- 1Department of Neurosurgery, The Affiliated Hospital of Kunming University of Science and Technology, Kunming, China
- 2Department of Neurosurgery, School of Medicine, Second Affiliated Hospital, Zhejiang University, Hangzhou, China
- 3College of Clinical Medicine, Jilin University, Changchun, China
- 4Medical Faculty, Kunming University of Science and Technology, Kunming, China
Introduction: As a common endovascular treatment for intracranial aneurysms, the pipeline embolization device (PED) is considered a standard treatment option, especially for large, giant, wide-necked, or dissecting aneurysms. A layer of phosphorylcholine biocompatible polymer added to the surface of the PED can substantially improve this technology. This PED with shield technology (pipeline shield) is relatively novel; its early technical success and safety have been reported. We conducted a systematic literature review with the aim of evaluating the efficacy and safety of the pipeline shield.
Methods: We searched the PubMed, Embase, and Cochrane databases, following the preferred reporting items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.
Results: We selected five prospective and two retrospective studies for review. A total of 572 aneurysms were included; of these, 506 (88.5%) were unruptured. The antiplatelet regimens were heterogeneous. The rate of perioperative and postoperative complications was 11.1% [95% confidence interval (CI): 6.5–18.9%]. The adequate occlusion rate at 6 months was 73.9% (95% CI: 69.1–78.7%). The adequate occlusion rate of more than 12 months was 80.9% (95% CI: 75.1–86.1%). The mortality rate was 0.7% (95% CI: 0.2–1.5%). Subgroup analyses showed that aneurysm rupture status had no effect on aneurysm occlusion rate, patient morbidity, or mortality.
Conclusion: This review demonstrates the safety and efficacy of the pipeline shield for treating intracranial aneurysms. However, direct comparisons of the pipeline shield with other flow diverters are needed to better understand the relative safety and effectiveness of different devices.
Introduction
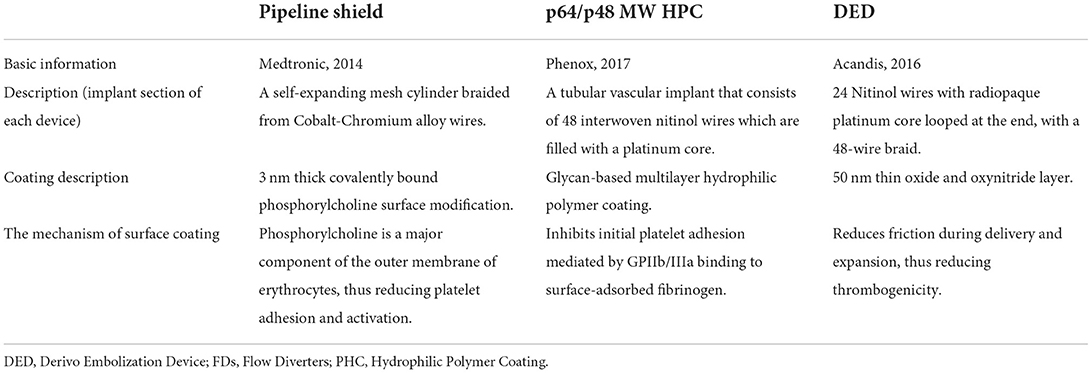
Flow diverters (FDs) enable the application of endovascular therapy for intracranial aneurysms in an increased number of indications. The utilization of FDs has become the preferred treatment option for various types of aneurysms (1–3). Despite their relatively recent development, numerous FDs have been introduced for clinical use. Currently available coating FDs include the pipeline embolization device (PED) with shield technology (referred to as the pipeline shield), derivo embolization device (DED), and p64/p48 MW HPC (Table 1). The pipeline shield incorporates a phosphorylcholine surface coating (4), which is a third-generation PED. It has been shown to reduce intimal hyperplasia (5) and increase early neointimal growth in preclinical studies (6). In ex vivo (4) and in vitro studies (7, 8), the pipeline shield significantly reduced thrombogenicity in comparison with other FDs. As a new therapeutic technique for intracranial aneurysms, the efficacy of complications associated with the pipeline shield remains unclear, and there is currently no relevant literature that summarizes existing findings. Therefore, this meta-analysis aimed to explore the efficacy and safety of the pipeline shield in treating intracranial aneurysms.
Methods
Search strategy
We searched the PubMed, Embase, and Cochrane databases to identify studies using the pipeline shield for treating intracranial aneurysms. We used the following search terms: “flow diverter,” “pipeline embolization device,” “PED,” “shield technology,” “surface modification,” and “aneurysm.” We followed the applicable Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (9). We reviewed literature published between device inception and March 2022 and carefully screened the search results to select studies that were particularly relevant to pipeline shield devices in the neurointerventional field.
Selection criteria
For this review, we included all English-language articles on the use of the pipeline shield for treating intracranial aneurysms. Case reports were excluded. Animal, in vitro, and cadaveric studies were excluded. We also excluded non-primitive research and conference abstracts. We assessed the center and time frame of included studies with the aim of excluding articles with overlapping cohorts and identifying the most recent and complete studies. We included studies on pipeline shield devices for treating intracranial aneurysms and pooled data on aneurysm occlusion rates, procedural complications, and mortality. The initial search results and screening process are shown in a PRISMA-based (9) flowchart (Figure 1).
Data selection
We extracted the following data from the included studies: the number of patients, sex ratio, mean age, total number of aneurysms, proportion of ruptured aneurysms at presentation, sizes and neck width of aneurysms, shapes of aneurysms (i.e., blister, fusiform, pseudoaneurysm, or dissecting), locations of aneurysms, devices per aneurysm, mortality rates, morbidity rates, adequate occlusion rate, antiplatelet regimens, and usage of detachable devices.
Statistical analysis
We used the R package “META” (https://cran.r-project.org) to analyze the acquired data. We calculated proportions across studies and performed meta-analyses using fixed- and random-effects (RE) models for the weighted estimation of the overall rates of each outcome of interest (i.e., periprocedural and postoperative complications, adequate occlusion, and mortality). We also estimated 95% confidence intervals (CIs) and event rates for each outcome. I2 statistics were used to assess statistical heterogeneity between studies. For data with I2 heterogeneity values >50%, RE models were used. Forest plots were generated based on the proportions and estimated overall rates (Figure 2). Subgroup analyses were conducted using Stata 14.0.
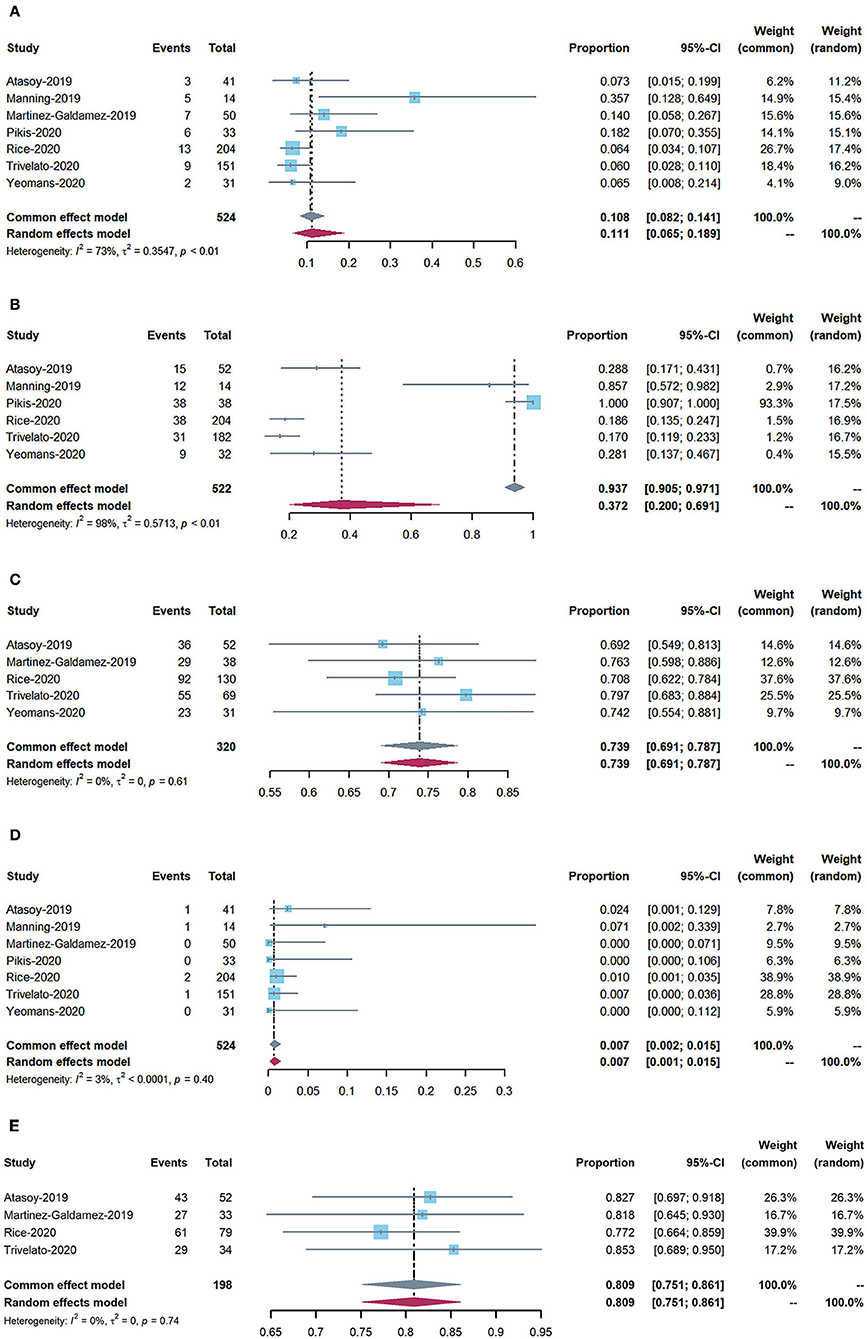
Figure 2. Forest plots: (A) periprocedural and postoperative complications; (B) use of adjunctive coiling; (C) adequate occlusion at 6-month follow-up (defined as Raymond–Roy class 1, O'Kelly–Marotta grade D, or Kamran grade 4); (D) mortality; and (E) adequate occlusion rate of more than 12 months follow-up.
Results
The preliminary search results contained 67 articles, 30 of which were duplicates. Ultimately, seven articles were selected for further analysis.
Study characteristics
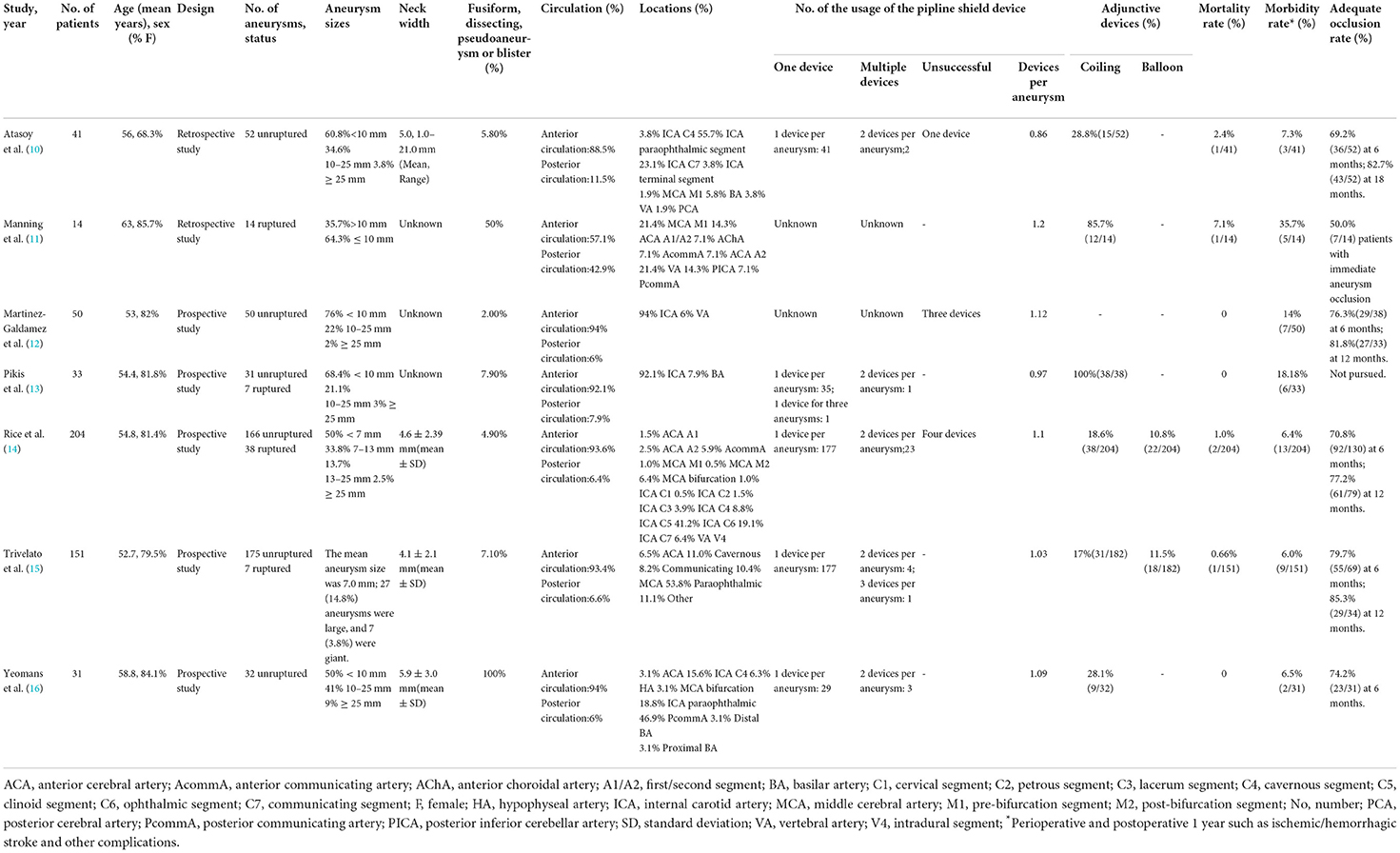
The characteristics of all included studies are presented in Table 2. Of the seven studies, two were retrospective (10, 17) and five were prospective (12–16). Adjunctive coiling was used in six studies, two of which also used adjunctive balloons. One study used the pipeline shield exclusively. A total of 524 patients with 572 intracranial aneurysms were included. A total of 11.5% of the aneurysms had ruptured before treatment. Most aneurysms were in the anterior rather than posterior circulation (92.1 vs. 7.9%). Aneurysm morphology was identified for all 572 aneurysms: 87.9% were saccular, with the remainder being fusiform, dissecting, blister, or pseudoaneurysms. Table 2 details aneurysm body diameter, neck dilation extent, and parent artery data.
Complications and mortality
The rate of perioperative and postoperative complications was 11.1% (95% CI: 6.5–18.9%). The overall mortality rate was 0.7% (95% CI: 0.2–1.5%).
Angiographic outcomes
The rate of adequate occlusion at 6-month follow-up was 73.9% (95% CI: 69.1–78.7%). The adequate occlusion rate of more than 12 months was 80.9% (95% CI: 75.1–86.1%). Moreover, the rate of adjunctive coiling use was 37.2% (95% CI: 20–69.1%).
Subgroup analysis
Subgroup analysis showed that, in the unruptured aneurysm group, the adequate occlusion rate was 80.6% (ES = 80.6%, 95% CI: 73.4–87.8%, I2 = 0%, p = 0.652; Figure 3A), the morbidity rate was 8.8% (ES = 80.6%, 95% CI: 3.8–13.8%, I2 = 0%, p = 0.463; Figure 3B), and the mortality rate was 0.4% (ES = 0.4%, 95% CI: 0.0–3.0%, I2 = 0%, p = 0.001; Figure 3C). The adequate occlusion rate, morbidity rate, and mortality rate in the ruptured aneurysm group were 50.0, 35.7, and 7.1%, respectively (Figure 3). Although the overall tendencies are noteworthy, the evidence is insufficient to draw any final conclusions.
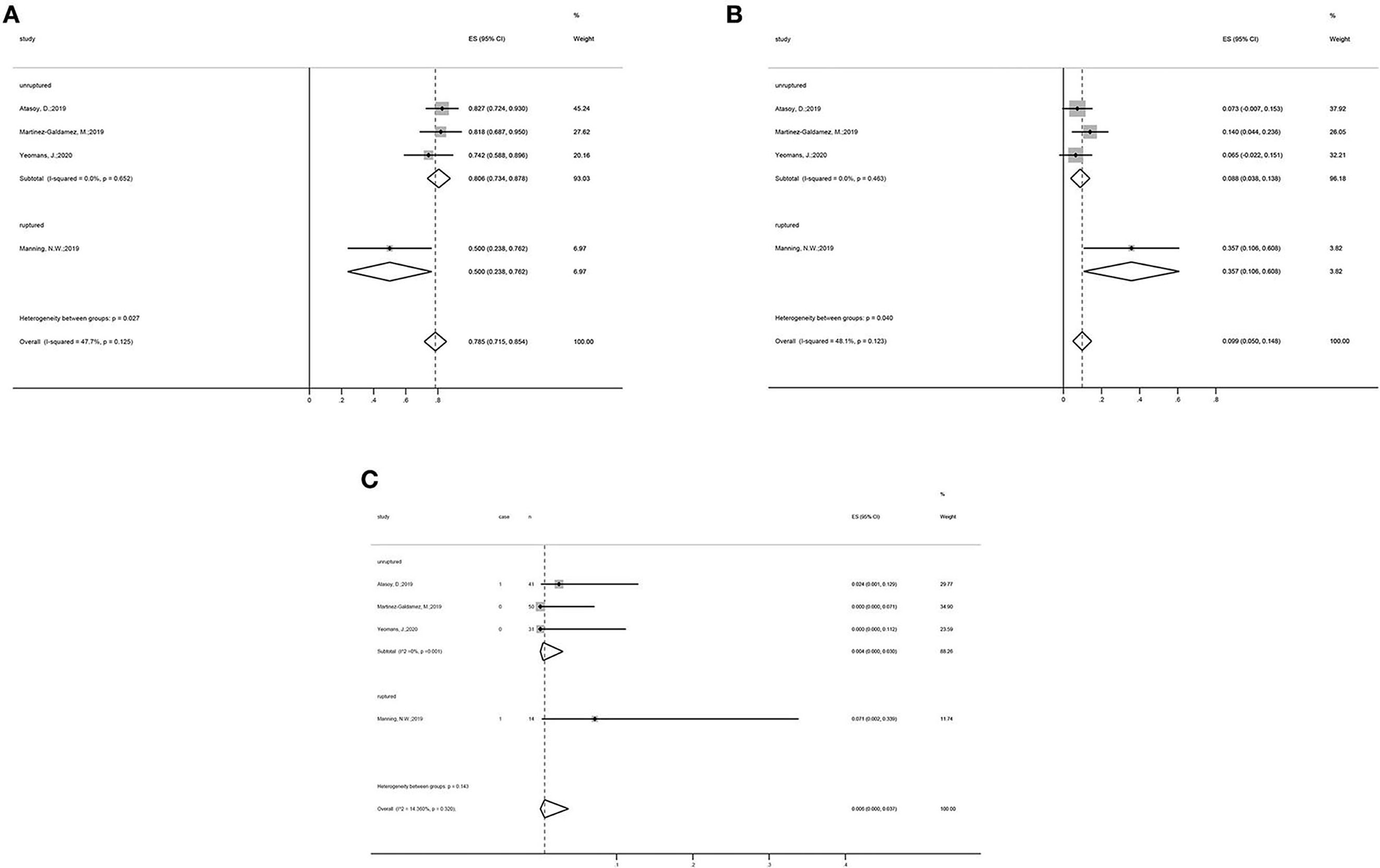
Figure 3. Subgroup analysis forest plots: Subgroup analysis on the (A) adequate occlusion rate, (B) morbidity rate, and (C) mortality rate, all categorized by the status of aneurysms (unruptured vs. ruptured group).
Discussion
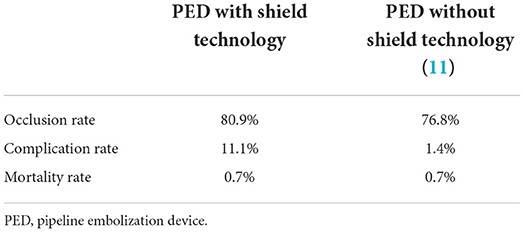
FDs are new important tools for treating intracranial aneurysms (18). Considering the novelty of these devices, the risk of thromboembolic events post-implant remains a concern. It is known that patients who have undergone flow shunt placement should be treated with prolonged dual antiplatelet therapy (DAPT) to prevent thrombosis. The pipeline shield is a surface-coated device that improves the hemocompatibility of PEDs and has been shown to reduce surface platelet and fibrin adhesion as well as thrombin generation (4, 7, 19). In our review, these benefits were indirectly verified. Compared to PEDs without shield technology (11), the pipeline shield was found to be associated with higher adequate occlusion and lower mortality rates (Table 3).
Few studies were controlled according to the rupture status of the aneurysms. In fact, the primary treatment for ruptured aneurysms, including antiplatelet and endovascular therapies, differs from that for unruptured aneurysms. For unruptured aneurysms, in addition to encouraging patients to quit smoking and control their blood pressure, clinical decisions are made using PHASES and unruptured intracranial aneurysm treatment scores (20). Unruptured aneurysms show that short-term growth should be treated rapidly (21). Ruptured aneurysms must be treated surgically. In these patients, in addition to basic supportive care, early aneurysm occlusion is critical (22, 23). The choice of treatment depends on the overall condition of the patient, the characteristics of the aneurysm, the presence of associated hematomas and mass effects, and the overall microsurgical and endovascular expertise of the treatment center.
The pipeline shield appears to have similar outcomes to those of other well-established and more widely used FDs. In a study evaluating Silk FDs, Florez et al. reported a mortality rate of 2.8%, total thromboembolic complication rate of 6.06%, and complete aneurysm occlusion rate of 80.4% (24). In another systematic review, the rate of complete or near-total occlusion of small intracranial aneurysms treated with a Silk Vista Baby FD was 72.1% at early follow-up. The postoperative mortality rate was 2.5%, including neurological death in three cases (1.8%) (25). Asnafi et al. reported that the rate of midterm complete occlusion of the Woven EndoBridge device was 22% in an unruptured aneurysm group compared with 45% in a ruptured group. Perioperative morbidity was 4%, and perioperative mortality was 1% (26). In a meta-regression
analysis predicting aneurysm treatment outcomes with PEDs, the estimated aneurysm occlusion rate was 76%, and the estimated death and modified Rankin Scale ≤ 2 rates at unspecified follow-up times were 2 and 92%, respectively (27). Wakhloo et al. performed a study evaluating Surpass devices and found intraprocedural in-stent clot formation in 3.7% of patients. The overall morbidity rate was 6%, and the mortality rate was 2.7% (28). In another systematic review on the utilization of pipeline flex devices for treating unruptured intracranial aneurysms, a low periprocedural risk of death (0.8%) or major complications (1.8%) was reported. The risk of major complications occurring was significantly higher for large/giant aneurysms (4.4%) than for small aneurysms <10 mm (0.9%) (29). Bhatia et al. performed a systematic review on the utilization of flow redirection endoluminal devices for treating intracranial aneurysms and reported that the occlusion rate between 4 and 6 months was 73.8%, the overall reported morbidity rate was 3.9%, and procedure-related mortality was 1.4%. Complication rates fell into five categories: technical (3.6%), ischemic (3.8%), thrombotic or stenotic (6%), hemorrhagic (1.5%), and non-neurological (0.8%) (30). The DED is another surface-modified FD. In a meta-analysis of its utilization, the rate of periprocedural ischemic and hemorrhagic complications was 4.9%, the complete angiographic occlusion rate was 81.4%, and the mortality rate was 2.1% (31). Moreover, Li et al. performed a meta-analysis on the outcome of FDs with surface modifications and determined that the rate of aneurysm occlusion was 80.5% at 6 months and 85.6% at 12 months. The pooled estimate for the total ischemia rate was 6.7%, of which the severe ischemia rate was 1.8%. Morbidity and mortality rates were 6.0 and 0.7%, respectively (32).
When we collated the data, we found that some aneurysms were treated using adjunctive devices in addition to FDs, but details about the patients requiring adjunctive devices were not provided; thus, we could not analyze whether such devices were beneficial. However, in a study on pipeline-assisted coiling vs. pipeline in FDs for treating intracranial aneurysms, the authors reported that joint PED and coiling were safe with no increase in complications when compared with PED alone. Aneurysm occlusion rates and functional outcomes with PED and coiling remained comparable to those of treatment with PED alone (33). Atassoy et al. purported that putative occlusion rate differences were unlikely to be caused by a difference in adjunctive coiling (10). The rates of adjunctive coil use did not appear beneficial for aneurysm occlusion, and evidence for potential benefits is currently lacking (33). Interestingly, adjunctive coiling may be more helpful for preventing aneurysm rupture during thrombosis than for increasing the occlusion rate. Moreover, additional overlapping devices may increase coverage by increasing mesh density, thereby affecting occlusion rate. In endovascular treatments, the aneurysm sac diameter may influence the occlusion rate, especially in aneurysm coiling. As mentioned above, however, a meta-analysis on FDs revealed no relationship between the sac diameter of aneurysms and occlusion rates (34). Compared with the coils alone, combining other techniques can treat complex aneurysms and reduce the recurrence rates. In a study by Lin et al., coils in conjunction with a PED yielded higher aneurysm occlusion rates and reduced the need for retreatment (35). Because FDs cannot provide direct dome protection, large and giant aneurysms could take longer to completely occlude when treated with percutaneous endovascular embolization alone (36). Therefore, until total occlusion is achieved, these aneurysms remain at risk of rupture during the follow-up period (37, 38). In addition, studies have found intraoperative device prolapse and postoperative device displacement/shortening (39, 40), which may lead to rupture and the need for retreatment (40). Therefore, for aneurysms at risk of imminent rupture, the combined use of coils and PEDs may be more effective and provide additional mechanical support, thereby reducing the risk of device dislocation and need for retreatment.
In a meta-analysis evaluating the efficacy of FDs in posterior compared to anterior circulation aneurysms, posterior circulation aneurysms were found to be effectively treated using FDs, with comparable occlusion rates to those in anterior circulation aneurysms. However, the risk of periprocedural complications was not negligible (41). Early studies have reported higher complication rates associated with the use of FDs in the posterior circulation (42–45). This may be due to the presence of numerous perforating arteries supplying the brainstem (46). We could not compare the treatment effects between anterior and posterior circulation aneurysms because we were unable to obtain more detailed information.
Owing to the complexity of patients' conditions and disagreements on antiplatelet regimens for pipeline shield utilization, protocols for antiplatelet therapy among the trials included in our review were not uniform (Table 4). The FDs need DAPT to prevent thrombosis and ischemic complications. However, DAPT increases the risk of hemorrhagic complications (47). Studies have shown that the pipeline shield can reduce platelet adhesion to the surface (19, 48, 49). In vivo, single antiplatelet therapy with pipeline shield had similar thrombogenicity to that of DAPT with PED-Flex (4). Therefore, pipeline shield devices may reduce the need for antiplatelet drugs, thereby reducing the risk of hemorrhage. The role of antiplatelet and anticoagulant medications in treating unruptured aneurysms has been controversial. Retrospective studies have reported that patients taking long-term aspirin exhibit a reduced risk of rupture, while those taking dipyridamole and new aspirin may be at risk of subarachnoid hemorrhage (50, 51). In another study, patients taking aspirin (28%) were found to have lower bleeding rates than those not taking aspirin (40%) (52). Aspirin was also not found to worsen outcomes after subarachnoid hemorrhage (51). In contrast, anticoagulants were associated with poor prognosis after subarachnoid hemorrhage (53) but did not increase the risk of aneurysm rupture (54, 55).
Our study has the following limitations. As some articles included in our review reported retrospective results based on small samples, our results may be biased. Further, as antiplatelet therapy regimens vary between studies and institutions, no reliable conclusions could be drawn regarding antiplatelet therapy.
Conclusion
Technological improvements have greatly improved endovascular treatment options for aneurysms. As a novel surface-modified PED, the pipeline shield is increasingly used to treat intracranial aneurysms. From our review, we determined that this intervention results in low rates of mortality and a high rate of occlusion.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
CL and LJin conceived the project and drafted the manuscript. EL, BL, and ZF searched the databases and analyzed data. JD, SY, PL, and LJia were responsible for the whole process of supervision. SZ and WH revised the manuscript. All authors read and approved the final version of the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant Number 81560227) and the Yunnan Health Training Project of High-level Talents (Grant Number H-2017030).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Arrese I, Sarabia R, Pintado R, Delgado-Rodriguez M. Flow-diverter devices for intracranial aneurysms: systematic review and meta-analysis. Neurosurgery. (2013) 73:193–9. doi: 10.1227/01.neu.0000430297.17961.f1
2. Ye G, Zhang M, Deng L, Chen X, Wang Y. Meta-analysis of the efficiency and prognosis of intracranial aneurysm treated with flow diverter devices. J Mol Neurosci. (2016) 59:158–67. doi: 10.1007/s12031-016-0723-x
3. Kallmes DF, Hanel R, Lopes D, Boccardi E, Bonafé A, Cekirge S, et al. International retrospective study of the pipeline embolization device: a multicenter aneurysm treatment study. AJNR Am J Neuroradiol. (2015) 36:108–15. doi: 10.3174/ajnr.A4111
4. Hagen MW, Girdhar G, Wainwright J, Hinds MT. Thrombogenicity of flow diverters in an ex vivo shunt model: effect of phosphorylcholine surface modification. J Neurointerv Surg. (2017) 9:1006–11. doi: 10.1136/neurintsurg-2016-012612
5. Caroff J, Tamura T, King RM, Lylyk PN, Langan ET, Brooks OW, et al. Phosphorylcholine surface modified flow diverter associated with reduced intimal hyperplasia. J Neurointerv Surg. (2018) 10:1097–101. doi: 10.1136/neurintsurg-2018-013776
6. Matsuda Y, Chung J, Lopes DK. Analysis of neointima development in flow diverters using optical coherence tomography imaging. J Neurointerv Surg. (2018) 10:162–7. doi: 10.1136/neurintsurg-2016-012969
7. Girdhar G, Li J, Kostousov L, Wainwright J, Chandler WL. In-vitro thrombogenicity assessment of flow diversion and aneurysm bridging devices. J Thromb Thrombolysis. (2015) 40:437–43. doi: 10.1007/s11239-015-1228-0
8. Girdhar G, Ubl S, Jahanbekam R, Thinamany S, Belu A, Wainwright J, et al. Thrombogenicity assessment of pipeline, pipeline shield, derivo and P64 flow diverters in an in vitro pulsatile flow human blood loop model. eNeurologicalSci. (2019) 14:77–84. doi: 10.1016/j.ensci.2019.01.004
9. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the prisma statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
10. Atasoy D, Kandasamy N, Hart J, Lynch J, Yang SH, Walsh D, et al. Outcome study of the pipeline embolization device with shield technology in unruptured aneurysms (Pedsu). AJNR Am J Neuroradiol. (2019) 40:2094–101. doi: 10.3174/ajnr.A6314
11. Hanel RA, Kallmes DF, Lopes DK, Nelson PK, Siddiqui A, Jabbour P, et al. Prospective study on embolization of intracranial aneurysms with the pipeline device: the premier study 1 year results. J Neurointerv Surg. (2020) 12:62–6. doi: 10.1136/neurintsurg-2019-015091
12. Martinez-Galdamez M, Lamin SM, Lagios KG, Liebig T, Ciceri EF, Chapot R, et al. Treatment of intracranial aneurysms using the pipeline flex embolization device with shield technology: angiographic and safety outcomes at 1-year follow-up. J Neurointerv Surg. (2019) 11:396–9. doi: 10.1136/neurintsurg-2018-014204
13. Pikis S, Mantziaris G, Mamalis V, Barkas K, Tsanis A, Lyra S, et al. Diffusion weighted image documented cerebral ischemia in the postprocedural period following pipeline embolization device with shield technology treatment of unruptured intracranial aneurysms: a prospective, single center study. J Neurointerv Surg. (2020) 12:407–11. doi: 10.1136/neurintsurg-2019-015363
14. Rice H, Martínez Galdámez M, Holtmannspötter M, Spelle L, Lagios K, Ruggiero M, et al. Periprocedural to 1-year safety and efficacy outcomes with the pipeline embolization device with shield technology for intracranial aneurysms: a prospective, post-market, multi-center study. J Neurointerv Surg. (2020) 12:1107–12. doi: 10.1136/neurintsurg-2020-015943
15. Trivelato FP, Wajnberg E, Rezende MTS, Ulhôa AC, Piske RL, Abud TG, et al. Safety and effectiveness of the pipeline flex embolization device with shield technology for the treatment of intracranial aneurysms: midterm results from a multicenter study. Neurosurgery. (2020) 87:104–11. doi: 10.1093/neuros/nyz356
16. Yeomans J, Sandu L, Sastry A. Pipeline flex embolisation device with shield technology for the treatment of patients with intracranial aneurysms: periprocedural and 6 month outcomes. Neuroradiol J. (2020) 33:471–8. doi: 10.1177/1971400920966749
17. Manning NW, Cheung A, Phillips TJ, Wenderoth JD. Pipeline shield with single antiplatelet therapy in aneurysmal subarachnoid haemorrhage: multicentre experience. J Neurointerv Surg. (2019) 11:694–8. doi: 10.1136/neurintsurg-2018-014363
18. Brinjikji W, Murad MH, Lanzino G, Cloft HJ, Kallmes DF. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke. (2013) 44:442–7. doi: 10.1161/STROKEAHA.112.678151
19. Marosfoi M, Clarencon F, Langan ET, King RM, Brooks OW, Tamura T, et al. Acute thrombus formation on phosphorilcholine surface modified flow diverters. J Neurointerv Surg. (2018) 10:406–11. doi: 10.1136/neurintsurg-2017-013175
20. Etminan N, Brown RD Jr, Beseoglu K, Juvela S, Raymond J, Morita A, et al. The unruptured intracranial aneurysm treatment score: a multidisciplinary consensus. Neurology. (2015) 85:881–9. doi: 10.1212/WNL.0000000000001891
21. Brinjikji W, Zhu YQ, Lanzino G, Cloft HJ, Murad MH, Wang Z, et al. Risk factors for growth of intracranial aneurysms: a systematic review and meta-analysis. AJNR Am J Neuroradiol. (2016) 37:615–20. doi: 10.3174/ajnr.A4575
22. Connolly ES Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2012) 43:1711–37. doi: 10.1161/STR.0b013e3182587839
23. Diringer MN, Bleck TP, Claude Hemphill J 3rd, Menon D, Shutter L, Vespa P, et al. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the neurocritical care society's multidisciplinary consensus conference. Neurocrit Care. (2011) 15:211–40. doi: 10.1007/s12028-011-9605-9
24. Florez WA, Garcia-Ballestas E, Quiñones-Ossa GA, Janjua T, Konar S, Agrawal A, et al. Silk® flow diverter device for intracranial aneurysm treatment: a systematic review and meta-analysis. Neurointervention. (2021) 16:222–31. doi: 10.5469/neuroint.2021.00234
25. Hanel RA, Cortez GM, Benalia VHC, Sheffels E, Sutphin DJ, Pederson JM, et al. Patient outcomes after treatment of brain aneurysm in small diameter vessels with the silk vista baby flow diverter: a systematic review. Interv Neuroradiol. (2022). doi: 10.1177/15910199221091645
26. Asnafi S, Rouchaud A, Pierot L, Brinjikji W, Murad MH, Kallmes DF. Efficacy and safety of the woven endobridge (Web) device for the treatment of intracranial aneurysms: a systematic review and meta-analysis. AJNR Am J Neuroradiol. (2016) 37:2287–92. doi: 10.3174/ajnr.A4900
27. Beydoun HA, Azarbaijani Y, Cheng H, Anderson-Smits C, Marinac-Dabic D. Predicting successful treatment of intracranial aneurysms with the pipeline embolization device through meta-regression. World Neurosurg. (2018) 114:e938–e58. doi: 10.1016/j.wneu.2018.03.120
28. Wakhloo AK, Lylyk P, de Vries J, Taschner C, Lundquist J, Biondi A, et al. Surpass flow diverter in the treatment of intracranial aneurysms: a prospective multicenter study. AJNR Am J Neuroradiol. (2015) 36:98–107. doi: 10.3174/ajnr.A4078
29. Bhatia KD, Kortman H, Orru E, Klostranec JM, Pereira VM, Krings T. Periprocedural complications of second-generation flow diverter treatment using pipeline flex for unruptured intracranial aneurysms: a systematic review and meta-analysis. J Neurointerv Surg. (2019) 11:817–24. doi: 10.1136/neurintsurg-2019-014937
30. Waqas M, Dossani RH, Alkhaldi M, Neveu J, Cappuzzo JM, Lim J, et al. Flow redirection endoluminal device (Fred) for treatment of intracranial aneurysms: a systematic review. Interv Neuroradiol. (2021). 28:347–357. doi: 10.1136/neurintsurg-2021-SNIS.224
31. Monteiro A, Burke SM, Baig AA, Khan S, Cappuzzo JM, Waqas M, et al. A systematic review and meta-analysis of the derivo embolization device: a novel surface-modified flow diverter for intracranial aneurysm treatment. J Neurointerv Surg. (2022). doi: 10.1136/neurintsurg-2021-018390
32. Li YL, Roalfe A, Chu EY, Lee R, Tsang ACO. Outcome of flow diverters with surface modifications in treatment of cerebral aneurysms: systematic review and meta-analysis. AJNR Am J Neuroradiol. (2021) 42:327–33. doi: 10.3174/ajnr.A6919
33. Sweid A, Atallah E, Herial N, Saad H, Mouchtouris N, Barros G, et al. Pipeline-assisted coiling versus pipeline in flow diversion treatment of intracranial aneurysms. J Clin Neurosci. (2018) 58:20–4. doi: 10.1016/j.jocn.2018.10.081
34. Becske T, Kallmes DF, Saatci I, McDougall CG, Szikora I, Lanzino G, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology. (2013) 267:858–68. doi: 10.1148/radiol.13120099
35. Lin N, Brouillard AM, Krishna C, Mokin M, Natarajan SK, Sonig A, et al. Use of coils in conjunction with the pipeline embolization device for treatment of intracranial aneurysms. Neurosurgery. (2015) 76:142–9. doi: 10.1227/NEU.0000000000000579
36. Saatci I, Yavuz K, Ozer C, Geyik S, Cekirge HS. Treatment of intracranial aneurysms using the pipeline flow-diverter embolization device: a single-center experience with long-term follow-up results. AJNR Am J Neuroradiol. (2012) 33:1436–46. doi: 10.3174/ajnr.A3246
37. Kan P, Siddiqui AH, Veznedaroglu E, Liebman KM, Binning MJ, Dumont TM, et al. Early postmarket results after treatment of intracranial aneurysms with the pipeline embolization device: a U.S. multicenter experience. Neurosurgery. (2012) 71:1080–7. doi: 10.1227/NEU.0b013e31827060d9
38. Siddiqui AH, Kan P, Abla AA, Hopkins LN, Levy EI. Complications after treatment with pipeline embolization for giant distal intracranial aneurysms with or without coil embolization. Neurosurgery. (2012) 71:E509–13. doi: 10.1227/NEU.0b013e318258e1f8
39. Crowley RW, Abla AA, Ducruet AF, McDougall CG, Albuquerque FC. Novel application of a balloon-anchoring technique for the realignment of a prolapsed pipeline embolization device: a technical report. J Neurointerv Surg. (2014) 6:439–44. doi: 10.1136/neurintsurg-2013-010806
40. Chalouhi N, Tjoumakaris SI, Gonzalez LF, Hasan D, Pema PJ, Gould G, et al. Spontaneous delayed migration/shortening of the pipeline embolization device: report of 5 Cases. AJNR Am J Neuroradiol. (2013) 34:2326–30. doi: 10.3174/ajnr.A3632
41. Abdel-Tawab M, Abdeltawab AK, Abdelmonem M, Moubark MA, Taha MA, Morsy A, et al. Efficacy and safety of flow diverters in posterior circulation aneurysms and comparison with their efficacy in anterior circulation aneurysms: a systematic review and meta-analysis. Interv Neuroradiol. (2021) 27:609–21. doi: 10.1177/15910199211003017
42. Chalouhi N, Tjoumakaris S, Starke RM, Gonzalez LF, Randazzo C, Hasan D, et al. Comparison of flow diversion and coiling in large unruptured intracranial saccular aneurysms. Stroke. (2013) 44:2150–4. doi: 10.1161/STROKEAHA.113.001785
43. Siddiqui AH, Abla AA, Kan P, Dumont TM, Jahshan S, Britz GW, et al. Panacea or problem: flow diverters in the treatment of symptomatic large or giant fusiform vertebrobasilar aneurysms. J Neurosurg. (2012) 116:1258–66. doi: 10.3171/2012.2.JNS111942
44. Munich SA, Tan LA, Keigher KM, Chen M, Moftakhar R, Lopes DK. The pipeline embolization device for the treatment of posterior circulation fusiform aneurysms: lessons learned at a single institution. J Neurosurg. (2014) 121:1077–84. doi: 10.3171/2014.7.JNS132595
45. Zhang Y, Yan P, Di Y, Liang F, Zhang Y, Liang S, et al. Reconsiderations on the use of pipeline embolization device in the treatment of intracerebral aneurysms with special angioarchitecture: fetal PCA, AVM, V-B junction and DAVF. Chin Neurosurg J. (2018) 4:25. doi: 10.1186/s41016-018-0133-8
46. Patel PD, Chalouhi N, Atallah E, Tjoumakaris S, Hasan D, Zarzour H, et al. Off-label uses of the pipeline embolization device: a review of the literature. Neurosurg Focus. (2017) 42:E4. doi: 10.3171/2017.3.FOCUS1742
47. Cagnazzo F, Di Carlo DT, Petrella G, Perrini P. Ventriculostomy-related hemorrhage in patients on antiplatelet therapy for endovascular treatment of acutely ruptured intracranial aneurysms. A Meta-Analysis Neurosurg Rev. (2020) 43:397–406. doi: 10.1007/s10143-018-0999-0
48. Campbell EJ, O'Byrne V, Stratford PW, Quirk I, Vick TA, Wiles MC, et al. Biocompatible surfaces using methacryloylphosphorylcholine laurylmethacrylate copolymer. ASAIO J. (1994) 40:M853–7. doi: 10.1097/00002480-199407000-00118
49. Matsuda Y, Jang DK, Chung J, Wainwright JM, Lopes D. Preliminary outcomes of single antiplatelet therapy for surface-modified flow diverters in an animal model: analysis of neointimal development and thrombus formation using Oct. J Neurointerv Surg. (2019) 11:74–9. doi: 10.1136/neurintsurg-2018-013935
50. Schmidt M, Johansen MB, Lash TL, Christiansen CF, Christensen S, Sørensen HT. Antiplatelet drugs and risk of subarachnoid hemorrhage: a population-based case-control study. J Thromb Haemost. (2010) 8:1468–74. doi: 10.1111/j.1538-7836.2010.03856.x
51. Toussaint LG 3rd, Friedman JA, Wijdicks EF, Piepgras DG, Pichelmann MA, McIver JI, et al. Influence of aspirin on outcome following aneurysmal subarachnoid hemorrhage. J Neurosurg. (2004) 101:921–5. doi: 10.3171/jns.2004.101.6.0921
52. Brown RD Jr, Broderick JP. Unruptured intracranial aneurysms: epidemiology, natural history, management options, and familial screening. Lancet Neurol. (2014) 13:393–404. doi: 10.1016/S1474-4422(14)70015-8
53. Rinkel GJ, Prins NE, Algra A. Outcome of aneurysmal subarachnoid hemorrhage in patients on anticoagulant treatment. Stroke. (1997) 28:6–9. doi: 10.1161/01.STR.28.1.6
54. Wiebers DO, Whisnant JP, Huston J 3rd, Meissner I, Brown RD Jr, Piepgras DG, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. (2003) 362:103–10. doi: 10.1016/S0140-6736(03)13860-3
Keywords: flow diverters, pipeline embolization device with shield technology, pipeline shield, intracranial aneurysm, endovascular therapy
Citation: Luo C, Jin L, Dong J, Fu Z, Liu E, Yin S, Jian L, Luo P, Liu B, Huang W and Zhou S (2022) Clinical outcomes of pipeline embolization devices with shield technology for treating intracranial aneurysms. Front. Neurol. 13:971664. doi: 10.3389/fneur.2022.971664
Received: 17 June 2022; Accepted: 24 October 2022;
Published: 14 November 2022.
Edited by:
Jianmin Liu, Second Military Medical University, ChinaReviewed by:
Yueqi Zhu, Shanghai Jiao Tong University, ChinaHua Lu, Nanjing Medical University, China
Shiwei Du, South China Hospital of Shenzhen University, China
Copyright © 2022 Luo, Jin, Dong, Fu, Liu, Yin, Jian, Luo, Liu, Huang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuai Zhou, enNodWFpX2ttdXN0QDE2My5jb20=; Wei Huang, MTI1ODg4NDk4QHFxLmNvbQ==
†These authors have contributed equally to this work
 Chao Luo
Chao Luo Lide Jin1†
Lide Jin1† Zaixiang Fu
Zaixiang Fu Erheng Liu
Erheng Liu Pengren Luo
Pengren Luo Bo Liu
Bo Liu Shuai Zhou
Shuai Zhou