- 1Department of Biomedical Sciences, College of Health Sciences, Debre Tabor University, Debre Tabor, Ethiopia
- 2School of Medicine, College of Medicine and Health Science, Bahir Dar University, Bahir Dar, Ethiopia
- 3School of Medicine, College of Health Sciences, Debre Tabor University, Debre Tabor, Ethiopia
- 4Department of Biomedical sciences, Goba Referral Hospital, Madda Walabu University, Bale-Goba, Ethiopia
- 5Department of Public Health, College of Health Sciences, Debre Tabor University, Debre Tabor, Ethiopia
- 6Department of Adult Health Nursing, College of Health Sciences, Debre Tabor University, Debre Tabor, Ethiopia
Background: Stroke is the second leading cause of death worldwide, with a significant increase in stroke burden over the last two and half decades, especially in developing countries. African countries are undergoing an epidemiological transition from being dominated by infectious diseases to being double-burdened by non-communicable diseases, with existing infectious diseases driven by sociodemographic and lifestyle changes and a weak healthcare system. Data on the risk profile, clinical presentation, and predictors of stroke subtypes are still limited. Therefore, the main aim of this study was to assess the risk profile, clinical presentation, and predictors of stroke in public referral hospitals of Northwest Ethiopia.
Methods: For this study, 554 patients with stroke admitted to three public referral hospitals were prospectively followed up. Data were collected using a pre-tested interviewer-administered questionnaire. STATA version 16 was used for data analyses. Candidate variables significant in bivariate analysis were selected for multivariate binary logistic regression, and statistical significance was set at a p < 0.05.
Results: Of the 554 patients with stroke, 60.3% had an ischemic stroke. The mean age of the participants was 61 ± 12.85 years, and more than half (53.25%) of them were women. The most common risk factor identified was hypertension (29.7%), followed by congestive heart failure. The most common clinical presentation was hemiparesis, which was reported by 57.7% of the patients, followed by loss of consciousness (20.7%) and aphasia (9%). Through multivariable logistic regression, age (AOR = 1.03, 95% CI:1.01–1.05), sedentary physical activity level (AOR = 6.78, 95% CI:1.97–23.32), absence of a family history of chronic illness (AOR = 3.79, 95% CI:2.21–6.48), hypertension (AOR=0.51, 95% CI:0.31–0.85), and past stroke (AOR = 3.54, 95% CI:0.93–13.49) were found to be independent determinants of the stroke subtype.
Conclusion: Age, the level of sedentary physical activity, absence of a family history of chronic illness, hypertension, and past stroke were independent determinants of stroke subtype.
Introduction
Stroke is the second leading cause of death worldwide, with a significant increase in stroke burden over the last two and half decades, especially in developing countries (1). African countries are undergoing an epidemiological transition from being dominated by infectious diseases to being double-burdened by non-communicable diseases, with existing infectious diseases driven by sociodemographic and lifestyle changes and poor healthcare system (2, 3). In 2013, an estimated 535,000 new cases and 2.1 million survivors of stroke were found in Africa (4). Currently, stroke morbidity, mortality, and disability are increasing worldwide (4, 5). Data from the Global Burden of Disease (GBD), Injuries, and Risk Factors study revealed that stroke is the leading cardiovascular disease (CVD) that causes mortality and disability in sub-Saharan Africa (SSA) and other low- and middle-income countries (LMICs) (6).
The prevalence of hemorrhagic stroke (HS) is higher in African countries than in Western countries. This disparity is usually ascribed to racial or genetic factors but may be due to differences in risk factor burden (7), which is largely driven by demographic changes and enhanced by an increasing magnitude of modifiable risk factors (8). Age, sex, family history, and ethnicity are non-modifiable risk factors, while hypertension, diabetes mellites, alcohol, smoking, diet, and physical inactivity are among some of the identified modifiable risk factors (9–13). Underdiagnosis or late diagnosis of hypertension and other risk factors like delayed presentation to the health institution, poor risk factor control, and failure to adhere to treatments are major challenges that need to be addressed (9, 14–17).
The burden, factors, and outcome of stroke vary in Ethiopia between regions and over various time periods (18–22). Risk factors of stroke can be classified into modifiable and non-modifiable factors. Age, sex, family history, and race/ethnicity are non-modifiable risk factors, while renal dysfunction, hyperlipidemia, hypertension, alcohol drinking, smoking, diet, obesity, and physical inactivity are among some of the identified modifiable risk factors (13, 22–24). Stroke can be prevented by lifestyle modification and controlling major risk factors.
Data on risk profiles, clinical presentations, and predictors of stroke subtypes are still limited. Therefore, the main aim of this study was to assess the risk profile, clinical presentation, and predictors of stroke subtypes in public referral hospitals of Northwest Ethiopia.
Methods
A cross-sectional study was conducted in three public referral hospitals located in Northwest Ethiopia: the University of Gondar Hospital located in Gondar, Tibebe Ghion Specialized Hospital, and Felege Hiwot Regional Referral Hospital located in Bahir Dar. The study was conducted from December to June 2020–2021. The study included 554 patients with stroke (age ≥ 18 years) admitted to and treated at the three public referral hospitals. The inclusion criterion was specified to include all patients with stroke whose diagnosis was confirmed by a CT scan. Patients who were dead before diagnosis confirmation by CT scan, whose initial assessment or diagnosis of stroke was later changed to another case (ruled out stroke) with further evaluation, and who were being treated by other health institutions and referred after treatment or due to complications were excluded.
Operational definitions
Diabetes mellitus
Patients who were previously on oral hypoglycemic agents/insulin treatment, who had a diagnosis of any type of DM or an FBS level ≥126 mg/dl, or who had a documented RBS level ≥200 mg/dl or a glycosylated hemoglobin level ≥6.5% (25).
Dyslipidemia or hyperlipidemia
Patients with a history of hyperlipidemia or using lipid-lowering medication or a total cholesterol level ≥200 mg/dl, an LDL cholesterol level ≥100 mg/ dl, an HDL cholesterol level <40 mg/dl in case of men or <50 mg/dl in case of women, and/or a serum triglyceride level ≥150 mg/dl (26–28).
Hypertension
Patients receiving antihypertensive medication, who were previously diagnosed with hypertension, or with a blood pressure level ≥140/90 mm/Hg for two measurements (28, 29).
Alcohol use
Patients consuming ≥2 drinks/day in the case of men and ≥1 drink in the case of women on average (previous drinker/ex-drinker for more than 1 year) (14, 30, 31).
Smoker
Patients taking two cigarettes per day in the case of men and one per day in the case of women on average (9, 30, 31).
Former smoker
Patients who abstained from smoking for >1 year (9, 30, 31).
Current smoker
Patients currently smoking for 1 year (9, 30, 31).
Physical activity level
It is the measure of planned or incidental activity, including mode or type of activity, frequency of performing an activity, duration of performing an activity, and intensity of performing an activity (32).
Extremely inactive
It is a PAL < 1.40 (e.g., cerebral palsy) (16, 33).
Sedentary
Patients with a PAL = 1.40–1.69 (e.g., an office worker with little or no exercise) (16, 33).
Moderately active
Patients with a PAL = 1.70–1.99 (e.g., construction worker or a person running 1 h daily) (16, 33).
Vigorously active
Patients with a PAL = 2–2.40 (e.g., agriculture worker non-mechanized or person swimming 2 h daily) (16, 33).
Extremely active
Patients with a PAL > 2.40 (e.g., competitive cyclist) (16, 33).
Data collection tool and procedure
Data collection was carried out by an (R-3) internal medicine resident in each hospital with training on the contents of the data collection tool. The data collection tool was developed based on previous research findings and the WHO STEPwise approach to stroke surveillance (34). All relevant data were retrieved from patients' charts, and interviews were conducted with the patients/caregivers using a prepared data extraction form and questionnaire. The patients' history used in the study was obtained from the patients and/or relatives in the language they understood (Amharic). The data collection form was used to collect data on the sociodemographic and behavioral characteristics and clinical characteristics of patients such as risk factors, clinical presentation, and subtypes of stroke.
Data management and analysis
The collected data were coded, manually checked, and entered into the data management system by using Epi-info version 7. The data were cleaned and edited by simple frequencies and cross-tabulations before analysis, and then, they were exported to STATA version 16 and checked for missing values. Descriptive statistics and numerical summary measures were presented using frequency distribution tables. During candidate selection, adequate significant variables were obtained at a P < 0.05, which was considered a cutoff point for multivariate logistic regression analysis using a backward stepwise approach to identify the independent predictors of stroke subtypes. The data were represented as odds ratios (ORs) and 95% confidence intervals.
Results
The study population comprised 554 patients who were admitted during the study period to the three public referral hospitals, among whom 334 cases (60.29%) had an ischemic stroke and 220 (39.71%) had a hemorrhagic stroke. All the patients were evaluated for stroke using a CT scan.
The mean age of the participants was 61 ± 12.85 years, ranging from 28 to 89 years, and 33% were in the age category of 60–69 years. More than half (53.25%) of the participants were women, and the remaining 46.75% were men. The majority of the participants (68.4%) were rural residents, and 49.64% of patients were housewives. Regarding the educational status of the patients, 67.69% have no formal education. The majority of the patients (84.66%) were married. Regarding behavioral characteristics, 95.49%, 34.3%, and 4.33% consume fruits and vegetables <2 times per week, were former drinkers, and were former smokers, respectively (Table 1).
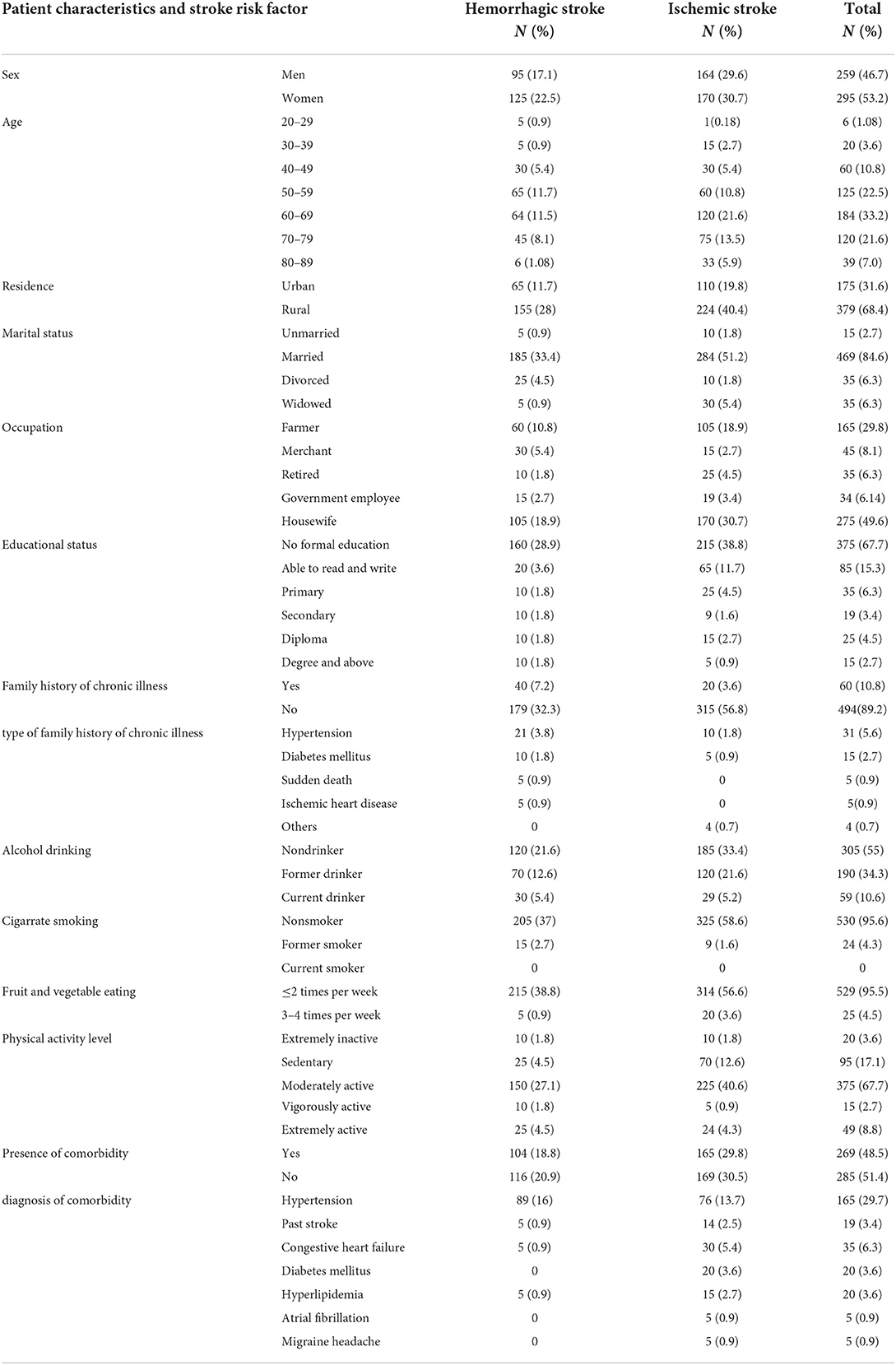
Table 1. Characteristics and risk profile of patients with stroke admitted to public referral hospitals, Northwest Ethiopia, 2020–2021.
Risk factors for stroke
Table 1 shows the frequency of identified risk factors by stroke subtype. The presence of comorbidity was identified in 269 (48.5%) patients, with 104 patients (47.3%) with HS and 165 patients (49.4%) with IS. The most common comorbidity identified was hypertension, which was in 165 patients (29.7%), followed by CHF in 35 (6.3%), DM in 20 (3.6%), hyperlipidemia in 20 (3.6%), past stroke in 19 (3.4%), and AF in 5 (0.9%). Among patients with hypertension, 89 (40.45%) had a hemorrhagic stroke and 76 (22.6%) had an ischemic stroke.
About 60 (10.8%) patients had a family history of chronic illness, with hypertension being the major chronic illness in the family in 31 patients (5.6%). A total of 190 patients (34.3%) were identified as former drinkers, 59 as current drinkers (10.6%), and the remaining as non-drinkers. Regarding physical activity, a significant share of patients, 95 (17.1%), were sedentary, and 20 (3.6%) were extremely inactive. In total, 529 patients (95.5%) eat fruit and vegetables <2 times per week.
Clinical presentation of patients with stroke
The most common clinical presentation was hemiparesis as reported by 320 (57.7%) patients, followed by loss of consciousness by 115 patients (20.7%) and aphasia by 50 patients (9%). Most patients with hemorrhage presented with hemiparesis (47.7%), loss of consciousness (34.1%), vomiting (6.8%), and aphasia (6.8%). Similarly, the chief complaint among patients with ischemic stroke was hemiparesis (64.4%), followed by coma (12%), aphasia (10.5%), and slurred speech (6%). Overall, of 225 (40.6%) patients with stroke, 59.1% of patients with hemorrhagic stroke, and 28.4% of patients with ischemic stroke had complications during hospital presentation. Aspiration pneumonia (28.9%) was the most common medical complication, and brain edema (17.1%) was the most common neurologic complication (Table 2).
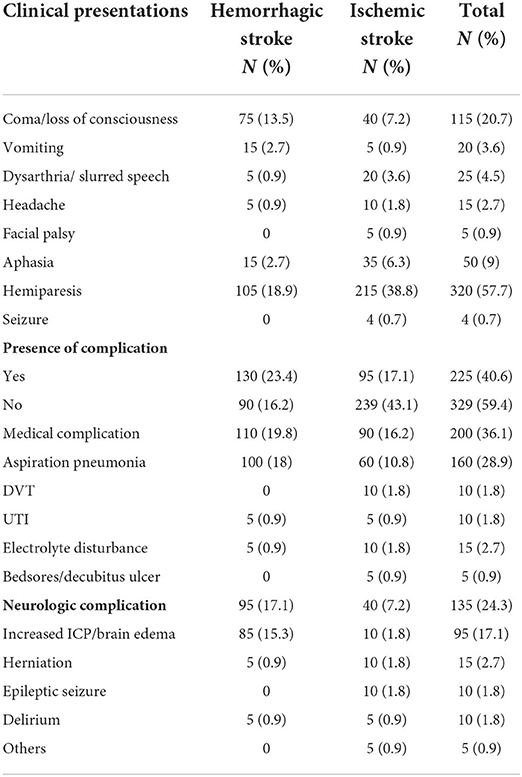
Table 2. Clinical presentation of patients with stroke admitted to public referral public hospitals, Northwest Ethiopia, 2020–2021.
Determinants of stroke subtypes
Through binary logistic regression, age, cigarette smoking, physical activity level, family history of chronic illness, type of comorbidity, complication during admission, and clinical presentation were selected as candidate variables to be included in multivariate logistic regression with a p < 0.05. Through multivariate logistic regression, age (AOR = 1.03, 95% CI = 1.01–1.05), the level of sedentary physical activity (AOR = 6.78, 95% CI = 1.97–23.32), absence of a family history of chronic illness (AOR = 3.79, 95% CI = 2.21–6.48), and past stroke (AOR = 3.54, 95% CI = 0.93–13.49) were found to be independent determinants of hemorrhagic stroke subtypes, while hypertension (AOR = 0.51, 95% CI = 0.31–0.85) was independently and strongly associated with an ischemic stroke compared with a hemorrhagic stroke.
With a 1-year increase in patients' age, the odds of having a hemorrhagic stroke compared with ischemic stroke will increase by 3%. Patients with sedentary activity levels were seven times more likely to experience a hemorrhagic stroke than an ischemic stroke. The odds of hemorrhagic stroke vs. ischemic stroke were 3.79 times higher in patients with the absence of a family history of chronic illness than in their counterparts. Furthermore, patients with a history of stroke were 3.54 times more likely to experience a hemorrhagic stroke than an ischemic stroke. Meanwhile, patients with hypertension were 0.51 times less likely to experience a hemorrhagic stroke than an ischemic stroke (Table 3).
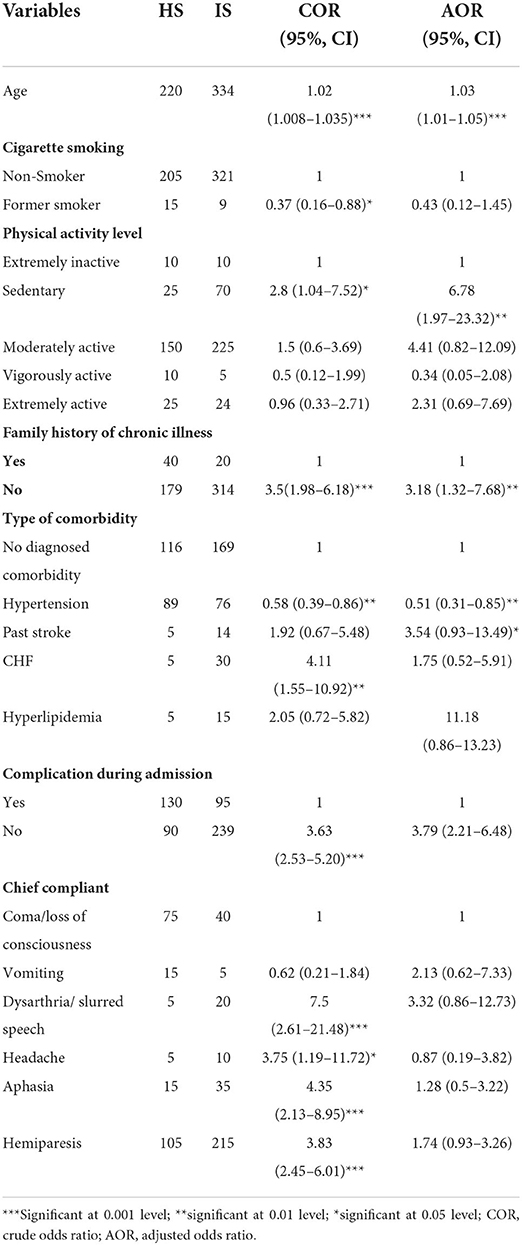
Table 3. Determinants of stroke subtypes among patients with stroke admitted to public referral hospitals, Northwest Ethiopia, 2020/2021.
Discussion
The study data were drawn from the large study on stroke conducted in public referral hospitals in Northwest Ethiopia. The findings of this study will lead to advances in and unique contributions to the previously published studies by exploring the risk factors, clinical presentations, and determinant subtypes of stroke.
The mean age of the participants was 61 ± 12.85 years, which was in line with studies conducted in other sub-Saharan African countries (19, 22, 35–37), but it was lower than that in studies conducted in developed countries (38, 39). This disagreement may be due to the Ethiopian population's lifestyle modification, abuse of alcohol, physical inactivity, cigarette smoking, and poor health-seeking behavior, which could result in the early onset of stroke.
In contrast to other previous studies, the percentage of stroke was higher in female patients than in male counterparts (9, 40). The possible reason for the higher percentage among female patients may be due to increased risk factors of stroke such as high use of contraception, pregnancy-related disorders, and migraine.
Almost half of the participants had comorbidity, which include both patients with an ischemic stroke and patients with a hemorrhagic stroke. Similar to other studies conducted in Ethiopia, Senegal, Nigeria, Iran, and China, hypertension was the most common comorbidity (9, 17, 22, 30, 41–43). This trend may reflect that there is still a limitation in screening for hypertension, underdiagnosis, poor blood pressure control in patients with hypertension, and a poor healthcare system.
Considering the habit of alcohol drinking and cigarette smoking, compared with previous studies conducted in Ethiopia, 34.3% former drinkers and 10.6% current drinkers, the habit was low in the current study, with 4.3% former smokers and no current smokers (9, 19). This could be due to underreporting of cigarette smoking, which is seen as being forbidden in the community in the catchment area of our study, although it has high abusers of alcohol. Alcoholism and the risk factor of stroke are associated with aggravating the effects that cause cardioembolism and hypertension.
At hospital presentation, the most common chief complaint was hemiparesis, which was reported by 57.7% of patients, followed by loss of consciousness (20.7%) and slurred speech (4.5%). A similar finding was reported in studies conducted in Ethiopia, India, and Pakistan (8, 35, 41, 44, 45). This finding was unlike other studies from Ethiopia, where a headache was the most common clinical presentation (9). The difference could be because this study was carried out among patients admitted to hospitals, which may contain more severe cases and late hospital presentations, thus leading to disease severity either at onset or in the later stages and complications.
Multivariate logistic regression showed that age, the level of sedentary physical activity, absence of a family history of chronic illness, and past stroke were identified as independent determinants of a hemorrhagic stroke compared with an ischemic stroke, while hypertension was associated with an ischemic stroke compared with a hemorrhagic stroke.
The results of our study revealed that an increase in age is associated with the odds of having a hemorrhagic stroke compared with an ischemic stroke, which is in contrast to studies from Central Africa, Nigeria, and China (7, 23, 46), but supported by a study from Australia (15). However, advanced age with heavy alcohol consumption could predispose individuals to hypertensive intraparenchymal hemorrhage.
Patients with sedentary activity levels were seven times more likely to experience a hemorrhagic stroke than an ischemic stroke (16, 47). The mechanistic basis of the effect of physical activity on stroke risk is likely to prevent obesity, hypertension, dyslipidemia, and the development of type 2 diabetes, all of which are implicated as risk factors of stroke.
The odds of hemorrhagic stroke vs. ischemic stroke were 3.79 times higher in patients with the absence of a family history of chronic illness than in their counterparts, which is supported by different studies (7, 48–50). This could be explained by the fact that a family history of DM, hypertension, and obesity are risk factors for ischemic stroke, largely by their link to atherosclerosis, but the effect of a family history of chronic illness may require further stratification and investigation.
Contradicting a study conducted in Singapore, out study showed that patients with a history of stroke were more likely to experience hemorrhagic stroke than an ischemic stroke (51), which could, in part, be explained by differences in the study population, stroke subtypes, stroke severity, and first-ever or recurrent strokes.
Furthermore, we observed hypertension was independently and strongly associated with ischemic stroke compared with hemorrhagic stroke, which is supported by other studies (7, 48, 49), but this finding contradicts the result of a study conducted in 22 countries (48). A possible explanation may be that, while hypertension predisposes patients to both strokes, atherosclerotic plaque formation (which favors IS) and higher blood pressures may be required for microaneurysmal rupture compared with plaque accident. Finally, in our study, there was no specific clinical presentation significantly associated with the stroke subtypes.
Strengths and limitations of the study
This study attempted to identify risk factors, clinical presentations, and predictors of stroke subtypes via a prospective follow-up of CT scan-confirmed cases in multiple health facilities to ascertain the reliability of the outcome of the study. This may reduce the false-positive and false-negative associations between different variables of the study. The limitation of the study is the study type being a hospital-based study rather than a longitudinal community-based study. Thus, attention should be given to the generalization of the findings to a large community. In addition, we relied on patient reports for some risk factors and other patient-related histories, which may introduce a recall bias.
Conclusion
The majority of the patients were middle-aged, women, rural residents, and participants with no formal education. The early-onset stroke poses a significant challenge for the healthcare system and the economy of low-income countries. Hypertension was the most common risk factor identified in the current study. The most common clinical presentation was hemiparesis, followed by coma and slurred speech. In addition, the majority of patients with stroke presented with complications during admission.
Age, the level of sedentary physical activity, absence of a family history of chronic illness, hypertension, and past stroke were independent determinants of stroke subtypes. This study has an important clinical implication that uncontrolled hypertension or undiagnosed hypertension are still the targets of wrestling in Ethiopia. Although this strategy in addition to lifestyle modification, screening first-degree relatives for chronic disease and rehabilitation of patients with stroke can be used to reduce the burden of the disease.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by College of Health Science Ethical Review Committee, Debre Tabor University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
GA, GY, EZ, ZE, ATA, BA, DA, AA, and MA contributed to the design of the study, analysis, interpretation, and wrote the manuscript. GA, GY, and ZE made the data analysis and interpretation of the data. YA, EZ, DA, AA, FA, and MA contributed to the design of the study, drafting, and edition of the manuscript. All authors critically revised the manuscript and have approved the final manuscript.
Acknowledgments
We would like to thank the participants of the study. We also thank and appreciate data collectors and staff of the University of Gondar Teaching Hospital, Tibebe Ghion Comprehensive Specialized Hospital, and Felege Hiwot Regional Referral Hospital.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AF, atrial fibrillation; CHF, congestive heart failure; CVD, cardiovascular disease; DM, diabetes mellitus; DVT, deep venous thrombosis; FBS, fasting blood sugar; GBD, global burden of disease; HDL, high-density lipoprotein; HS, hemorrhagic stroke; ICP, intra-cranial pressure; IS, ischemic stroke; LDL, low-density lipoprotein; LMICs, low- and middle-income countries; PAL, physical activity level; RBS, random blood sugar; SSA, sub-Saharan Africa; UTI, urinary tract infection.
References
1. Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res. (2017) 120:439–48. doi: 10.1161/CIRCRESAHA.116.308413
2. Owolabi MO, Akarolo-anthony S, Akinyemi R, Arnett D, Gebregziabher M, Jenkins C, et al. The burden of stroke in Africa : a glance at the present and a glimpse into the future. Cardiovasc J Afr. (2015) 26:s27–38. doi: 10.5830/CVJA-2015-038
3. Boutayeb A, Boutayeb S. The burden of non communicable diseases in developing countries. Int J Equity Health. (2005) 4:2. doi: 10.1186/1475-9276-4-2
4. Adeloye D. An estimate of the incidence and prevalence of stroke in Africa : a systematic review and meta-analysis. PLoS ONE. (2014) 9:e0100724. doi: 10.1371/journal.pone.0100724
5. Healey JS, Oldgren J, Ezekowitz M, Zhu J, Pais P, Wang J, et al. Occurrence of death and stroke in patients in 47 countries 1 year after presenting with atrial fibrillation: a cohort study. Lancet. (2016) 388:1161–9. doi: 10.1016/S0140-6736(16)30968-0
6. Feigin V, Krishnamurthi R, Parmar P, Norrving B, Mensah G, Bennett D, et al. Update on the global burden of ischaemic and haemorrhagic stroke in 1990–2013: the GBD 2013 study. Neuroepidemiology. (2016) 45:161–76. doi: 10.1159/000441085
7. Owolabi MO, Agunloye AM. Which risk factors are more associated with ischemic rather than hemorrhagic stroke in black Africans? Clin Neurol Neurosurg. (2013) 115:2069–74. doi: 10.1016/j.clineuro.2013.07.015
8. Patne S, Chintale K. Study of clinical profile of stroke patients in rural tertiary health care centre. Int J Adv Med. (2016) 3:666–70. doi: 10.18203/2349-3933.ijam20162514
9. Fekadu G, Chelkeba L, Kebede A. Risk factors, clinical presentations and predictors of stroke among adult patients admitted to stroke unit of Jimma university medical center, south west Ethiopia: prospective observational study. BMC Neurol. (2019) 19:183. doi: 10.1186/s12883-019-1409-0
10. Li RC, Xu WD, Lei YL, Bao T, Yang HW, Huang WX, et al. The risk of stroke and associated risk factors in a health examination population: a cross-sectional study. Medicine. (2019) 98:e17218. doi: 10.1097/MD.0000000000017218
11. Fekadu G, Wakassa H, Tekle F. Stroke event factors among adult patients admitted to stroke unit of Jimma university medical center: prospective observational study. Stroke Res Treat. (2019) 2019:4650104. doi: 10.1155/2019/4650104
12. Jensen MB, Yoo B, Clarke WR, Davis PH, Adams HP. Blood pressure as an independent prognostic factor in acute ischemic stroke. Can J Neurol Sci. (2006) 33:34–8. doi: 10.1017/S0317167100004662
13. Bikila Gedefa, Takele Menna TB HA. Assessment of risk factors and treatment outcome of stroke admissions at St. Paul's teaching hospital, Addis Ababa, Ethiopia. J Neurol Neurophysiol. (2017) 8:6–11. doi: 10.4172/2155-9562.1000431
14. Campbell NRC, Ashley MJ, Carruthers SG, Lacourcière Y, McKay DW. Recommendations on alcohol consumption. CMAJ. (1999) 160 (9 Suppl.):13–20.
15. Hankey GJ, Anderson NE, Ting RD, Veillard AS, Romo M, Wosik M, et al. Rates and predictors of risk of stroke and its subtypes in diabetes: a prospective observational study. J Neurol Neurosurg Psychiatry. (2013) 84:281–7. doi: 10.1136/jnnp-2012-303365
16. Wendel-Vos GCW, Schuit AJ, Feskens EJM, Boshuizen HC, Verschuren WMM, Saris WHM, et al. Physical activity and stroke. A meta-analysis of observational data. Int J Epidemiol. (2004) 33:787–98. doi: 10.1093/ije/dyh168
17. Sagui E, M'Baye PS, Dubecq C, Fall KB, Niang A, Gning S, et al. Ischemic and hemorrhagic strokes in Dakar, Senegal: a hospital-based study. Stroke. (2005) 36:1844–7. doi: 10.1161/01.STR.0000177864.08516.47
18. Referral H, Dar B, Mulat B, Mohammed J, Yeseni M, Alamirew M, et al. Magnitude of stroke and associated factors among patients who attended the medical ward of Felege Hiwot Referral Hospital, Bahir Dar town, Northwest Ethiopia. Ethiop J Heal Dev. (2016) 30:103–76. Available online at: https://ejhd.org/index.php/ejhd/article/view/821
19. Mulugeta H, Yehuala A, Haile D, Mekonnen N, Dessie G, Kassa GM, et al. Magnitude, risk factors and outcomes of stroke at Debre Markos Referral Hospital, Northwest Ethiopia: a retrospective observational study. Egypt J Neurol Psychiatry Neurosurg. (2020) 56:41. doi: 10.1186/s41983-020-00173-4
20. Sultan M, Debebe F, Azazh A, Hassen GW. Epidemiology of stroke patients in Tikur Anbessa specialized hospital : emphasizing clinical characteristics of hemorrhagic stroke patients. Ethiopia J Health Dev. (2017) 31:13–7. Available online at: https://www.ejhd.org/index.php/ejhd/article/view/966
21. Fekadu G, Chelkeba L, Kebede A. Burden, clinical outcomes and predictors of time to in hospital mortality among adult patients admitted to stroke unit of Jimma university medical center: a prospective cohort study. BMC Neurol. (2019) 19:213. doi: 10.1186/s12883-019-1439-7
22. Gebremariam SA, Yang HS. Types, risk profiles, and outcomes of stroke patients in a tertiary teaching hospital in northern Ethiopia. eNeurologicalSci. (2016) 3:41–7. doi: 10.1016/j.ensci.2016.02.010
23. Tshikwela ML, Londa FB, Tongo SY. Stroke subtypes and factors associated with ischemic stroke in Kinshasa, Central Africa. Afr Health Sci. (2015) 15:68–73. doi: 10.4314/ahs.v15i1.9
24. Setyopranoto I, Bayuangga HF, Panggabean AS, Alifaningdyah S, Lazuardi L, Dewi FST, et al. Prevalence of stroke and associated risk factors in sleman district of Yogyakarta Special Region, Indonesia. Stroke Res Treat. (2019) 2019:2642458. doi: 10.1155/2019/2642458
25. American Diabetes Association. Diagnosis and classification of diabetes mellitus. In: Diabetes Care. Arlington, VA: American Diabetes Association (2008). doi: 10.2337/dc10-S062
26. Zhang P, Jin H, Guo ZN, Sun HJ, Zhang FL, Sun X, et al. The accumulation of key stroke risk factors and its association with the characteristics of subjects: a population based cross sectional study. Front Neurol. (2018) 9:949. doi: 10.3389/fneur.2018.00949
27. Manorenj S, Inturi S, Jyotsna B, Savya V, Areli D, Reddy B. Prevalence, pattern, risk factors and outcome of stroke in women: a clinical study of 100 cases from a tertiary care center in South India. Int J Res Med Sci. (2016) 4:2388–93. doi: 10.18203/2320-6012.ijrms20161819
28. Nkoke C, Lekoubou A, Balti E, Pascal A. Stroke mortality and its determinants in a resource-limited setting : a prospective cohort study in Yaounde, Cameroon. J Neurol Sci. (2015) 358:113–7. doi: 10.1016/j.jns.2015.08.033
29. Tiruneh SA, Bukayaw YA, Yigizaw ST, Angaw DA. Prevalence of hypertension and its determinants in Ethiopia: a systematic review and meta-analysis. PLoS ONE. (2020) 15:e0244642. doi: 10.1371/journal.pone.0244642
30. Zhang FL, Guo ZN, Wu YH, Liu HY, Luo Y, Sun MS, et al. Prevalence of stroke and associated risk factors: a population based cross sectional study from northeast China. BMJ Open. (2017) 7:e015758. doi: 10.1136/bmjopen-2016-015758
31. Owolabi MO, Sarfo F, Akinyemi R, Gebregziabher M, Akpa O, Akpalu A, et al. Dominant modifiable risk factors for stroke in Ghana and Nigeria (SIREN): a case-control study. Lancet Glob Health. (2018) 6:e436–46. doi: 10.1016/S2214-109X(18)30002-0
32. Strath SJ, Kaminsky LA, Ainsworth BE, Ekelund U, Freedson PS, Gary RA, et al. Guide to the assessment of physical activity: clinical and research applications: a scientific statement from the American heart association. Circulation. (2013) 128:2259–79. doi: 10.1161/01.cir.0000435708.67487.da
33. González K, Fuentes J, Márquez JL. Physical inactivity, sedentary behavior and chronic diseases. Korean J Fam Med. (2017) 38:111–5. doi: 10.4082/kjfm.2017.38.3.111
35. Vaidya C, Majmudar D. A retrospective study of clinical profile of stroke patients from GMERS medical college and hospital, Gandhinagar, Gujarat. Int J Clin Trials. (2014) 1:62–6. doi: 10.5455/2349-3259.ijct20140805
36. Okeng'o K, Chillo P, Gray WK, Walker RW, Matuja W. Early mortality and associated factors among patients with stroke admitted to a large teaching hospital in Tanzania. J Stroke Cerebrovasc Dis. (2017) 26:871–8. doi: 10.1016/j.jstrokecerebrovasdis.2016.10.037
37. Sanya E, Wahab K, Bello A, Alaofin W, Ademiluyi B. In-hospital stroke mortality and its predictors within one month of ictus: result from a tertiary hospital in Ilorin, middle belt Nigeria. Sub Saharan Afr J Med. (2015) 2:165–9. doi: 10.4103/2384-5147.172439
38. Cabral NL, Muller M, Franco SC, Longo A, Moro C, Nagel V, et al. Three-year survival and recurrence after first-ever stroke: the Joinville stroke registry. BMC Neurol. (2015) 15:70. doi: 10.1186/s12883-015-0317-1
39. Heuschmann PU, Kolominsky-Rabas PL, Misselwitzet B. Predictors of in-hospital mortality and attributable risks of death after ischemic stroke: the German stroke registers study group. ACC Curr J Rev. (2004) 13:19–20. doi: 10.1016/j.accreview.2004.11.080
40. Tirschwell DL, Ton TGN, Ly KA, Van Ngo Q, Vo TT, Pham CH, et al. A prospective cohort study of stroke characteristics, care, and mortality in a hospital stroke registry in Vietnam. BMC Neurol. (2012) 12:150. doi: 10.1186/1471-2377-12-150
41. Greffie ES, Mitiku T, Getahun S. Risk factors, clinical pattern and outcome of stroke in a referral hospital, risk factors, clinical pattern and outcome of stroke in a referral hospital, Northwest Ethiopia. Clin Med Res. (2015) 4:182–8. doi: 10.11648/j.cmr.20150406.13
42. Obiako OR, Oparah SK, Ogunniyi A. Prognosis and outcome of acute stroke in the university college hospital Ibadan, Nigeria. Niger J Clin Pract. (2011) 14:359–62. doi: 10.4103/1119-3077.86784
43. Sariaslani P, Sultanabadi R, Hosseini F, Mohammadi H. The incidence of ischemic stroke and its associated factors in young adults in Kermanshah over a seven-year period. J Kermanshah Univ Med Sci. (2019) 23:e90139. doi: 10.5812/jkums.90139
44. Alemayehu CM. Assessment of stoke patients: occurrence of unusually high number of haemorrhagic stroke cases in tikur anbessa specialized hospital, Addis Ababa, Ethiopia. Clin Med Res. (2013) 2:94. doi: 10.11648/j.cmr.20130205.11
45. Masood CT, Hussain M, Anis-ur-Rehman, Abbasi S. Clinical presentation, risk factors and outcome of stroke at a district level teaching hospital. J Ayub Med Coll Abbottabad. (2013) 25:49–51.
46. Gan Y, Wu J, Zhang S, Li L, Yin X, Gong Y, et al. Prevalence and risk factors associated with stroke in middle-aged and older Chinese: a community-based cross-sectional study. Sci Rep. (2017) 7:9501. doi: 10.1038/s41598-017-09849-z
47. Gallanagh S, Quinn TJ, Alexander J, Walters MR. Physical activity in the prevention and treatment of stroke. ISRN Neurol. (2011) 2011:953818. doi: 10.5402/2011/953818
48. O'Donnell MJ, Denis X, Liu L, Zhang H, Chin SL, Rao-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. (2010) 376:112–23. doi: 10.1016/S0140-6736(10)60834-3
49. Namale G, Kamacooko O, Kinengyere A, Yperzeele L, Cras P, Ddumba E, et al. Risk factors for hemorrhagic and ischemic stroke in Sub-Saharan Africa. J Trop Med. (2018) 2018:4650851. doi: 10.1155/2018/4650851
50. Fahimfar N, Khalili D, Mohebi R, Azizi F, Hadaegh F. Risk factors for ischemic stroke; results from 9 years of follow-up in a population based cohort of Iran. BMC Neurol. (2012) 12:117. doi: 10.1186/1471-2377-12-117
Keywords: risk profile, clinical presentation, determinants, stroke subtype, Ethiopia
Citation: Ayehu GW, Yitbarek GY, Zewdie EA, Amsalu BT, Abie Y, Atlaw D, Agegnehu A, Admasu FT, Azanaw MM, Amare AT and Emiru ZA (2022) Risk profile, clinical presentation, and determinants of stroke subtypes among patients with stroke admitted to public referral hospitals, Northwest Ethiopia in 2021: A cross-sectional study. Front. Neurol. 13:988677. doi: 10.3389/fneur.2022.988677
Received: 07 July 2022; Accepted: 22 August 2022;
Published: 25 October 2022.
Edited by:
Tapas Banerjee, National Neurosciences Centre, IndiaCopyright © 2022 Ayehu, Yitbarek, Zewdie, Amsalu, Abie, Atlaw, Agegnehu, Admasu, Azanaw, Amare and Emiru. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gashaw Walle Ayehu, Z2FzaGF3d2FsbGUwMUBnbWFpbC5jb20=
 Gashaw Walle Ayehu
Gashaw Walle Ayehu Getachew Yideg Yitbarek
Getachew Yideg Yitbarek Edgiet Abebe Zewdie
Edgiet Abebe Zewdie Bedemariam Tadesse Amsalu2
Bedemariam Tadesse Amsalu2 Daniel Atlaw
Daniel Atlaw Assefa Agegnehu
Assefa Agegnehu Fitalew Tadele Admasu
Fitalew Tadele Admasu Melkalem Mamuye Azanaw
Melkalem Mamuye Azanaw Abraham Tsedalu Amare
Abraham Tsedalu Amare