- 1Henan University People's Hospital, Henan Provincial People's Hospital, Zhengzhou, China
- 2Fuwai Central China Cardiovascular Hospital, Henan Provincial People's Hospital, Zhengzhou, China
- 3Henan University Joint National Laboratory for Antibody Drug Engineering, Kaifeng, China
- 4Henan Provincial People's Hospital, Zhengzhou University People's Hospital, Zhengzhou, China
Background: Restless Legs Syndrome (RLS) is a common neurological disorder. Growing evidence shows that dopaminergic dysfunction and iron deficiency are associated with the pathogenesis of RLS. Additionally, the dopaminergic system is linked with the hypothalamic-pituitary-thyroid (HPT) axis. Thus, the current study aimed to compare thyroid function between RLS patients and healthy subjects and investigate the associations with clinical characteristics of RLS.
Methods: Serum levels of thyroid hormones were investigated in 102 first-episode drug-naïve RLS patients and 80 matched healthy controls (HCs). Baseline data and clinical characteristics were performed by professional personnel. In addition, multivariate regression was used to analyze the relationship between thyroid function and RLS.
Results: Compared with control group, RLS patients had significantly higher serum thyroid-stimulating hormone (TSH) levels (p < 0.001), and higher prevalence of subclinical hypothyroidism [Odds ratio (OR) 8.00; 95% confidence interval (CI) = 3.50–18.30; p < 0.001]. The Subclinical hypothyroidism rate (47.1 vs. 10%, p < 0.001) in RLS patients was higher than the HCs group. Regression analysis revealed that serum TSH (OR = 1.77; 95% CI = 1.41–2.23; p < 0.001) was independently associated with RLS. There was a statistically significant positive correlation between TSH and the Pittsburgh sleep quality index (PSQI) scores (r = 0.728, p < 0.001), and the International Restless Legs Scales (IRLS) points (r = 0.627, p < 0.001). Spearman correlation analysis showed that FT3 was positive correlated with HAMA14 score (r = 0.239, p = 0.015). In addition, compared with the good-sleeper group, poor-sleeper patients had significantly higher serum TSH levels (p < 0.001).
Conclusion: Serum levels of TSH and the prevalence of subclinical hypothyroidism were higher in RLS patients, indicating the imbalance between thyroid hormones (TH) and the dopaminergic system may contribute to the development of primary RLS. Additionally, the TH axis may influence the quality of sleep in RLS patients.
Introduction
Restless Legs Syndrome (RLS), also known as Willis-Ekbom disease (1), is a common neurological sensory-motor disorder, mainly characterized by strong discomfort of the lower limbs at night or at rest and the irresistible desire to move the legs (2), which is estimated to affect 0.1 ~ 15% of the general population (3). RLS patients also suffer from sleep disturbance and depression (4), which can strongly impact the quality of life (5). Currently, RLS can be divided into idiopathic and secondary forms (6). Alternately, secondary RLS may be related to other medical causes, such as multiple sclerosis, Parkinson's disease (PD), anemia, iron depletion, chronic renal failure, and pregnancy (7). Genetic studies had identified MEIS1 as an RLS-predisposing gene (8, 9), which increases the risk of RLS (10). Although the exact pathophysiology of RLS is not fully understood, there is robust evidence that the neurotransmitter dopamine (DA) and iron deficiency are associated with the pathogenesis of RLS (3).
Previous studies on RLS have revealed a decreased fluorine-18-L-dihydroxyphenylalanine (18F-dopa) uptake in the substantia nigra along with the reduction in presynaptic dopaminergic, providing insight into the role of dopaminergic dysfunction in RLS (11–13). In an autopsy study, compared with controls, the dopamine 2 (D2) receptors in the putamina of idiopathic RLS were decreased, which is also associated with the severity of the disease (14). Numerous studies have also shown that DA agonists can alleviate RLS symptoms (15). These findings indicated dopaminergic dysfunction in RLS patients. Interestingly, there are signs that the dopaminergic system is may be associated with the hypothalamic-pituitary-thyroid (HPT) axis (16). Specifically, DA can downregulate thyroid hormones (TH) and thyroid stimulating hormone (TSH) while upregulating-thyrotropin releasing hormone (TRH) (17). Therefore, based on these findings, we hypothesized that the imbalance between thyroid hormones and the dopaminergic system may contribute to the development of RLS.
Currently, on the one hand, it has been reported that the daily pattern of TSH levels is similar to the fluctuation of symptoms in RLS patients (16). On the other hand, the relationship between thyroid function and RLS has been less studied, and inconsistency in conclusions. There are no studies that associate thyroid function and symptom severity of RLS. Therefore, to explore this connection further, the current study aimed to compare thyroid function between RLS patients and healthy subjects, and to investigate the associations with clinical characteristics and symptom severity of RLS, which may contribute to the development of therapeutic strategies for managing and preventing RLS.
Materials and methods
Research subjects
Our cross-sectional cohort study was conducted from January 2018 to October 2021, including 102 patients with RLS who were recruited from the neurology department of the Henan Provincial People's Hospital. Additionally, during the same period, 80 sex- and age-matched healthy subjects screened from the healthy examination center of our hospital were selected as healthy controls (HCs).
The study was approved by the Research Ethics Committee of Henan Provincial People's Hospital according to the principles of the Helsinki Declaration. Additionally, all the participants signed the written informed consent forms.
Inclusion criteria
The patients met the diagnostic criteria of RLS according to the International Restless Legs Syndrome Study Group (IRLSSG) (18). To make the diagnosis, the four essential features were confirmed by the interview. Additionally, to avoid interference from DA agonist treatment, we included subjects who were first-episode drug-naïve RLS patients.
Exclusion criteria
Studies that met in the following criteria were excluded: (1) RLS secondary to chronic kidney disease, diabetes, rheumatoid arthritis, renal failure, iron deficiency, peripheral neuropathy, PD, drug-induced factors; (2) combined with other sleep disorders such as narcolepsy, rapid eye movement sleep behavior disorder (RBD), narcolepsy, or obstructive sleep apnea syndrome (OSAS); (3) patients with a history of thyroidectomy, neck radiotherapy, 131I treatment for hyperthyroidism, or current consumption of antithyroid drugs were also excluded; (4) history of mental disorders (e.g., schizophrenia) or family history of mental disorders; (5) pregnant and lactating women.
Research methods
Collecting general information
Self-reported general information included: gender, age, the age of onset, disease duration (years), and education duration. Their body mass index (BMI), heart rate, and blood pressure were directly measured.
Clinical and neuropsychological assessment
For all patients who met the diagnostic criteria, we used the International Restless Legs Scales (IRLS) to assess the severity of RLS (19). For the assessment of the disease-specific quality of life, the Restless Legs Syndrome Quality of Life Questionnaire (QoL-RLS) was used by professional physicians (20). In addition, for neuropsychological assessment, the 14-item Hamilton Anxiety Scale (HAMA14), and the 24-item Hamilton Depression Rating Scale (HAMD24) (21) were used to assess the anxiety and depression symptoms of patients with RLS.
Sleep conditions and grouping
The Pittsburgh sleep quality index (PSQI) has been widely used to measure sleep quality over the past month (22), which consists of 23 items. The global points range from 0 to 21. The higher scores, the worse the quality of sleep. According to the PSQI score, subjects can be divided into poor-sleeper (PSQI > 5) group and good-sleeper (PSQI ≤ 5) group (22).
Laboratory assessment
All subjects fasted overnight for 12 h and 5 ml venous blood samples were collected in serum separator tubes and immediately centrifuged at 3 000 r/min and stored at −80° C. According to standard operating procedures, serum TSH, free triiodothyronine (FT3), and free thyroxine (FT4) levels were measured using electrochemiluminescence immunoassays. In addition, anti-thyroid peroxidase antibodies (anti-TPO) and anti-thyroglobulin antibodies (anti-TG) were also detected. In our hospital, the reference value of TSH, FT3, and FT4 has been estimated at 3.1–6.8, 12.0–22.0 pmol/L, and 0.27–4.2 uIU/mL, respectively.
Additionally, high TSH and normal FT4 can be diagnosed with subclinical hypothyroidism; High TSH and low FT4 were diagnosed with primary hypothyroidism (23).
Statistical analysis
For data processing and statistical analysis, the SPSS Statics 24.0 computer software was conducted. Continuous variables were presented as mean ± standard deviation (SD) or median and interquartile range (M, P25, P75) based on the normal or non-normal distribution of the data determined by the Kolmogorov-Smirnov test. For normally distributed continuous data, we used the student's t-test, and for non-normally distributed continuous variables, we used the Mann-Whitney U-test. The Chi-square test or Fisher's exact test was used to compare the groups. Categorical data are expressed in amount (%). In order to identify predictive indicators of RLS, a multivariate logistic regression analysis was performed, adjusting for disease duration sex, age, BMI, SBP, and DBP. To compare the prevalence of thyroid dysfunction between RLS patients and controls, the odds ratio (OR) and 95% confidence interval (CI) were calculated. The two–by–two frequency table was performed to calculate OR. The associations between thyroid function and clinical characteristics in RLS were analyzed by Spearman or Pearson. P < 0.05 were considered statistically significant.
Results
Baseline characteristics
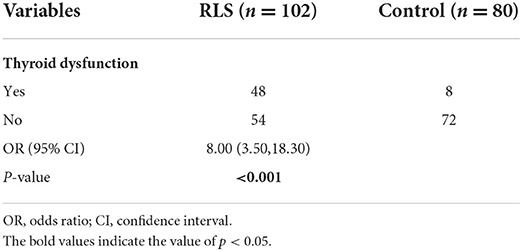
A total of 102 RLS patients (70 female; mean age, 51.58 ± 6.79 years), and 80 HCs (22 female; mean age, 50.86 ± 6.15 years) were recruited for this study. Differences in age, gender, BMI, smoking, and alcohol consumption were not significant between the RLS group and the HCs group (all p > 0.05). The Subclinical hypothyroidism rate (47.1 vs. 10%, p < 0.001) in RLS patients was higher than those in the HCs group. However, no patients with hypothyroidism were found in the two groups. As compared with the HCs group, serum TSH was significantly higher in the RLS group (p < 0.001). Additionally, there was no statistically significant different between both groups in either FT4 or FT3 (all p > 0.05). The demographic and clinical characteristics of all subjects are shown in Table 1.
The prevalence of the thyroid dysfunction
According to the TSH, FT3, and FT4 levels, 47.1% of the patients (n = 48) in the RLS group with high TSH and normal FT4 were diagnosed with subclinical hypothyroidism, whereas, in the HCs group, 10% (n = 8) of the subjects with subclinical hypothyroidism. In addition, there was no subject with hypothyroidism (high TSH and low FT4). Patients with RLS were up to 8 times more likely to develop thyroid dysfunction compared to the HCs group (OR = 8.00, 95% CI = 3.50–18.30, p < 0.001; Table 2).
Logistic regression analysis
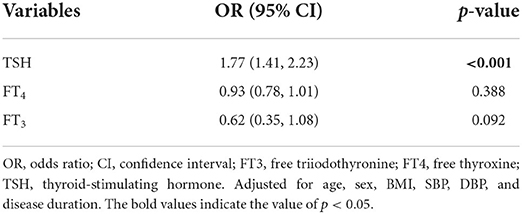
The results of the multiple stepwise logistic regression analysis were shown in Table 3. After adjusting for disease duration sex, age, BMI, SBP, and DBP, serum TSH was independently associated with RLS (OR = 1.77; 95% CI = 1.41–2.23; p < 0.001).
Correlation analysis
A significant positive correlation was found between PSQI scores and serum TSH levels in RLS patients by Spearman correlation analysis (r = 0.728, p < 0.001). In addition, there was also a statistically significant positive correlation between TSH serum level and IRLS points (r = 0.627, p < 0.001). Spearman correlation analysis showed that FT3 was positive correlated with HAMA14 score (r = 0.239, p = 0.015; Table 4).
Subgroup analysis by sleep disorder
One hundred two patients with RLS, were divided into poor-sleepers (PSQI points > 5) and good-sleepers (PSQI points ≤ 5) groups. As compared with the good-sleepers group, the serum TSH level was higher in the poor-sleepers group (p < 0.001). No significant difference was found in FT4 or FT3 between the two groups (p > 0.05; Table 5).
Discussion
To the best of our knowledge, our study is the first to investigate the associations between clinical characteristics and thyroid function among RLS patients. Our study found that serum TSH levels and prevalence of subclinical hypothyroidism were higher in RLS patients, compared with the control group. Regression analysis revealed that serum TSH was independently associated with RLS. Our further spearman correlation analysis found that serum TSH was positively correlated with the PSQI scores and the IRLS points. In addition, compared with the poor-sleeper group, good-sleeper patients had significantly higher serum TSH levels.
DA system and iron deficiency and HPT axis
Increasing evidence has indicated that dopaminergic dysfunction plays a vital role in RLS pathophysiology (24). There are many studies had showed that DA agonists can be beneficial to RLS patients (15). Evidence indicated that iron deficiency is associated with the pathophysiology of RLS (25).
Studies have shown that iron is involved in processes of DA synthesis, which is a cofactor of tyrosine hydroxylase (26). Similarly, there is evidence that iron deficiency can increase TH levels (27). Notably, it is known that DA depresses the TH axis (28). On the other hand, TRH can be able to stimulate DA release by activating presynaptic dopaminergic neurons (17). Patients with typical RLS have the most severe symptoms at night (29). Interestingly, a previous study found that TSH secretion pattern varies in a circadian rhythm (30), which coincides with the diurnal fluctuations in symptom worsening among RLS patients (16).
RLS and thyroid function
Currently, the link between hypothyroidism and RLS has been less studied, and the conclusions were inconsistent. There are many studies indicating that patients with pregnancy and Graves' disease had higher levels of TH, which may contribute to the higher prevalence of RLS symptoms (31). A study including 600 pregnant women by Ozer et al. (32) revealed that the prevalence of hypothyroidism was higher in pregnant women with RLS compared to those without RLS (p < 0.05), which was consistent with another finding (33). Tan et al. (34) found that there was no significant difference in the prevalence of RLS between thyroid disorders patients and normal individuals. On the contrary, a case–control study of 353 RLS by Ahmed et al. (33) showed that RLS patients had an increased prevalence of hypothyroidism than the healthy controls. Our results also showed an elevated prevalence of subclinical hypothyroidism in RLS patients and serum TSH was independently related to RLS. Considering these findings, thyroid dysfunction can influence RLS symptoms despite the lack of direct evidence that it directly causes RLS. Since thyroid dysfunction is clinically easier to detect as well as treat, it should be taken seriously given that it may be a potentially modifiable risk factor for RLS.
Epidemiological studies have found that, compared with the male population, the prevalence of RLS was higher in the female population (35, 36), which was consistent with our finding that the ratio of female to male RLS patients was 2:1 in our paper. Currently, few studies have elucidated the pathological mechanisms underlying sex-specific differences in RLS patients. According to subgroup analysis by sex, we found no difference in serum TSH, FT3, and FT4 levels between male and female RLS patients, which we speculate may be associated with the relatively small sample size.
In this study, we found a statistically significant positive correlation between TSH serum levels and IRLS points. Although, we have no direct evidence of a correlation between serum TSH levels and iron levels as well as DA levels. However, we can speculate that the abnormal correlation may exacerbate RLS symptoms.
Thyroid dysfunction and sleep disorders
There are many studies indicating that hypothyroidism may affect sleep quality (31). A Population-Based Study of 2,224 patients with subclinical hypothyroidism by Song et al. (37) found that patients with subclinical hypothyroidism typically had shorter sleep duration, longer sleep latency, and higher PSQI scores compared to 12,622 controls with normal thyroid function. On the contrary, Akatsu et al. (38) showed that there was no significant association between sleep quality and subclinical hypothyroidism or hyperthyroidism patients. However, the pathophysiological mechanism between hypothyroidism and insomnia is not known.
Epidemiological studies have shown that the incidence of sleep disorders was higher in RLS patients than in the general population (4, 39). In this study, there was also a statistically significant positive correlation between TSH serum levels and PSQI scores. We speculate that sleep quality is closely related to serum TSH levels in RLS patients. Additionally, this study also found that the serum levels of TSH were higher in the poor-sleepers group as compared to the good-sleepers group, indicating a possible association between thyroid dysfunction and sleep disorders.
Limitations and recommendations
Our study has several limitations. First, our study could not clarify the causal relationship between thyroid dysfunction and RLS, which was a cross-sectional descriptive study. Future large-scale cohort studies should be conducted to confirm the causal relationship. Second, we did not quantify TH with dopamine receptors in the brain, but only used a scale to measure the severity of the disease. Third, we assessed the sleep quality of RLS patients using only the PSQI scale and did not correlate TH with the arousal index (AI) in objective polysomnography to improve the accuracy of our conclusions. Finally, we included all patients with idiopathic first-episode drug-naïve RLS, and the effects of DA agonists on patients' thyroid hormones after their use for treatment should be compared in future studies.
Conclusion
In conclusion, our results showed that serum levels of TSH and the prevalence of subclinical hypothyroidism was higher in RLS patients, indicating the imbalance between thyroid hormones and the dopaminergic system may contribute to the development of primary RLS. In addition, more research evidence is needed to investigate whether the TH axis may affect sleep quality in patients with RLS.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Research Ethics Committee of Henan Provincial People's Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CG and ZY: wrote first draft and statistics. ZY and XK: statistics and data collection. CG and HZ: conceptualization, resources, and supervision. All authors approved the submitted version.
Funding
This work was supported by the Henan Medical Science and Technology Research Program (No. 202102310082), and Henan Province Medical Science and Technology Tackling Provincial Ministry Key Projects (SBGJ202102033).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Trenkwalder C, Allen R, Hogl B, Clemens S, Patton S, Schormair B, et al. Comorbidities, treatment, and pathophysiology in restless legs syndrome. Lancet Neurol. (2018) 17:994–1005. doi: 10.1016/S1474-4422(18)30311-9
2. Sun S, Liu C, Jia Y, Wu J, Li H, Li X, et al. Association between migraine complicated with restless legs syndrome and vitamin D. Front Neurol. (2021) 12:777721. doi: 10.3389/fneur.2021.777721
3. Liu HM, Chu M, Liu CF, Zhang T, Gu P. Analysis of serum vitamin D level and related factors in patients with restless legs syndrome. Front Neurol. (2021) 12:782565. doi: 10.3389/fneur.2021.782565
4. Xu Y, Wen H, Li J, Yang J, Luo K, Chang L. The relationship between sleep disorders, anxiety, depression, and cognitive function with restless legs syndrome (RLS) in the elderly. Sleep Breath. (2021). doi: 10.1007/s11325-021-02477-y. [Epub ahead of print].
5. Didato G, Di Giacomo R, Rosa GJ, Dominese A, de Curtis M, Lanteri P. Restless legs syndrome across the lifespan: symptoms, pathophysiology, management and daily life impact of the different patterns of disease presentation. Int J Environ Res Public Health. (2020) 17:3658. doi: 10.3390/ijerph17103658
6. Oran M, Unsal C, Albayrak Y, Tulubas F, Oguz K, Avci O, et al. Possible association between vitamin D deficiency and restless legs syndrome. Neuropsychiatr Dis Treat. (2014) 10:953–8. doi: 10.2147/NDT.S63599
7. Balaban H, Yildiz OK, Cil G, Senturk IA, Erselcan T, Bolayir E, et al. Serum 25-hydroxyvitamin D levels in restless legs syndrome patients. Sleep Med. (2012) 13:953–7. doi: 10.1016/j.sleep.2012.04.009
8. Catoire H, Dion PA, Xiong L, Amari M, Gaudet R, Girard SL, et al. Restless legs syndrome-associated MEIS1 risk variant influences iron homeostasis. Ann Neurol. (2011) 70:170–5. doi: 10.1002/ana.22435
9. Schulte EC, Kousi M, Tan PL, Tilch E, Knauf F, Lichtner P, et al. Targeted resequencing and systematic in vivo functional testing identifies rare variants in MEIS1 as significant contributors to restless legs syndrome. Am J Hum Genet. (2014) 95:85–95. doi: 10.1016/j.ajhg.2014.06.005
10. Ergun U, Say B, Ergun SG, Percin FE, Inan L, Kaygisiz S, et al. Genome-wide association and whole exome sequencing studies reveal a novel candidate locus for restless legs syndrome. Eur J Med Genet. (2021) 64:104186. doi: 10.1016/j.ejmg.2021.104186
11. Michaud M, Soucy JP, Chabli A, Lavigne G, Montplaisir J. SPECT imaging of striatal pre- and postsynaptic dopaminergic status in restless legs syndrome with periodic leg movements in sleep. J Neurol. (2002) 249:164–70. doi: 10.1007/PL00007859
12. Turjanski N, Lees AJ, Brooks DJ. Striatal dopaminergic function in restless legs syndrome: 18F-dopa and 11C-raclopride PET studies. Neurology. (1999) 52:932–7. doi: 10.1212/WNL.52.5.932
13. Ruottinen HM, Partinen M, Hublin C, Bergman J, Haaparanta M, Solin O, et al. An FDOPA PET study in patients with periodic limb movement disorder and restless legs syndrome. Neurology. (2000) 54:502–4. doi: 10.1212/WNL.54.2.502
14. Connor JR, Wang XS, Allen RP, Beard JL, Wiesinger JA, Felt BT, et al. Altered dopaminergic profile in the putamen and substantia nigra in restless leg syndrome. Brain. (2009) 132(Pt 9):2403–12. doi: 10.1093/brain/awp125
15. Winkelman JW. High national rates of high-dose dopamine agonist prescribing for restless legs syndrome. Sleep. (2022) 45:zsab212. doi: 10.1093/sleep/zsab212
16. Pereira JC Jr, Pradella-Hallinan M, Lins Pessoa H. Imbalance between thyroid hormones and the dopaminergic system might be central to the pathophysiology of restless legs syndrome: a hypothesis. Clinics. (2010) 65:548–54. doi: 10.1590/S1807-59322010000500013
17. Mohammadi S, Dolatshahi M, Rahmani F. Shedding light on thyroid hormone disorders and Parkinson disease pathology: mechanisms and risk factors. J Endocrinol Invest. (2021) 44:1–13. doi: 10.1007/s40618-020-01314-5
18. Allen RP, Picchietti DL, Garcia-Borreguero D, Ondo WG, Walters AS, Winkelman JW, et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated international restless legs syndrome study group (IRLSSG) consensus criteria–history, rationale, description, and significance. Sleep Med. (2014) 15:860–73. doi: 10.1016/j.sleep.2014.03.025
19. Sharon D, Allen RP, Martinez-Martin P, Walters AS, Ferini Strambi L, Hogl B, et al. Validation of the self-administered version of the international restless legs syndrome study group severity rating scale - the sIRLS. Sleep Med. (2019) 54:94–100. doi: 10.1016/j.sleep.2018.10.014
20. Walters AS, Frauscher B, Allen R, Benes H, Chaudhuri KR, Garcia-Borreguero D, et al. Review of quality of life instruments for the restless legs syndrome/Willis-Ekbom Disease (RLS/WED). Critique and recommendations. J Clin Sleep Med. (2014) 10:1351–7. doi: 10.5664/jcsm.4300
21. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. (1960) 23:56–62. doi: 10.1136/jnnp.23.1.56
22. Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
23. Bednarczuk T, Brix TH, Schima W, Zettinig G, Kahaly GJ. 2021 european thyroid association guidelines for the management of iodine-based contrast media-induced thyroid dysfunction. Eur Thyroid J. (2021) 10:269–84. doi: 10.1159/000517175
24. Romero-Peralta S, Cano-Pumarega I, Garcia-Borreguero D. Emerging concepts of the pathophysiology and adverse outcomes of restless legs syndrome. Chest. (2020) 158:1218–29. doi: 10.1016/j.chest.2020.03.035
25. Beliveau V, Stefani A, Birkl C, Kremser C, Gizewski ER, Hogl B, et al. Revisiting brain iron deficiency in restless legs syndrome using magnetic resonance imaging. Neuroimage Clin. (2022) 34:103024. doi: 10.1016/j.nicl.2022.103024
26. Olgun Yazar H, Yazar T, Ozdemir S, Kasko Arici Y. Serum C-reactive protein/albumin ratio and restless legs syndrome. Sleep Med. (2019) 58:61–5. doi: 10.1016/j.sleep.2019.02.022
27. Pereira JC Jr, Rocha e Silva IR, Pradella-Hallinan M. Transient Willis-Ekbom's disease (restless legs syndrome) during pregnancy may be caused by estradiol-mediated dopamine overmodulation. Med Hypotheses. (2013) 80:205–8. doi: 10.1016/j.mehy.2012.11.030
28. Scanlon MF, Weetman AP, Lewis M, Pourmand M, Rodriguez-Arnao MD, Weightman DR, et al. Dopaminergic modulation of circadian thyrotropin rhythms and thyroid hormone levels in euthyroid subjects. J Clin Endocrinol Metab. (1980) 51:1251–6. doi: 10.1210/jcem-51-6-1251
29. Pienczk-Reclawowicz K, Pilarska E, Olszewska A, Reclawowicz D, Konieczna S, Slawek J. The prevalence of the restless legs Syndrome/Willis-Ekbom disease among teenagers, its clinical characteristics and impact on everyday functioning. Sleep Med. (2022) 89:48–54. doi: 10.1016/j.sleep.2021.10.004
30. Brabant G, Prank K, Ranft U, Schuermeyer T, Wagner TO, Hauser H, et al. Physiological regulation of circadian and pulsatile thyrotropin secretion in normal man and woman. J Clin Endocrinol Metab. (1990) 70:403–9. doi: 10.1210/jcem-70-2-403
31. Green ME, Bernet V, Cheung J. Thyroid dysfunction and sleep disorders. Front Endocrinol. (2021) 12:725829. doi: 10.3389/fendo.2021.725829
32. Ozer I, Guzel I, Orhan G, Erkilinc S, Oztekin N, Ak F, et al. A prospective case control questionnaire study for restless leg syndrome on 600 pregnant women. J Matern Fetal Neonatal Med. (2017) 30:2895–9. doi: 10.3109/14767058.2016.1170801
33. Ahmed N, Kandil M, Elfil M, Jamal A, Koo BB. Hypothyroidism in restless legs syndrome. J Sleep Res. (2021) 30:e13091. doi: 10.1111/jsr.13091
34. Tan EK, Ho SC, Eng P, Loh LM, Koh L, Lum SY, et al. Restless legs symptoms in thyroid disorders. Parkinsonism Relat Disord. (2004) 10:149–51. doi: 10.1016/j.parkreldis.2003.11.003
35. Beladi-Mousavi SS, Jafarizade M, Shayanpour S, Bahadoram M, Moosavian SM, Houshmand G. Restless legs syndrome: associated risk factors in hemodialysis patients. Nephrourol Mon. (2015) 7:e31967. doi: 10.5812/numonthly.31967
36. Almeneessie AS, Alyousefi N, Alzahrani M, Alsafi A, Alotaibi R, Olaish AH, et al. Prevalence of restless legs syndrome among pregnant women: a case-control study. Ann Thorac Med. (2020) 15:9–14. doi: 10.4103/atm.ATM_206_19
37. Song L, Lei J, Jiang K, Lei Y, Tang Y, Zhu J, et al. The association between subclinical hypothyroidism and sleep quality: a population-based study. Risk Manag Healthc Policy. (2019) 12:369–74. doi: 10.2147/RMHP.S234552
38. Akatsu H, Ewing SK, Stefanick ML, Fink HA, Stone KL, Barrett-Connor E, et al. Association between thyroid function and objective and subjective sleep quality in older men: the osteoporotic fractures in men (MrOS) study. Endocr Pract. (2014) 20:576–86. doi: 10.4158/EP13282.OR
Keywords: restless legs syndrome, thyroid dysfunction, sleep disorder, TSH, FT3, FT4
Citation: Geng C, Yang Z, Kong X, Xu P and Zhang H (2022) Association between thyroid function and disease severity in restless legs syndrome. Front. Neurol. 13:974229. doi: 10.3389/fneur.2022.974229
Received: 21 June 2022; Accepted: 25 July 2022;
Published: 12 August 2022.
Edited by:
Yo Oishi, University of Tsukuba, JapanReviewed by:
Keisuke Suzuki, Dokkyo Medical University, JapanTatsuya Yamamoto, Chiba Prefectural University of Health Sciences, Japan
Copyright © 2022 Geng, Yang, Kong, Xu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongju Zhang, aG9uZ2p1ekBzaW5hLmNvbQ==
 Chaofan Geng
Chaofan Geng Zhenzhen Yang2
Zhenzhen Yang2