- 1Concussion Research Lab, University of British Columbia, Kelowna, BC, Canada
- 2Faculty of Kinesiology, University of Calgary, Calgary, AB, Canada
- 3Sport Injury Prevention Research Centre, University of Calgary, Calgary, AB, Canada
- 4Human Performance Laboratory, University of Calgary, Calgary, AB, Canada
- 5Faculty of Medicine, Hotchkiss Brain Institute, University of Calgary, Calgary, AB, Canada
- 6Integrated Concussion Research Program, University of Calgary, Calgary, AB, Canada
- 7Alberta Children's Hospital Research Institute, University of Calgary, Calgary, AB, Canada
- 8Libin Cardiovascular Institute, University of Calgary, Calgary, AB, Canada
- 9Southern Medical Program, University of British Columbia, Kelowna, BC, Canada
- 10Division of Neurology, Department of Pediatrics, BC Children's Hospital, Vancouver, BC, Canada
- 11University of British Columbia, Vancouver, BC, Canada
- 12Experimental Medicine, University of British Columbia, Vancouver, BC, Canada
- 13School of Physiotherapy, Faculty of Health, Dalhousie University, Halifax, NS, Canada
Objectives: There is elevated unease regarding how repetitive head impacts, such as those associated with soccer heading, contribute to alterations in brain function. This study examined the extent heart rate variability (HRV) and cardiac baroreceptor sensitivity (BRS) metrics are altered immediately following an acute bout of soccer heading.
Methods: Seven male elite soccer players (24.1 ± 1.5 years) completed 40 successful soccer headers in 20-min. The headers were performed under controlled circumstances using a soccer ball launcher located 25 meters away and using an initial ball velocity of 77.5 ± 3.7 km/h (heading condition). An accelerometer (xPatch) on the right mastoid process quantified linear/rotational head accelerations. Participants also completed sham (body contact) and control (non-contact) sessions. A three-lead ECG and finger photoplethysmography characterized short-term spontaneous HRV/cardiac BRS, before and after each condition. The SCAT3 indexed symptom scores pre-post exposures to all three conditions.
Results: During the heading condition, cumulative linear and rotational accelerations experienced were 1,574 ± 97.9 g and 313,761 ± 23,966 rad/s2, respectively. Heart rate trended toward an increase from pre- to post-heading (p = 0.063), however HRV metrics in the time-domain (ps > 0.260) and frequency-domain (ps > 0.327) as well as cardiac BRS (ps > 0.144) were not significantly changed following all three conditions. Following the heading condition, SCAT3 symptom severity increased (p = 0.030) with a trend for symptom score augmentation (p = 0.078) compared to control and sham.
Conclusion: Whereas, symptoms as measured by the SCAT3 were induced following an acute bout of controlled soccer heading, these preliminary findings indicate they were not accompanied by alterations to autonomic function. Ultimately, this demonstrates further research is needed to understand the physiological underpinnings of alterations in brain function occurring immediately after a bout of soccer heading and how these may, over time, contribute to long-term neurological impairments.
Introduction
Around the globe, more than 265 million individuals partake in soccer making it the world's most prominent sport (1) and one that also results in substantial risk for head injuries to occur (2–4). This is very likely at least partially due to the unique aspect of this sport in which players use their heads to contact the ball and direct it during play. Previous research has demonstrated soccer heading occurs ~4 to 10 times per game with variations dependent upon age, sex, and player position [e.g., (5)]. Beyond the immediate effects of soccer heading on brain function, there is emerging concern that the number of repetitive sub-concussive impacts a soccer player has over their career may be associated with the development of long-term neurodegenerative disorders (6–9). Nevertheless, there is currently a paucity of data providing insight into the magnitude and total exposure necessary to cause alterations in brain function in the immediate aftermath of a bout of soccer heading.
The autonomic nervous system functions to maintain homeostasis through the unconscious regulation of various bodily functions (e.g., heart rate, respiration, digestion, etc.) (10). Previous research has demonstrated autonomic function can be aberrant in certain pathophysiological states [e.g., Parkinson disease (11), multiple sclerosis (12), traumatic brain injury (13–15), etc.], through heart rate variability (HRV) and baroreceptor sensitivity (BRS) domains. HRV is a technique designed to examine the activity of the autonomic nervous system through the variations in consecutive R-R intervals (16). Conversely, BRS maintains cardiovascular homeostasis through a negative feedback loop by balancing input from the sympathetic and parasympathetic nervous systems during acute alterations in blood pressure (17).
Autonomic function has been found to be dysregulated following concussion (13–15, 18), though the exact physiological underpinning of this phenomenon remains unknown. Furthermore, there is a relative void within the current literature examining the effects repetitive heading impacts has on autonomic function. To our knowledge there has only been one study which investigated an acute bout of five soccer head impacts and found minimal alterations in HRV parameters (19). As such, there is a substantial lack of understanding regarding the physiological changes that occur to the autonomic nervous system from both concussive and sub-concussive head impacts. Therefore, this investigation sought to examine the acute effects a controlled bout of soccer heading using a soccer ball launcher has on the autonomic nervous system relative to sham (body contact) and control (non-contact) sessions. It was hypothesized repeated sub-concussive impacts in the form of soccer heading would lead to an elevated sympathetic response in HRV and cardiac BRS metrics that would not be present during sham (non-head impacts) and control (no impacts) conditions.
Materials and methods
Participants
Seven male soccer players (age: 24.1 ± 1.5 years; BMI: 25.5 ± 1.6 kg/m2) participated in this investigation, which used a crossover design that has also examined cerebral autoregulation (20), neurovascular coupling (21), and blood-based biomarkers (22) within the same three randomized conditions (heading, sham, and control). All individuals had a minimum of 5 years of soccer playing experience and refrained from caffeine, alcoholic beverages, smoking, and exercise for 12 h prior to testing. Participants were healthy with no history of cardiac, respiratory, neurological, vascular, or severe neurodevelopmental disorders. Testing procedures were explained prior to data collection to ensure all participants were familiar with them. Written informed consent was obtained and ethical approval was through the University of British Columbia clinical research ethics board (H14-00368). Participants were compensated $50 CAD for each testing session, for a total compensation of $150 CAD across the duration of the study.
Study design
On each testing day, spontaneous short-term HRV and cardiac BRS was quantified using a pre-test, exposure, post-test design through electrocardiography and finger plethysmography (23, 24). These variables were measured while each individual quietly stood for 5 min, before and roughly 15–20 min after each condition. Participants completed these conditions with an average of 26.1 ± 25.2 days between testing conditions in a pseudo-random order, in brief the conditions consisted of:
i) Heading—participants performed 40 headers in 20 min with ~30 s between each trial. They stood ~25 m from a JUGS machine (JUGS International, Taulatin, Oregon, USA). In the case of an unsuccessful trial, a second soccer ball was launched within the 30 s time frame. The soccer balls used were FIFA regulation size 5 ball inflated to 13 psi and propelled from the JUGS machine at an average of 77.5 ± 3.7 km/h, which was recorded with a Bushnell Velocity Speed Gun (Bushnell Outdoor Products, Richmond Hill, Ontario, Canada).
ii) Sham—Participants performed 40 ball contacts in 20 min with any part of the body other than the head. Other than this requirement, all the other details of this condition were the same as the Heading condition. This intervention was performed to determine if alterations to the autonomic nervous system require head contract or if they could arise from body contact or “whiplash-like” effects (25).
iii) Control—No soccer balls were launched in this condition, as participants were taken to the testing area, and completed 20 min of quiet rest, before returning to the laboratory for post-condition data collections.
For a greater description of the heading, sham, and control protocols the reader is directed to (21).
Lab-based instrumentation
A three-lead electrocardiogram (ECG) and finger photoplethysmography, with a brachial cuff to adjust finger and brachial artery height differences (Finometer; Finapres Medical Systems, Amsterdam, The Netherlands), was attached to each individual before and following the three aforementioned conditions (26, 27). All data were sampled at 1,000 Hz (PowerLab 8/30 ML880; AD Instruments) and stored for offline analysis using commercially available software (LabChart version 7.1; AD Instruments). All measurements were collected at the same time of day to control for diurnal variation (28, 29).
Consistent with other research in the soccer heading field, linear and angular acceleration were measured using the xPatch (X2 Biosystems; Seattle, WA) system placed over the right mastoid process of participants during both the header and sham conditions (20–22, 25, 30–32). These sensors monitor three axes of translational acceleration as well as three axes of angular velocity with a 1,000 Hz sampling frequency. Head impact accelerations exceeding the threshold of 10 g were automatically recorded by the device and the associated peak linear/rotation accelerations (PLA, PRA) as well as the average impact duration were quantified. In the event, the impact from heading the soccer ball (or contact to the body in the sham condition) did not meet or exceed the standard 10 g threshold of the device, the impact was then coded as 0 g for interpretations of average/cumulative impact exposure levels. For this paper, we analyzed average and total linear and rotational acceleration in each condition. Others have shown soccer heading effects based on number of head impacts (33, 34) or time between impacts (35), however, because these variables were held relatively constant in the current study, we felt that average and cumulative impact magnitude variables would capture head impact exposure most appropriately.
The total number of symptoms and symptom severity scores were recorded with the third edition of the Sport Concussion Assessment Tool (SCAT3) before and after the three interventions (36). This tool contains a Likert scale ranging from 0 (no symptom) to 6 (severe) with 22 symptoms regarding somatic, cognitive, and neurobehavioral functions. The total number of symptoms (range: 0–22) and symptom severity were calculated by totaling the severity for each symptom (range: 0–132). Participants had a follow up call in the evening following soccer heading to assess symptom persistence. No individuals reported any lingering symptomology at this time point.
Data processing
Following at least 1 min of standing, short-term HRV/cardiac BRS measures were collected through 5 min of quiet standing in accordance with established guidelines (37, 38) using commercially available software (Version 1.0, Ensemble, Elucimed, Wellington, NZ). The outcome variables for HRV included the root mean square of successive normal sinus QRS complexes interval (R-R interval) differences (RMSSD), number of successive R-R intervals that differ by more than 50 ms (NN50), the percentage of R-R intervals that differ by more than 50 ms (pNN50), and low frequency (LF) and high frequency (HF) power (23, 37–40). The cardiac BRS was quantified through the LF gain (0.07–0.20 Hz) metric (23, 24, 41).
Statistical analysis
Statistical analyses were conducted with SPSS v.25.0 (IBM Crop, Armonk, NY). A three (condition: heading, sham, control) by two (time: pre, post) repeated measures analysis of variance was performed. Bonferroni post-hoc analyses were conducted to determine significant condition effects, with a priori Bonferroni corrected simple effects comparisons for condition or time. Data are presented as mean ± standard deviation. Significance was set a priori at p < 0.05.
Results
xPatch impact sensor
Greater than 98% of the headers were recorded by the xPatch Sensor as above the threshold of 10 g, with an average of 40.7 ± 3.6 g and 8097.8 ± 807.3 rad/s2 of linear and rotational acceleration, respectively. This translates to 1574.7 ± 97.9 g of total linear acceleration and 313760.6 ± 23966.4 rad/s2 of total rotational acceleration during the soccer heading intervention. Conversely, during the sham condition, <1% of the body contacts registered an impact >10 g, with an average linear acceleration of 3.2 ± 5.7 g and rotational acceleration of 827.9 ± 1424.5 rad/s2 (these values are underestimates as any impact below 10 g was registered as 0 g). Thus, the cumulative exposure during the sham condition was 1574.7 ± 97.9 g and 1284.8 ± 2453.2 rad/s2, for linear and rotational acceleration, respectively.
Sport concussion assessment test – 3rd edition (SCAT3)
At baseline prior to the soccer heading, total symptom score and severity were not different between the three conditions (all p > 0.80) (Figure 1). There was no significant change in the number or severity of symptoms following sham or control exposures (all p > 0.23, Figure 1). By contrast, following soccer heading, there was an increased symptom severity (p = 0.03) and a trend toward an elevated number of symptoms (p = 0.08) (Figure 1). Five of the seven participants reported a greater number of symptoms and symptom severity following soccer heading, which returned to baseline in all participants at the evening follow-up phone call. The most commonly reported symptoms following soccer heading were headache (71%, 1.3 ± 1.1); pressure in the head (57%, 0.7 ± 0.8); and don't feel right (57%, 0.7 ± 0.8).
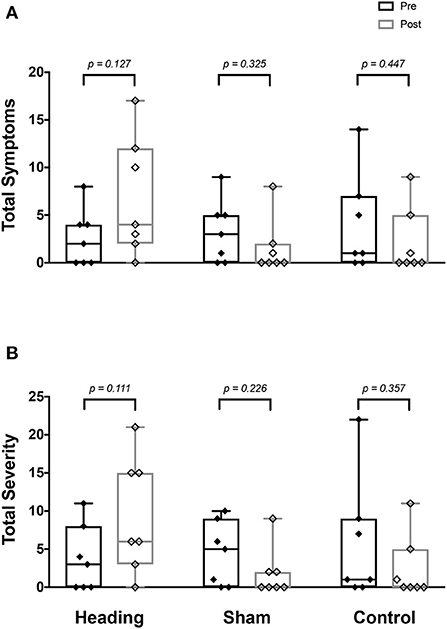
Figure 1. SCAT3 symptom metrics measured before and after exposure to heading, sham, and control conditions across all participants. Boxes span upper and lower quartile with median indicated; whiskers represent range. SCAT3 metrics describe (A) total number of symptoms reported, and (B) summed severity of all symptoms reported.
Heart rate variability and baroreceptor sensitivity metrics
A trend toward an increase in heart rate was noted during the soccer heading condition (p = 0.063) when comparing pre (74.8 ± 7.1 bpm) to post (82.3 ± 6.6 bpm) heart rate (Figure 2). Contrarily, there was no significant heart rate change following sham (p = 0.439) or control exposures (p = 0.666) (Figure 2). Moreover, following all three conditions, no significant change was present in time-domain (all p > 0.260) or frequency-domain (all p > 0.327: Figures 3, 4) metrics. Congruently, cardiac BRS LF gain was not significantly changed from pre- to post-exposure in all three interventions (all p > 0.144) (Figure 5). All of the aforementioned metrics had comparable pre-measures (all p > 0.427) between the three interventions (Figures 2–5). Detailed information on the resting physiologic parameters, SAC scores, and HRV and BRS metrics in each condition can be found in Tables 1, 2.
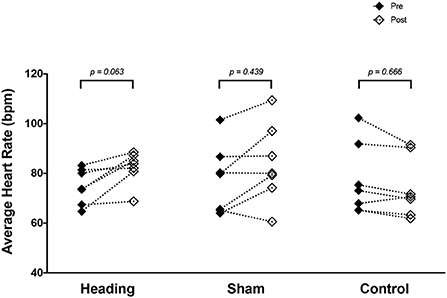
Figure 2. Heart rate measured before and after exposure to heading, sham, and control conditions across all participants. p-values are for a priori simple effects comparisons.
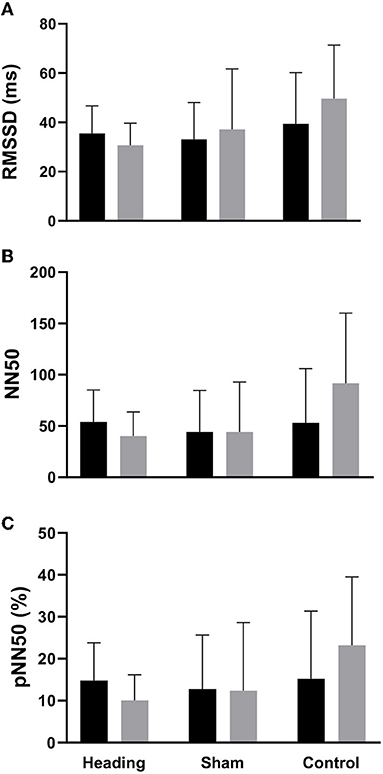
Figure 3. Time-domain HRV metrics before and after exposure to heading, sham, and control conditions across all participants. (A) Root-mean square differences of successive R-R intervals (RMSSD), (B) Number of adjacent R-R intervals that differ from each other by more than 50 ms (NN50), and (C) Percentage of successive R-R intervals that differ by more than 50 ms (pNN50). The columns are average values for the group with the standard deviation being represented with the error bars (p-values all >0.05).

Figure 4. Frequency-domain HRV metrics comparing before and after exposure to heading, sham, and control conditions across all participants. (A) relative lower frequency (LF) power. (B) relative high frequency (HF) power, and (C) LF/HF ratio. The columns are average values for the group with the standard deviation being represented with the error bars (p-values all >0.05).
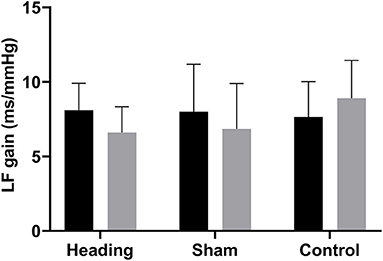
Figure 5. Cardiac baroreceptor sensitivity comparing before and after exposure to heading, sham, and control conditions across all participants, which is represented by the low frequency (LF) gain metrics. The columns are average values for the group with the standard deviation being represented with the error bars (p-values all >0.05).
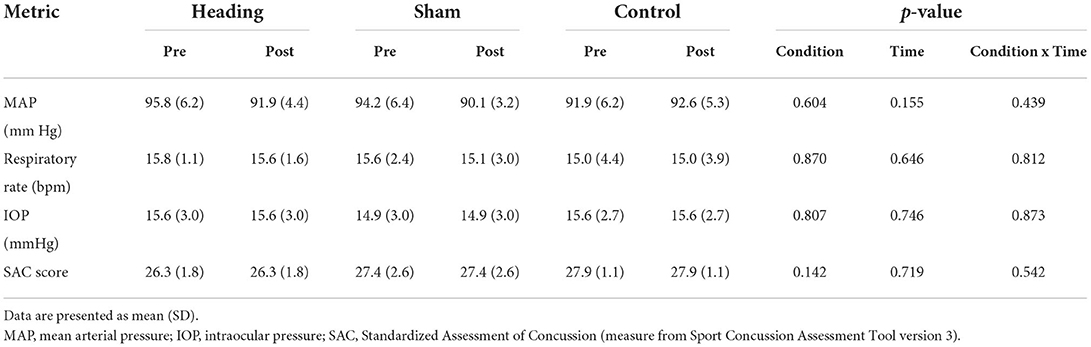
Table 1. Resting physiologic parameters and Standardized Assessment of Concussion recorded before and after heading, sham, and control exposures.
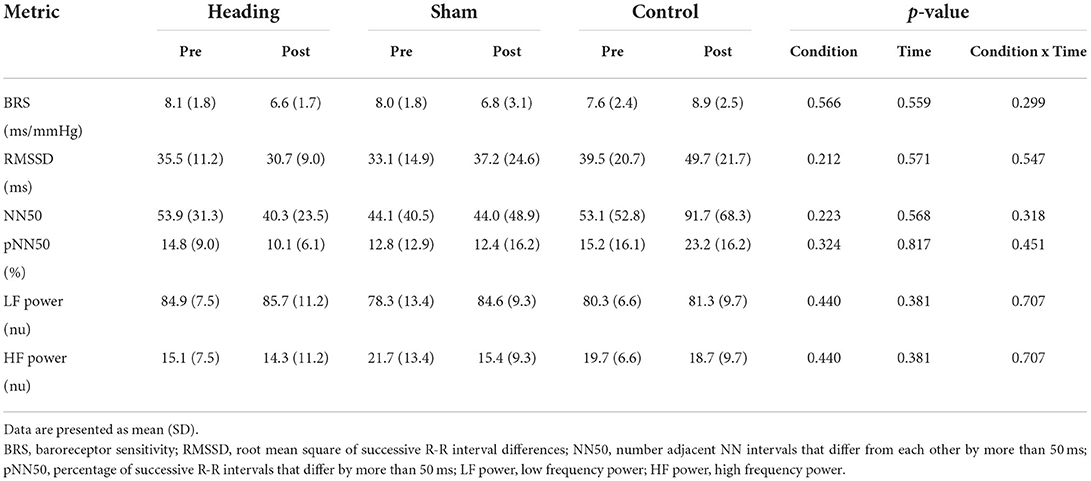
Table 2. Baroreceptor sensitivity and heartrate variability before and after heading, sham, and control exposures.
Discussion
This investigation examined how an acute bout of controlled soccer heading affects autonomic function. The main findings were an acute bout of soccer heading resulted in: (1) an elevation in symptom severity that resolved within 24 h; (2) a trend toward augmented absolute heart rate levels, but no significant change in time- and frequency- HRV domains; and (3) no alterations in cardiac BRS. Taken together, the current data suggest a controlled bout of soccer heading is insufficient to cause short-term alterations in autonomic nervous system function, although it did appear to result in a short-term worsening of symptoms as measured with the SCAT3.
Comparison to previous research
Several studies have examined the effect acute soccer heading has on various physiological parameters (42–53). This study varies substantially from much of the prior work in that it induced large cumulative linear and rotational impact exposure levels that were beyond those typically observed in match play. We have previously shown in a series of studies that the magnitude of head impact in the current investigation alters blood-based biomarkers associated with concussion (22), neurovascular coupling metrics (21), and cerebral autoregulation metrics (20). Nevertheless, despite these previous findings using the same protocol, there were minimal changes observed with respect to the autonomic nervous system function, aside from a trend toward increased heart rate (Figure 2). The relative lack of agreement between the current findings and those from our previous publications using the same protocol suggests that autonomic nervous system function is less sensitive to the effects of head impact exposure in an acute bout of soccer heading than either cerebrovascular function or blood biomarkers associated with neurological disruption.
The current findings with respect to the autonomic nervous system have some similarities to a study in 2019 by Harriss et al. (19), who noted small and moderate effect size (Cohen's d) differences in HRV metrics following acute soccer heading. However, the two studies vary in several key aspects. The current investigation had individuals successfully perform 40 headers with a 20-min span at a distance of ~25 m, whereas Harriss et al. (19), had players engage in just 5 headers, although these were performed in under a minute at a distance of just ~8 m. Moreover, they propelled the ball at 21.6 km/h toward the participants, whereas the current protocol used initial velocity of 77.5 ± 3.7 km/h, resulting in average linear and rotational head accelerations near the upper values previously reported (54). Finally, the previous investigation examined spontaneous HRV and cardiac BRS measures in a supine position; whereas in this study they were taken in an upright position, which is known to reduce variation and enhance reproducibility (55). Given this context, one might have expected larger effects in the current study than in that of Harriss et al. (19). Despite this, both studies found that acute soccer heading has minimal effects on autonomic function.
Furthermore, other reports have noted perturbations in HRV (18, 56, 57) and cardiac BRS (58) metrics following more substantive head impacts (e.g., concussions) among athletes. The exact physiological underpinnings that occur to the autonomic nervous system following concussion are largely unknown; however, this may in part be explained through the metabolic cascade known to occur following concussion (59, 60). This cascade involves diffuse axonal dysfunction and altered neurotransmission. After an initial period of hypermetabolism, there is altered mitochondrial function leading to reduced glucose utilization. Cardiac BRS and HRV are known to be influenced through output from the brain stem and the hypothalamus, respectively (10). Therefore, a cellular or neurometabolic disruption in networks associated with these regions could result in dysautonomia which may partially explain these deficits seen more often following concussion, as opposed to the controlled soccer-heading performed in the current investigation. Future research is needed to understand the total exposure required to cause immediate changes in different aspects of brain function.
Whether and how such alterations are related to the development of longer-term neurodegenerative disorders observed in former soccer players (6–9) is currently unknown. The results from this study suggest changes in autonomic function as probed by HRV are unlikely to be involved at least as they relate to sub-concussive soccer heading impacts. Clearly, other pathophysiological processes and/or exposure to concussive impacts must play a role in these longer-term clinical outcomes.
Limitations
A prominent limitation of the current study is the small sample size, which is likely linked to the non-significant findings. Nonetheless, a pseudo-random crossover design was used to reduce the likelihood any covariates (i.e., concussion history, genetics, soccer experience, etc.) (61, 62) influenced the findings, and also optimized statistical power while exposing fewer participants to potentially hazardous conditions. Therefore, we believe the results provide pertinent knowledge to the literature, given the cumulative impact of heading exposure and the comparable sample size to previous soccer heading studies. Additionally, the group recruited were exclusively male soccer players which is important as female soccer players have one of the highest rates of concussion among female athletes (63). However, despite methodological differences, these results are similar to a published study examining HRV in female adolescents (19). Furthermore, we also had no way of blinding participants to their exposure (heading, sham, or control), which could induce alterations to sympathetic and parasympathetic tone, thereby changing absolute heart rate and potentially HRV metrics. The SCAT3 has recently been shown to have only moderate reliability and specificity in diagnosing concussion and post-concussive symptoms (64) and, thus, its utility in the context of this study can be questioned. Moreover, as the SCAT3 relies on subjective self-report, participants may have been more likely to report symptoms knowing they had just been exposed to a controlled bout of repeated head impacts. Nonetheless, as the methodology in this study used objective outcomes [short-term HRV/cardiac BRS: (23, 24, 37–39)] and validated questionnaires (36) the risk of bias from this is minimal. Finally, skin-worn impact sensors can potentially overestimate head-impact exposure levels as a result of skin-based movement artifact during impacts (65–67). It is possible this may have occurred during the current investigation and the absolute values reported in this manuscript may have overestimated the exposure levels. Despite all these, the present findings are important given the lack of autonomic data presented within the current literature on soccer heading.
Conclusion
This investigation found exposure to 40 soccer head impacts elevates symptom severity and a trend toward an increase in absolute heart rate, although no alterations were found in HRV and cardiac BRS. These findings add to the evolving body of literature regarding the alterations in brain structure and function resulting from an acute bout of soccer heading. Future experiments are needed to comprehensively understand acute changes that occur to the autonomic nervous system and the associated structures across the lifetime of heading in a soccer career. Finally, investigations into the impact of soccer heading on HRV and cardiac BRS response changes with respect to age, sex, soccer skill level, and accumulative exposure are also warranted.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by University of British Columbia Clinical Research Ethics Board (H14-00368). The patients/participants provided their written informed consent to participate in this study.
Author contributions
JS, AW, and PvD designed the study. JS, JB, AW, KB, and JD collected data. All authors contributed to data interpretation and writing and/or editing the manuscript.
Funding
This research was funded by grants from CIHR (183304) and CFI (30979) awarded to PvD. AW was supported by the Vanier Canada Graduate Scholarships program, the O'Brien Foundation Fellowship, and the Vancouver Coastal Health-CIHR-UBC MD/PhD Studentship. JS was supported by the Innovations in Wellness post-doctoral fellowship.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Rodrigues AC, Lasmar RP, Caramelli P. Effects of soccer heading on brain structure and function. Front Neurol. (2016) 7:38. doi: 10.3389/fneur.2016.00038
2. Baldwin GT, Breiding MJ, Dawn Comstock R. Epidemiology of sports concussion in the United States. Handb Clin Neurol. (2018) 158:63–74. doi: 10.1016/B978-0-444-63954-7.00007-0
3. Coronado VG, Haileyesus T, Cheng TA, Bell JM, Haarbauer-Krupa J, Lionbarger MR, et al. Trends in sports- and recreation-related traumatic brain injuries treated in us emergency departments: the national electronic injury surveillance system-all injury program (neiss-aip) 2001-2012. J Head Trauma Rehabil. (2015) 30:185–97. doi: 10.1097/HTR.0000000000000156
4. Putukian M, D'Alonzo BA, Campbell-McGovern CS, Wiebe DJ. The ivy league-big ten epidemiology of concussion study: a report on methods and first findings. Am J Sports Med. (2019) 47:1236–47. doi: 10.1177/0363546519830100
5. Langdon SE, Goedhart E, Oosterlaan J, Konigs M. Heading exposure in elite football (soccer): A study in adolescent, young adult, and adult male and female players. Med Sci Sports Exerc. (2022) 54:1459–65. doi: 10.1249/MSS.0000000000002945
6. Grinberg LT, Anghinah R, Nascimento CF, Amaro E, Leite RP., Martin MdGM, et al. Chronic traumatic encephalopathy presenting as Alzheimer's disease in a retired soccer player. J Alzheimers Dis. (2016) 54:169–74. doi: 10.3233/JAD-160312
7. Hales C, Neill S, Gearing M, Cooper D, Glass J, Lah J. Late-stage CTE pathology in a retired soccer player with dementia. Neurology. (2014) 83:2307–9. doi: 10.1212/WNL.0000000000001081
8. Chio A, Benzi G, Dossena M, Mutani R, Mora G. Severely increased risk of amyotrophic lateral sclerosis among Italian professional football players. Brain J Neurol. (2005) 128(Pt 3):472–6. doi: 10.1093/brain/awh373
9. Ling H, Morris HR, Neal JW, Lees AJ, Hardy J, Holton JL, et al. Mixed pathologies including chronic traumatic encephalopathy account for dementia in retired association football (soccer) players. Acta Neuropathol. (2017) 133:337–52. doi: 10.1007/s00401-017-1680-3
10. Karemaker JM. An introduction into autonomic nervous function. Physiol Meas. (2017) 38:R89–r118. doi: 10.1088/1361-6579/aa6782
11. Yoon JH, Kim MS, Lee SM, Kim HJ, Hong JM. Heart rate variability to differentiate essential tremor from early-stage tremor-dominant Parkinson's disease. J Neurol Sci. (2016) 368:55–8. doi: 10.1016/j.jns.2016.06.059
12. Damla O, Altug C, Pinar KK, Alper K, Dilek IG, Kadriye A. Heart rate variability analysis in patients with multiple sclerosis. Mult Scler Relat Disord. (2018) 24:64–8. doi: 10.1016/j.msard.2018.06.012
13. Bishop S, Dech R, Baker T, Butz M, Aravinthan K, Neary JP. Parasympathetic baroreflexes and heart rate variability during acute stage of sport concussion recovery. Brain Inj. (2017) 31:247–59. doi: 10.1080/02699052.2016.1226385
14. Blake TA, McKay CD, Meeuwisse WH, Emery CA. The impact of concussion on cardiac autonomic function: a systematic review. Brain Inj. (2016) 30:132–45. doi: 10.3109/02699052.2015.1093659
15. Bishop SA, Dech RT, Guzik P, Neary JP. Heart rate variability and implication for sport concussion. Clin Physiol Funct Imaging. (2018) 38:733–42. doi: 10.1111/cpf.12487
16. Rajendra Acharya U, Paul Joseph K, Kannathal N, Lim CM, Suri JS. Heart rate variability: a review. Med Biol Eng Comput. (2006) 44:1031–51. doi: 10.1007/s11517-006-0119-0
17. Di Rienzo M, Parati G, Radaelli A, Castiglioni P. Baroreflex contribution to blood pressure and heart rate oscillations: time scales, time-variant characteristics and nonlinearities. Philos Trans A Math Phys Eng Sci. (2009) 367:1301–18. doi: 10.1098/rsta.2008.0274
18. Johnson BD, O'Leary MC, McBryde M, Sackett JR, Schlader ZJ, Leddy JJ. Face cooling exposes cardiac parasympathetic and sympathetic dysfunction in recently concussed college athletes. Physiol Rep. (2018) 6:e13694. doi: 10.14814/phy2.13694
19. Harriss AB, Abbott K, Kimpinski K, Holmes JD, Johnson AM, Walton DM, et al. An evaluation of heart rate variability in female youth soccer players following soccer heading: a pilot study. Sports. (2019) 7:229. doi: 10.3390/sports7110229
20. Smirl JD, Peacock D, Burma JS, Wright AD, Bouliane KJ, Dierijck J, et al. An acute bout of controlled subconcussive impacts can alter dynamic cerebral autoregulation indices: a preliminary investigation. Eur J Appl Physiol. (2022) 122:1059–70. doi: 10.1007/s00421-022-04908-4
21. Smirl JD, Peacock D, Wright AD, Bouliane KJ, Dierijck J, Burma JS, et al. An acute bout of soccer heading subtly alters neurovascular coupling metrics. Front Neurol. (2020) 11:738. doi: 10.3389/fneur.2020.00738
22. Wallace C, Smirl JD, Zetterberg H, Blennow K, Bryk K, Burma J, et al. Heading in soccer increases serum neurofilament light protein and SCAT3 symptom metrics. BMJ Open Sport Exe Med. (2018) 4:e000433. doi: 10.1136/bmjsem-2018-000433
23. Burma JS, Copeland PV, Macaulay A, Khatra O, Smirl JD. Effects of high-intensity intervals and moderate-intensity exercise on baroreceptor sensitivity and heart rate variability during recovery. Appl Physiol Nutr Metab. (2020) 45:1156–64. doi: 10.1139/apnm-2019-0810
24. Burma JS, Kennedy CM, Penner LC, Miutz LN, Galea OA, Ainslie PN, et al. Long-term heart transplant recipients: Heart rate related effects on augmented transfer function coherence during repeated squat-stand maneuvers in males. Am J Physiol Regul Integr Comp Physiol (. (2021). doi: 10.1152/ajpregu.00177.2021
25. Lynall RC, Clark MD, Grand EE, Stucker JC, Littleton AC, Aguilar AJ, et al. Head impact biomechanics in women's college soccer. Med Sci Sports Exerc. (2016) 48:1772–8. doi: 10.1249/MSS.0000000000000951
26. Omboni S, Parati G, Frattola A, Mutti E, Di Rienzo M, Castiglioni P, et al. Spectral and sequence analysis of finger blood pressure variability. Comparison with analysis of intra-arterial recordings. Hypertension. (1993) 22:26–33. doi: 10.1161/01.HYP.22.1.26
27. Sammons EL, Samani NJ, Smith SM, Rathbone WE, Bentley S, Potter JF, et al. Influence of noninvasive peripheral arterial blood pressure measurements on assessment of dynamic cerebral autoregulation. J Appl Physiol. (2007) 103:369–75. doi: 10.1152/japplphysiol.00271.2007
28. Burma JS, Copeland P, Macaulay A, Khatra O, Smirl JD. Comparison of diurnal variation, anatomical location, and biological sex within spontaneous and driven dynamic cerebral autoregulation measures. Physiol Rep. (2020) 8:e14458. doi: 10.14814/phy2.14458
29. Labrecque L, Burma JS, Roy MA, Smirl JD, Brassard P. Reproducibility and diurnal variation of the directional sensitivity of the cerebral pressure-flow relationship in men and women. J Appl Physiol. (2022) 132:154–66. doi: 10.1152/japplphysiol.00653.2021
30. Saunders TD, Le RK, Breedlove KM, Bradney DA, Bowman TG. Sex differences in mechanisms of head impacts in collegiate soccer athletes. Clin Biomech. (2020) 74:14–20. doi: 10.1016/j.clinbiomech.2020.02.003
31. McCuen E, Svaldi D, Breedlove K, Kraz N, Cummiskey B, Breedlove EL, et al. Collegiate women's soccer players suffer greater cumulative head impacts than their high school counterparts. J Biomech. (2015) 48:3720–3. doi: 10.1016/j.jbiomech.2015.08.003
32. Willmott C, McIntosh AS, Howard T, Mitra B, Dimech-Betancourt B, Donovan J, et al. SCAT3 changes from baseline and associations with X2 Patch measured head acceleration in amateur Australian football players. J Sci Med Sport. (2018) 21:442–6. doi: 10.1016/j.jsams.2017.09.591
33. Zonner SW, Ejima K, Fulgar CC, Charleston CN, Huibregtse ME, Bevilacqua ZW, et al. Oculomotor response to cumulative subconcussive head impacts in US high school football players: A pilot longitudinal study. JAMA Ophthalmol. (2019) 137:265–70. doi: 10.1001/jamaophthalmol.2018.6193
34. Harriss A, Johnson AM, Thompson JWG, Walton DW, Dickey JP. Cumulative soccer heading amplifies the effects of brain activity observed during concurrent moderate exercise and continuous performance task in female youth soccer players. J Concuss. (2020) 4:1–9. doi: 10.1177/2059700220912654
35. Merchant-Borna K, Asselin P, Narayan D, Abar B, Jones CM, Bazarian JJ. Novel method of weighting cumulative helmet impacts improves correlation with brain white matter changes after one football season of sub-concussive head blows. Ann Biomed Eng. (2016) 44:3679–92. doi: 10.1007/s10439-016-1680-9
36. McCrory P, Meeuwisse WH, Aubry M, Cantu B, Dvorak J, Echemendia RJ, et al. Consensus statement on concussion in sport: the 4th international conference on concussion in sport held in Zurich, November 2012. Br J Sports Med. (2013) 47:250–8. doi: 10.1136/bjsports-2013-092313
37. Burma JS, Graver S, Miutz LN, Macaulay A, Copeland PV, Smirl JD. The validity and reliability of ultra-short-term heart rate variability parameters and the influence of physiological covariates. J Appl Physiol. (2021) 130:1848–67. doi: 10.1152/japplphysiol.00955.2020
38. Burma JS, Lapointe AP, Soroush A, Oni IK, Smirl JD, Dunn JF. Insufficient sampling frequencies skew heart rate variability estimates: Implications for extracting heart rate metrics from neuroimaging and physiological data. J Biomed Inform. (2021) 123:103934. doi: 10.1016/j.jbi.2021.103934
39. Nunan D, Sandercock GRH, Brodie DA, A. quantitative systematic review of normal values for short-term heart rate variability in healthy adults. Pacing Clin Electrophysiol. (2010) 33:1407–17. doi: 10.1111/j.1540-8159.2010.02841.x
40. Burma JS, Lapointe AP, Soroush A, Oni IK, Smirl JD, Dunn JF. The validity and reliability of an open source biosensing board to quantify heart rate variability. Heliyon. (2021) 7:e07148. doi: 10.1016/j.heliyon.2021.e07148
41. Smirl JD, Haykowsky MJ, Nelson MD, Tzeng YC, Marsden KR, Jones H, et al. Relationship between cerebral blood flow and blood pressure in long-term heart transplant recipients. Hypertension. (2014) 64:1314–20. doi: 10.1161/HYPERTENSIONAHA.114.04236
42. Bevilacqua ZW, Huibregtse ME, Kawata K. In vivo protocol of controlled subconcussive head impacts for the validation of field study data. J Vis Exp. (2019) 146:59381. doi: 10.3791/59381
43. Bretzin AC, Covassin T, Fox ME, Petit KM, Savage JL, Walker LF, et al. Sex differences in the clinical incidence of concussions, missed school days, and time loss in high school student-athletes: part 1. Am J Sports Med. (2018) 46:2263–9. doi: 10.1177/0363546518778251
44. Broglio SP, Guskiewicz KM, Sell TC, Lephart SM. No acute changes in postural control after soccer heading. Br J Sports Med. (2004) 38:561–7. doi: 10.1136/bjsm.2003.004887
45. Caccese JB, Buckley TA, Tierney RT, Arbogast KB, Rose WC, Glutting JJ, et al. Head and neck size and neck strength predict linear and rotational acceleration during purposeful soccer heading. Sports Biomech. (2018) 17:462–76. doi: 10.1080/14763141.2017.1360385
46. Caccese JB, Buckley TA, Tierney RT, Rose WC, Glutting JJ, Kaminski TW. Postural control deficits after repetitive soccer heading. Clin J Sport Med. (2018) 31:266–72. doi: 10.1097/JSM.0000000000000709
47. Caccese JB, Buckley TA, Tierney RT, Rose WC, Glutting JJ, Kaminski TW. Sex and age differences in head acceleration during purposeful soccer heading. Res Sports Med. (2018) 26:64–74. doi: 10.1080/15438627.2017.1393756
48. Di Virgilio TG, Hunter A, Wilson L, Stewart W, Goodall S, Howatson G, et al. Evidence for acute electrophysiological and cognitive changes following routine soccer. Heading. (2016) 13:66–71. doi: 10.1016/j.ebiom.2016.10.029
49. Dorminy M, Hoogeveen A, Tierney RT, Higgins M, McDevitt JK, Kretzschmar J. Effect of soccer heading ball speed on S100B, sideline concussion assessments and head impact kinematics. Brain Inj. (2015) 29:1158–64. doi: 10.3109/02699052.2015.1035324
50. Elbin RJ, Beatty A, Covassin T, Schatz P, Hydeman A, Kontos AP, et al. preliminary examination of neurocognitive performance and symptoms following a bout of soccer heading in athletes wearing protective soccer headbands. Res Sports Med. (2015) 23:203–14. doi: 10.1080/15438627.2015.1005293
51. Haran FJ, Tierney R, Wright WG, Keshner E, Silter M. Acute changes in postural control after soccer heading. Int J Sports Med. (2013) 34:350–4. doi: 10.1055/s-0032-1304647
52. Kawata K, Tierney R, Phillips J, Jeka JJ. Effect of repetitive sub-concussive head impacts on ocular near point of convergence. Int J Sports Med. (2016) 37:405–10. doi: 10.1055/s-0035-1569290
53. Tierney RT, Higgins M, Caswell SV, Brady J, McHardy K, Driban JB, et al. Sex differences in head acceleration during heading while wearing soccer headgear. J Athl Train. (2008) 43:578–84. doi: 10.4085/1062-6050-43.6.578
54. Basinas I, McElvenny DM, Pearce N, Gallo V, Cherrie JW, A. systematic review of head impacts and acceleration associated with soccer. Int J Environ Res Public Health. (2022) 19:5488. doi: 10.3390/ijerph19095488
55. Lord SW, Clayton RH, Hall MC, Gray JC, Murray A, McComb JM, et al. Reproducibility of three different methods of measuring baroreflex sensitivity in normal subjects. Clin Sci. (1998) 95:575–81. doi: 10.1042/cs0950575
56. Purkayastha S, Williams B, Murphy M, Lyng S, Sabo T, Bell KR. Reduced heart rate variability and lower cerebral blood flow associated with poor cognition during recovery following concussion. Auton Neurosci. (2019) 220:102548. doi: 10.1016/j.autneu.2019.04.004
57. Senthinathan A, Mainwaring LM, Hutchison M. Heart rate variability of athletes across concussion recovery milestones: a preliminary study. Clin J Sport Med. (2017) 27:288–95. doi: 10.1097/JSM.0000000000000337
58. La Fountaine MF, Hohn AN, Testa AJ, Weir JP. Attenuation of spontaneous baroreceptor sensitivity following concussion. Med Sci Sports Exerc. (2019) 51:792–7. doi: 10.1249/MSS.0000000000001833
60. Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery. (2014) 75(Suppl 4):S24–33. doi: 10.1227/NEU.0000000000000505
61. Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. (1991) 133:144–53. doi: 10.1093/oxfordjournals.aje.a115853
62. Mills EJ, Chan AW, Wu P, Vail A, Guyatt GH, Altman DG. Design, analysis, and presentation of crossover trials. Trials. (2009) 10:27. doi: 10.1186/1745-6215-10-27
63. Daneshvar DH, Nowinski CJ, McKee AC, Cantu RC. The epidemiology of sport-related concussion. Clin Sports Med. (2011) 30:1–17. doi: 10.1016/j.csm.2010.08.006
64. Ferris LM, Kontos AP, Eagle SR, Elbin RJ, Collins MW, Mucha A, et al. Utility of VOMS, SCAT3, and ImPACT baseline evaluations for acute concussion identification in collegiate athletes: findings from the NCAA-DoD concussion assessment, research and education (CARE) consortium. Am J Sports Med. (2022) 50:1106–19. doi: 10.1177/03635465211072261
65. Schussler E, Stark D, Bolte JH, Kang YS, Onate JA. Comparison of a head mounted impact measurement device to the hybrid III anthropomorphic testing device in a controlled laboratory setting. Int J Sports Phys Ther. (2017) 12:592–600.
66. Wu LC, Nangia V, Bui K, Hammoor B, Kurt M, Hernandez F, et al. In vivo evaluation of wearable head impact sensors. Ann Biomed Eng. (2016) 44:1234–45. doi: 10.1007/s10439-015-1423-3
Keywords: repetitive football/soccer heading, heart rate variability, autonomic function, sub-concussive impacts, sport concussion assessment tool 3, SCAT3
Citation: Smirl JD, Peacock D, Burma JS, Wright AD, Bouliane KJ, Dierijck J and van Donkelaar P (2022) Repetitive bout of controlled soccer heading does not alter heart rate variability metrics: A preliminary investigation. Front. Neurol. 13:980938. doi: 10.3389/fneur.2022.980938
Received: 29 June 2022; Accepted: 09 November 2022;
Published: 25 November 2022.
Edited by:
Kerry Peek, The University of Sydney, AustraliaReviewed by:
Kimbra Kenney, Uniformed Services University of the Health Sciences, United StatesJim Dickey, Western University, Canada
Copyright © 2022 Smirl, Peacock, Burma, Wright, Bouliane, Dierijck and van Donkelaar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jonathan David Smirl, am9uYXRoYW4uc21pcmxAdWNhbGdhcnkuY2E=
†These authors share first authorship
 Jonathan David Smirl
Jonathan David Smirl Dakota Peacock
Dakota Peacock Joel Stephen Burma
Joel Stephen Burma Alexander D. Wright
Alexander D. Wright Kevin J. Bouliane1
Kevin J. Bouliane1 Paul van Donkelaar
Paul van Donkelaar