
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 17 August 2022
Sec. Neurotrauma
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.988854
This article is part of the Research TopicInsights in Neurotrauma: 2021View all 11 articles
To determine the possible role of matrix metallopeptidase (MMP)-8 and MMP-9 in the development of chronic subdural hematoma (CSDH), we investigated their expression in CSDH. In our previous study, we analyzed hematoma fluid and peripheral blood of 83 patients with CSDH, including 17 postoperative patients. Based on these results, we included 50 people in the normal group and analyzed 20 markers in the peripheral blood of each person. In order to identify representative markers, it was assessed by using overall differential gene expression. The concentration of MMP-8 was significantly higher in the normal group than that in the preoperative and postoperative groups. The concentration of MMP-9 was significantly lower in the normal group than in both preoperative and postoperative groups. Immunohistochemistry confirmed the expression of MMP-8 and MMP-9 in CSDH membranes. In conclusion, our results provide evidence of the expression of MMP-8 and MMP-9 in CSDH. In addition, the expression of MMP-8 and MMP-9 suggests angiogenesis in CSDH formation.
As a common neurosurgical disease, chronic subdural hematoma (CSDH) involves the collection of blood in the subdural space (1). The incidence rates of CSDH have been rising on account of the aging population and the increasing use of anticoagulants and antiplatelet medications (2). However, the development of CSDH is still poorly understood (3, 4). Previous research has investigated the mechanisms underlying CSDH, including angiogenesis, fibrinolysis, and inflammation (5).
In a rat model, inflammation and angiogenesis were found to play important roles in the formation of CSDH (6, 7). Numerous biomarkers have been identified in patients with CSDH, including interleukin (IL)-1, IL-6, IL-8, IL-10, vascular endothelial growth factor (VEGF), matrix metallopeptidase (MMP)-2, and MMP-9 (5, 8–10). In our previous study, we found that MMP-8 and MMP-9 may play a key role in the pathophysiology of CSDH (11).
MMPs are a family of zinc-dependent proteolytic enzymes that contribute to pathological inflammation and endothelial dysfunction (12). This appears to be related to CSDH formation. However, there have been few studies on the expression and activity of MMPs in CSDH. Based on our previous study, we used immunohistochemistry to analyze CSDH membranes to determine the possible role of MMP-8 and MMP-9 in the development of CSDH (11).
We recruited 50 individuals to the normal group based on our previous study (11). In addition, the membranes of CSDHs were obtained from 10 patients (eight males and two females) who underwent surgeries at the Shenzhen Second People's Hospital. The patients ranged from 35 to 84 years of age (mean: 65.3 years) and had not been previously treated for CSDH. This clinical study was approved by the Ethics Committee of the Shenzhen Second People's Hospital (20200422003-XZ2021-XZ2021). Informed consent was obtained from all the participants involved in this study.
Peripheral blood samples were collected from 50 patients in the control group. All samples were collected in tubes containing a coagulator and immediately centrifuged at 2,000 rpm for 15 min. After centrifugation, the supernatants were stored in sealed polypropylene tubes at −80 °C until further analysis.
We analyzed the hematoma fluid and preoperative and postoperative peripheral blood samples using the 20-plex human panel A system (R&D Systems, Minneapolis, MN, USA), Luminex system (Luminex, Austin, TX, USA), and Bioplex software (BioRad, Hercules, CA, USA). We evaluated IL-1α, IL-6, IL-8, IL-10, Angiopoietin-2, platelet-derived growth factor-BB (PDGF-BB), MMP-1, MMP-2, MMP-3, MMP-8, MMP-9, D-dimer, epidermal growth factor (EGF), C-C motif chemokine ligand 2 (CCL2), tumor necrosis factor (TNF-α), hepatocyte growth factor (HGF), VEGF, insulin-like growth factor binding protein-3 (IGFBP-3), prolactin, and VEGF receptor-2 (VEGFR2) levels.
We searched the genes corresponding to cytokines through PubMed. The overall differential gene expression was evaluated by using the ggplot2 package in the R statistical software. Furthermore, the LIMMA package was used to select DEGs. We used the empirical Bayes moderated t-test to calculate the p-values for each cytokine. The adjusted p-values were calculated based on the false discovery rate. Only genes with a log2 fold change > 2 and false discovery rate < 0.01 were considered DEGs.
CSDH membrane tissues were placed in 4% paraformaldehyde for 20 h at 4 °C. The tissue was embedded in paraffin, and 4 μm serial sections were cut. The sections were deparaffinized in xylene and dehydrated using descending dilutions of ethanol (100% and 95%). The primary antibodies (Goat monoclonal, Anti-MMP-8, No: AF908-SP, 1:50, R&D Systems; Rabbit monoclonal, Anti-MMP-9, No: ab76003, 1:50, Abcam, Cambridge, UK) were incubated for 30 min at 25 °C and overnight at 4 °C and stained using the avidin-biotin peroxidase method and 3, 3'-diaminobenzidine as a chromogen. Finally, the sections were counterstained with Mayer's hematoxylin and mounted using an aqueous mounting medium.
Statistical analyses were performed using the Mann–Whitney U-test. Data are presented as mean ± standard deviation. All analyses were performed using the SPSS software. Statistical significance was set at p < 0.05.
The mean age in the preoperative CSDH group was 67.16 ± 4.13 years. The general characteristics of each group are presented in Table 1.
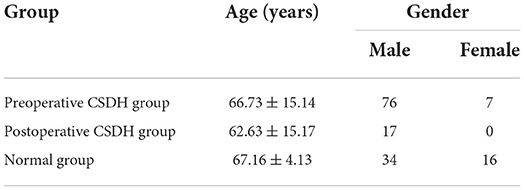
Table 1. Age and sex in preoperative chronic subdural hematoma (CSDH), postoperative CSDH, and normal groups.
All peripheral blood samples obtained from the preoperative CSDH and normal groups were analyzed. An overview of the differential gene expression is presented in Table 2. MMP-2, PDGF-BB, IL-10, EGF, IL-1α, CCL2, Angiopoietin-2, IL-6, MMP-8, VEGF, VEGFR2, HGF, MMP-9, IGFBP-3, TNF-α, IL-8, and Prolactin were DEGs between the preoperative and normal groups. MMP-1, MMP-3, and D-dimer were not significantly different between the preoperative and normal groups. All peripheral blood samples obtained from the postoperative and normal groups were analyzed. An overview of the differential gene expression is presented in Table 3. CCL2, EGF, IL-10, IL-1α, Angiopoietin-2, IL-6, MMP-8, HGF, TNF-α, MMP-9, IL-8, VEGFR2, VEGF, prolactin, D-dimer, IGFBP-3, MMP-1, and PDGF-BB were identified as DEGs between postoperative and normal groups. MMP-2 and MMP-3 were not significantly different between the postoperative and normal groups.
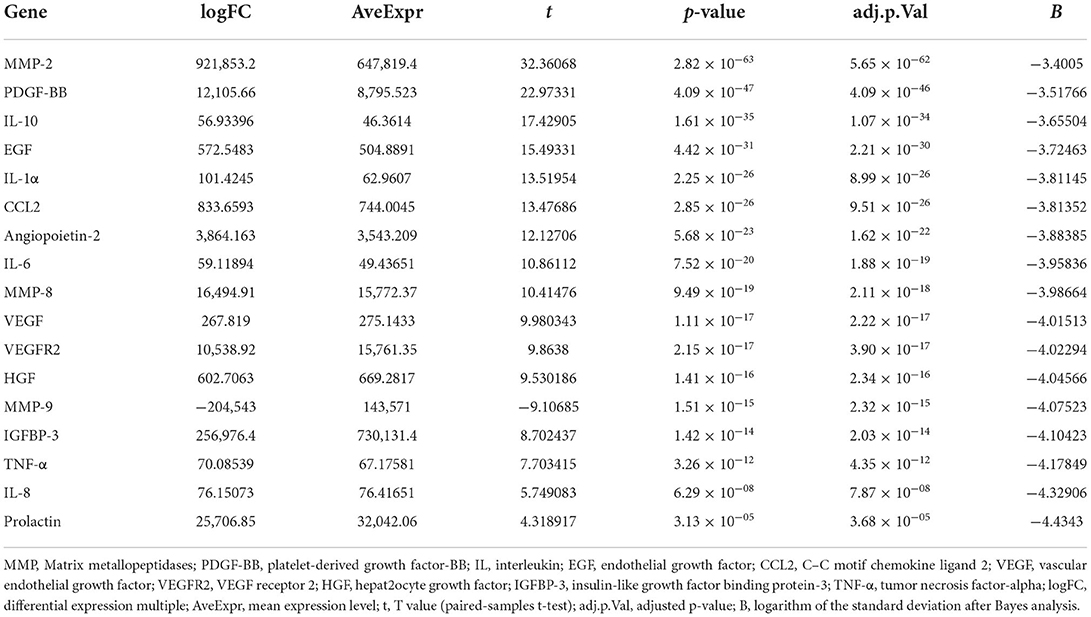
Table 2. Differential expression of all cytokines in peripheral blood samples between the preoperative CSDH and normal groups.
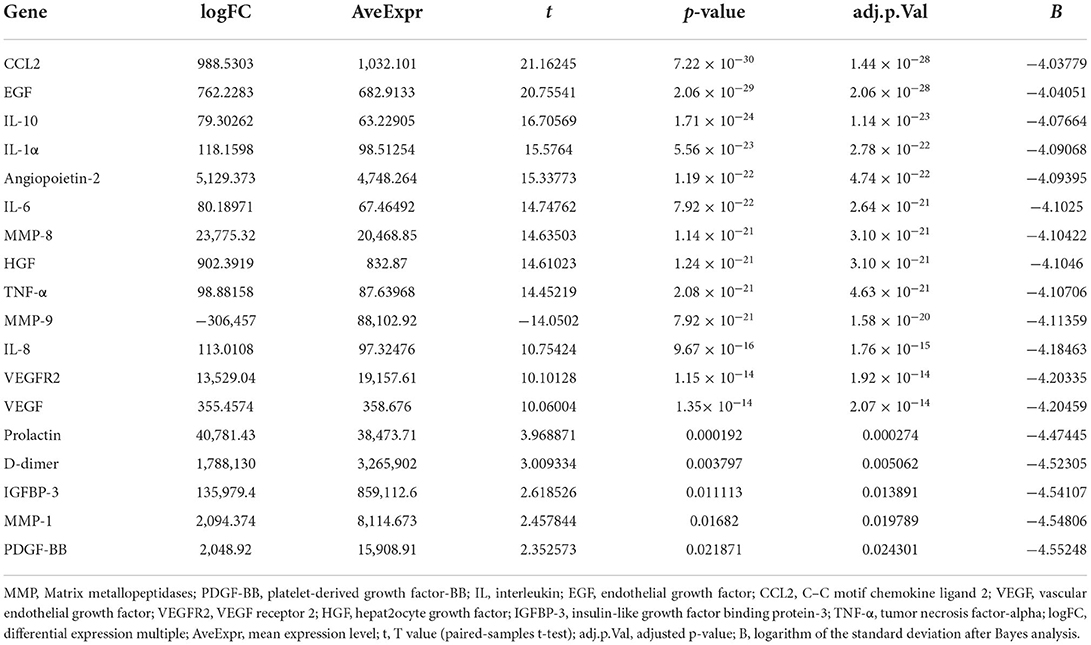
Table 3. Differential expression of all cytokines in peripheral blood samples between the postoperative CSDH and normal groups.
The concentrations of MMP-8 and MMP-9 in the control (normal) group are shown in Table 4 (p < 0.01). Based on our previous study (11), the concentration of MMP-8 was significantly higher in the normal group compared to that in both preoperative and postoperative CSDH groups, while that of MMP-9 was significantly lower. All CSDH membranes were immunostained for MMP-8 and MMP-9 in all samples. These proteins were positively immunostained in vascular endothelial cells. The results for the immunostained membranes are shown in Figures 1, 2.
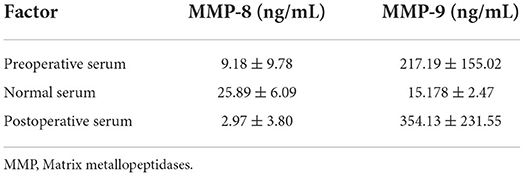
Table 4. Concentration of MMP-8 and MMP-9 in the normal, preoperative, and postoperative groups (p < 0.01).
Based on our previous study, MMP-8 and MMP-9 may contribute to CSDH pathogenesis in different ways (11). We found that the MMP-8 concentration was significantly higher in the normal group compared to that in preoperative and postoperative groups, whereas the MMP-9 concentration was significantly lower. Simultaneously, we observed that both MMP-8 and MMP-9 were expressed in vascular endothelial cells. Nowadays, an increasing number of studies consider that CSDH formation may be related to the growth of new vessels and inflammation (1, 13). In addition, MMPs significantly contribute to blood vessel formation, remodeling, and angiogenesis by regulating the functions or behaviors of stem/progenitor and vascular cells (14).
Although CSDH is the most common neurosurgical disease, a few questions remain to be answered. Tamura et al. (15) indicated that the split dural border cell layer produced a dural hematoma by forming the inner and outer membranes in CSDH. The outer membranes drive inflammation and angiogenesis. After studying the role of MMPs in the development of CSDH, Nakagawa et al. (16) found that MMPs degrade the integrity of the extracellular matrix in the outer membranes of CSDH, which can cause the exudation of interstitial edematous fluid from membrane vessels into the hematoma cavity. This seemed to be the reason for CSDH enlargement. Some studies have shown that MMP-8 plays an important role in endothelial cell angiogenesis (12, 14). In our study, the change in the MMP-8 concentration indicated that MMP-8 might be inhibited when neovascularization proceeds and increases after the formation of new vessels are suspended. In future studies, we will focus on MMP-8 in CSDH formation.
The role of MMP-9 in the pathophysiological pathways of CSDH has been reported in many studies (1, 10, 11, 13). MMP-9 expression in vascular endothelial cells confirmed that CSDH formation was related to MMP-9 expression. MMP-9 is regarded as one of the factors contributing to the formation of fragile, leaky capillaries (5). Hua et al. (10) considered that the MMP/VEGF system may be involved in angiogenesis associated with CSDH, because of the relation of the concentrations of MMP-2 and MMP-9 and the VEGF concentration in the hematoma fluid. Xu et al. (6) found that the formation of neovessels might be correlated with the increased production of pro-angiogenic factors, including MMP-9 and VEGF, in rats. Furthermore, studies have indicated that MMP-9 can reduce CSDH absorption by increasing vascular permeability, enhancing inflammation, and reducing vascular maturation (13). Huang et al. (3) found that atorvastatin plays a similar role to MMP-9 and can promote hematoma absorption, reduce tissue inflammation, and improve neurological function. We propose that when the trigger began, the MMP-9 concentration increased rapidly, while that of MMP-8 decreased. These biomolecules interact with and may influence each other. With the development and treatment of CSDH, the concentration of MMP-9 also increased, indicating that it could promote the formation of new normal vessels to repair normal physiological function.
Our understanding of CSDH pathogenesis is constantly being updated, and researchers are currently focusing on developing appropriate treatments. It has been found that atorvastatin inhibits inflammation and angiogenesis (17, 18). Based on a study of SDH in rats, it may eliminate SDH and improve neural function by using atorvastatin (19). In addition, Soleman et al. (20) concluded that atorvastatin is valid and safe for treating asymptomatic or mildly symptomatic patients with CSDH. Atorvastatin may enhance angiogenesis to reduce CSDH-related inflammation (21). Fan et al. (22) found that Krüppel-like factor 2 (KLF-2) plays a key role in drug therapy for CSDH. It was found that combination therapy with atorvastatin and low-dose dexamethasone can inhibit the expression of KLF-2 to attenuate robust endothelial inflammation and permeability (22). In a randomized placebo-controlled trial, effective function was confirmed in patients treated with atorvastatin and low-dose dexamethasone (23). Our findings indicate that MMP-8 and MMP-9 may serve as accessible markers of CSDH formation. Future studies should focus on the relationship between MMP-8 and MMP-9 in CSDH fluid and their membrane activity. Moreover, we wish to determine the relationship between these two cytokines and CSDH prognosis and identify a way to prevent recurrence in patients with CSDH. More important in the clinic, understanding CSDH mechanisms would help physicians restore neurological deficits in patients with CSDH and improve their quality of life (24).
There were some limitations in this study. Firstly, from our limited number of cases, it is difficult to represent the wide spectrum of patients with CSDH. Further studies including a larger sample of patients will be needed to clarify this point. Secondly, the experimental analysis of the CSDH membrane was relatively limited, and so the results may not be completely convincing. More approaches are needed to verify the expression and role of MMP-8 and MMP-9 on the membrane.
Our study provides evidence of MMP-8 and MMP-9 expression in CSDH. In addition, the expression of MMP-8 and MMP-9 is indicative of angiogenesis in CSDH formation. Identification of the biomolecules involved in CSDH formation may be helpful in the development of new clinical therapies. However, further studies are necessary to clarify the association between MMP-8 and MMP-9 expression during the transformation and progression of CSDH.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Institutional Ethics Committee of Shenzhen Second People's Hospital. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Conceptualization, methodology, and project administration: G-JS, DZ, and X-JH. Validation, resources, and investigation: G-JS and DZ. Software: J-NW, C-WW, and X-JZ. Formal analysis: Y-HD, C-WW, and X-JZ. Data curation, visualization, and writing—original draft preparation: G-JS. Writing—review and editing and supervision: X-JH. Funding acquisition: X-JH and X-JZ. All authors contributed to the article and approved the submitted version.
This research was funded by the Natural Science Foundation of China, Grant Number 81301062, Shenzhen Double Chain Grant, Grant Number (2018) 256, Discipline Construction Capacity Improvement Project of the Shenzhen Municipal Health Commission, Grant Number SZXJ2018057, and Basic Research Fund of the Shenzhen Science and Technology Program, Grant Numbers JCYJ20180228163034627 and JCYJ20190806162210754.
The authors would like to thank the physicians and nurses of Shenzhen Second People's Hospital Department of Neurosurgery.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Feghali J, Yang W, Huang J. Updates in chronic subdural hematoma: epidemiology, etiology, pathogenesis, treatment, and outcome. World Neurosurg. (2020) 141:339–45. doi: 10.1016/j.wneu.2020.06.140
2. Kolias AG, Chari A, Santarius T, Hutchinson PJ. Chronic subdural haematoma: Modern management and emerging therapies. Nat Rev Neurol. (2014) 10:570–8. doi: 10.1038/nrneurol.2014.163
3. Huang J, Gao C, Dong J, Zhang J, Jiang R. Drug treatment of chronic subdural hematoma. Expert Opin Pharmacother. (2020) 21:435–44. doi: 10.1080/14656566.2020.1713095
4. Iliescu IA, Constantinescu AI. Clinical evolutional aspects of chronic subdural haematomas–Literature review. J Med Life. (2015) 8:26–33.
5. Edlmann E, Giorgi-Coll S, Whitfield PC, Carpenter KLH, Hutchinson PJ. Pathophysiology of chronic subdural haematoma: inflammation, angiogenesis and implications for pharmacotherapy. J Neuroinflammation. (2017) 14:108. doi: 10.1186/s12974-017-0881-y
6. Xu X, Wang D, Han Z, Wang B, Gao W, Fan Y, et al. A novel rat model of chronic subdural hematoma: Induction of inflammation and angiogenesis in the subdural space mimicking human-like features of progressively expanding hematoma. Brain Res Bull. (2021) 172:108–19. doi: 10.1016/j.brainresbull.2021.04.024
7. Quan W, Zhang Z, Tian Q, Wen X, Yu P, Wang D, et al. A rat model of chronic subdural hematoma: Insight into mechanisms of revascularization and inflammation. Brain Res. (2015) 1625:84–96. doi: 10.1016/j.brainres.2015.08.017
8. Wada T, Kuroda K, Yoshida Y, Ogasawara K, Ogawa A, Endo S. Local elevation of the anti-inflammatory interleukin-10 in the pathogenesis of chronic subdural hematoma. Neurosurg Rev. (2006) 29:242–5. doi: 10.1007/s10143-006-0019-7
9. Pripp AH, Stanišić M. Association between biomarkers and clinical characteristics in chronic subdural hematoma patients assessed with Lasso regression. PLoS One. (2017) 12:e0186838. doi: 10.1371/journal.pone.0186838
10. Hua C, Zhao G, Feng Y, Yuan H, Song H, Bie L. Role of matrix metalloproteinase-2, matrix metalloproteinase-9, and vascular endothelial growth factor in the development of chronic subdural hematoma. J Neurotrauma. (2016) 33:65–70. doi: 10.1089/neu.2014.3724
11. Su GJ, Gao J, Wu CW, Zou JF, Zhang D, Zhu DL, et al. Serum levels of MMP-8 and MMP-9 as markers in chronic subdural hematoma. J Clin Med. (2022) 11:902. doi: 10.3390/jcm11040902
12. Bassiouni W, Ali MAM, Schulz R. Multifunctional intracellular matrix metalloproteinases: Implications in disease. FEBS J. (2021) 288:7162–82. doi: 10.1111/febs.15701
13. Holl DC, Volovici V, Dirven CMF, Peul WC, van Kooten F, Jellema K, et al. Pathophysiology and nonsurgical treatment of chronic subdural hematoma: From past to present to future. World Neurosurg. (2018) 116:402–11.e2. doi: 10.1016/j.wneu.2018.05.037
14. Chen Q, Jin M, Yang F, Zhu J, Xiao Q, Zhang L. Matrix metalloproteinases: Inflammatory regulators of cell behaviors in vascular formation and remodeling. Mediators Inflamm. (2013) 2013:928315. doi: 10.1155/2013/928315
15. Tamura R, Sato M, Yoshida K, Toda M. History and current progress of chronic subdural hematoma. J Neurol Sci. (2021) 429:118066. doi: 10.1016/j.jns.2021.118066
16. Nakagawa T, Kodera T, Kubota T. Expression of matrix metalloproteinases in the chronic subdural haematoma membrane. Acta Neurochir. (2000) 142:61–6. doi: 10.1007/s007010050008
17. Wang B, Sun L, Tian Y, Li Z, Wei H, Wang D, et al. Effects of atorvastatin in the regulation of circulating EPCs and angiogenesis in traumatic brain injury in rats. J Neurol Sci. (2012) 319:117–23. doi: 10.1016/j.jns.2012.04.015
18. Araújo FA, Rocha MA, Mendes JB, Andrade SP. Atorvastatin inhibits inflammatory angiogenesis in mice through down regulation of VEGF, TNF-alpha and TGF-beta1. Biomed Pharmacother. (2010) 64:29–34. doi: 10.1016/j.biopha.2009.03.003
19. Li T, Wang D, Tian Y, Yu H, Wang Y, Quan W, et al. Effects of atorvastatin on the inflammation regulation and elimination of subdural hematoma in rats. J Neurol Sci. (2014) 341:88–96. doi: 10.1016/j.jns.2014.04.009
20. Soleman J, Nocera F, Mariani L. The conservative and pharmacological management of chronic subdural haematoma. Swiss Med Wkly. (2017) 147:w14398. doi: 10.4414/smw.2017.14398
21. Fu S, Li F, Bie L. Drug therapy for chronic subdural hematoma: Bench to bedside. J Clin Neurosci. (2018) 56:16–20. doi: 10.1016/j.jocn.2017.07.034
22. Fan Y, Wang D, Rao C, Li Y, Rong H, Wang Z, et al. Atorvastatin combined with low-dose dexamethasone treatment protects endothelial function impaired by chronic subdural hematoma via the transcription factor KLF-2. Drug Des Dev Ther. (2020) 14:3291–9. doi: 10.2147/DDDT.S256050
23. Wang D, Gao C, Xu X, Chen T, Tian Y, Wei H, et al. Treatment of chronic subdural hematoma with atorvastatin combined with low-dose dexamethasone: Phase II randomized proof-of-concept clinical trial. J Neurosurg. (2020) 134:235–243. doi: 10.3171/2019.11.JNS192020
Keywords: chronic subdural hematoma, matrix metallopeptidase, immunoexpression, differential gene expression, disease development
Citation: Su G-J, Zhang D, Wu J-N, Deng Y-H, Wu C-W, Zhang X-J and Huang X-J (2022) Immunoexpression of MMP-8 and MMP-9 in chronic subdural hematoma. Front. Neurol. 13:988854. doi: 10.3389/fneur.2022.988854
Received: 07 July 2022; Accepted: 01 August 2022;
Published: 17 August 2022.
Edited by:
Darrin Jason Lee, University of Southern California, United StatesReviewed by:
Guangzhi Ning, Tianjin Medical University General Hospital, ChinaCopyright © 2022 Su, Zhang, Wu, Deng, Wu, Zhang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xian-Jian Huang, eGpodWFuZ0BlbWFpbC5zenUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.