stroke. N Engl J Med. (2017) 376:2437–47. doi: 10.1056/NEJMoa1615715
7. Wojner-Alexander AW, Garami Z, Chernyshev OY, Alexandrov AV. Heads down: flat positioning improves blood flow velocity in acute ischemic stroke. Neurology. (2005) 64:1354–7. doi: 10.1212/01.WNL.0000158284.41705.A5
8. Fan JY. Effect of backrest position on intracranial pressure and cerebral perfusion pressure in individuals with brain injury: a systematic review. J Neurosci Nurs. (2004) 36:278–88. doi: 10.1097/01376517-200410000-00007
9. Munoz Venturelli P, Olavarria V, Gonzalez F, Brunser A, Lavados P, Arima H, et al. Head position in the early phase of acute ischemic stroke: an international survey of current practice. J Stroke Cerebrovasc Dis. (2015) 24:1564–9. doi: 10.1016/j.jstrokecerebrovasdis.2015.03.023
10. Schwarz S, Georgiadis D, Aschoff A, Schwab S. Effects of body position on intracranial pressure and cerebral perfusion in patients with large hemispheric stroke. Stroke. (2002) 33:497–501. doi: 10.1161/hs0202.102376
11. Schwarz S, Georgiadis D, Aschoff A, Schwab S. Effects of induced hypertension on intracranial pressure and flow velocities of the middle cerebral arteries in patients with large hemispheric stroke. Stroke. (2002) 33:998–1004. doi: 10.1161/01.STR.0000014584.17714.2E
12. Brunser AM, Munoz Venturelli P, Lavados PM, Gaete J, Martins S, Arima H, et al. Head position and cerebral blood flow in acute ischemic stroke patients: protocol for the pilot phase, cluster randomized, head position in acute ischemic stroke trial (headpost pilot). Int J Stroke. (2016) 11:253–9. doi: 10.1177/1747493015620808
13. Olavarria VV, Lavados PM, Munoz-Venturelli P, Gonzalez F, Gaete J, Martins S, et al. Flat-head positioning increases cerebral blood flow in anterior circulation acute ischemic stroke. A cluster randomized phase iib trial. Int J Stroke. (2018) 13:600–11. doi: 10.1177/1747493017711943
14. Alexandrov AW, Tsivgoulis G, Hill MD, Liebeskind DS, Schellinger P, Ovbiagele B, et al. Headpost: rightly positioned, or flat out wrong? Neurology. (2018) 90:885–9. doi: 10.1212/WNL.0000000000005481
15. Katsanos AH, Kargiotis O, Safouris A, Psychogios K, Magoufis G, Tsivgoulis G. Letter to the editor for the article “flat-head positioning increases cerebral blood flow in anterior circulation acute ischemic stroke: a cluster randomized phase iib trial”. Int J Stroke. (2019) 14:NP7. doi: 10.1177/1747493018816461
16. Telischak NA, Detre JA, Zaharchuk G. Arterial spin labeling MRI: clinical applications in the brain. J Magn Reson Imaging. (2015) 41:1165–80. doi: 10.1002/jmri.24751
17. de Havenon A, Haynor DR, Tirschwell DL, Majersik JJ, Smith G, Cohen W, et al. Association of collateral blood vessels detected by arterial spin labeling magnetic resonance imaging with neurological outcome after ischemic stroke. JAMA Neurol. (2017) 74:453–8. doi: 10.1001/jamaneurol.2016.4491
18. Richter V, Helle M, van Osch MJ, Lindner T, Gersing AS, Tsantilas P, et al. Mr imaging of individual perfusion reorganization using superselective pseudocontinuous arterial spin-labeling in patients with complex extracranial steno-occlusive disease. AJNR Am J Neuroradiol. (2017) 38:703–11. doi: 10.3174/ajnr.A5090
19. Yu SL, Wang R, Wang R, Wang S, Yao YQ, Zhang D, et al. Accuracy of vessel-encoded pseudocontinuous arterial spin-labeling in identification of feeding arteries in patients with intracranial arteriovenous malformations. AJNR Am J Neuroradiol. (2014) 35:65–71. doi: 10.3174/ajnr.A3638
20. Ni WW, Christen T, Rosenberg J, Zun Z, Moseley ME, Zaharchuk G. Imaging of cerebrovascular reserve and oxygenation in moyamoya disease. J Cereb Blood Flow Metab. (2017) 37:1213–22. doi: 10.1177/0271678X16651088
21. Jezzard P, Chappell MA, Okell TW. Arterial spin labeling for the measurement of cerebral perfusion and angiography. J Cereb Blood Flow Metab. (2018) 38:603–26. doi: 10.1177/0271678X17743240
22. Nikoubashman O, Dekeyzer S, Riabikin A, Keulers A, Reich A, Mpotsaris A, et al. True first-pass effect. Stroke. (2019) 50:2140–6. doi: 10.1161/STROKEAHA.119.025148
23. Mehagnoul-Schipper DJ, Vloet LC, Colier WN, Hoefnagels WH, Jansen RW. Cerebral oxygenation declines in healthy elderly subjects in response to assuming the upright position. Stroke. (2000) 31:1615–20. doi: 10.1161/01.STR.31.7.1615
24. Nagatani K, Nawashiro H, Takeuchi S, Otani N, Wada K, Shima K. Effects of a head-down tilt on cerebral blood flow in mice during bilateral common carotid artery occlusion. Asian J Neurosurg. (2012) 7:171–3. doi: 10.4103/1793-5482.106648
25. Hunter AJ, Snodgrass SJ, Quain D, Parsons MW, Levi CR. Hoboe (head-of-bed optimization of elevation) study: association of higher angle with reduced cerebral blood flow velocity in acute ischemic stroke. Phys Ther. (2011) 91:1503–12. doi: 10.2522/ptj.20100271
26. Han M, Kwon I, Ha J, Kim J, Cha MJ, Kim YD, et al. Collateral augmentation treatment with a combination of acetazolamide and head-down tilt in a rat ischemic stroke model. J Clin Neurosci. (2020) 73:252–8. doi: 10.1016/j.jocn.2020.01.079
27. Beretta S, Versace A, Carone D, Riva M, Dell'Era V, Cuccione E, et al. Cerebral collateral therapeutics in acute ischemic stroke: a randomized preclinical trial of four modulation strategies. J Cereb Blood Flow Metab. (2017) 37:3344–54. doi: 10.1177/0271678X16688705
28. Olavarria VV, Arima H, Anderson CS, Brunser AM, Munoz-Venturelli P, Heritier S, et al. Head position and cerebral blood flow velocity in acute ischemic stroke: a systematic review and meta-analysis. Cerebrovasc Dis. (2014) 37:401–8. doi: 10.1159/000362533
29. Hou X, Chen H. Proposed antithrombotic strategy for acute ischemic stroke with large-artery atherosclerosis: focus on patients with high-risk transient ischemic attack and mild-to-moderate stroke. Ann Transl Med. (2020) 8:16. doi: 10.21037/atm.2019.10.111
30. Treger I, Streifler JY, Ring H. The relationship between mean flow velocity and functional and neurologic parameters of ischemic stroke patients undergoing rehabilitation. Arch Phys Med Rehabil. (2005) 86:427–30. doi: 10.1016/j.apmr.2004.09.004
Keywords: arterial spin labeling, cerebral blood flow, head-down tilt, stroke, early neurological deterioration
Citation: Zhao Z-A, Zhang N-N, Tao L, Cui Y, Li M, Qi S-L and Chen H-S (2022) Effect of head-down tilt on clinical outcome and cerebral perfusion in ischemic stroke patients: A case series. Front. Neurol. 13:992885. doi: 10.3389/fneur.2022.992885
Received: 13 July 2022; Accepted: 08 September 2022;
Published: 26 September 2022.
Edited by:
Michael J. Schneck, Loyola University Chicago, United StatesReviewed by:
Simone Beretta, San Gerardo Hospital, ItalyCopyright © 2022 Zhao, Zhang, Tao, Cui, Li, Qi and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui-Sheng Chen, Y2hzemhAYWxpeXVuLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.