
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol., 20 September 2022
Sec. Multiple Sclerosis and Neuroimmunology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.970383
This article is part of the Research TopicCNS autoimmune disorders and COVID-19View all 12 articles
Background: Viral infections are a proposed possible cause of inflammatory central nervous system (CNS) demyelinating diseases, including multiple sclerosis (MS), neuromyelitis optica spectrum disorder (NMOSD), and myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD). During the past 2 years, CNS demyelinating events associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection have been reported, but causality is unclear.
Objective: To investigate the relationship between CNS demyelinating disease development and exacerbation with antecedent and/or concurrent SARS-CoV-2 infection.
Methods: A systematic literature review of all publications describing either a new diagnosis or relapse of CNS demyelinating diseases (MS, NMOSD, MOGAD) in association with SARS-CoV-2 infection was performed utilizing PRISMA guidelines. Descriptive statistics were used for data analysis, using a case analysis approach.
Results: Sixty-seven articles met the inclusion criteria for the study. Most of the reported cases of NMOSD (n = 13, 72.2% of reported cases) and MOGAD (n = 27, 96.5% of reported cases) were of new disease onset, presenting with typical clinical and radiographic features of these conditions, respectively. In contrast, reported MS cases varied amongst newly diagnosed cases (n = 10, 10.5% of reported cases), relapses (n = 63, 66.4%) and pseudo-relapses (n = 22, 23.2%). The median duration between COVID-19 infection and demyelinating event onset was 11.5 days (range 0–90 days) in NMOSD, 6 days (range−7 to +45 days) in MOGAD, and 13.5 days (range−21 to +180 days) in MS. Most cases received high-dose corticosteroids with a good clinical outcome.
Conclusion: Based upon available literature, the rate of CNS demyelinating events occurring in the setting of preceding or concurrent SARS-CoV-2 infection is relatively low considering the prevalence of SARS-CoV-2 infection. The clinical outcomes of new onset or relapsing MS, NMOSD, or MOGAD associated with antecedent or concurrent infection were mostly favorable. Larger prospective epidemiological studies are needed to better delineate the impact of COVID-19 on CNS demyelinating diseases.
Multiple sclerosis (MS), neuromyelitis optica spectrum disorder (NMOSD), and myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) are immune-mediated inflammatory demyelinating diseases of the central nervous system (CNS). While the cause of these conditions is unknown, it is proposed that an interaction between genetic predisposition and behavioral, environmental, and personal factors contribute to disease development. Among the environmental factors involved, viral infections are considered a possible triggering factor.
Prior studies have shown a higher rate of multiple sclerosis (MS) exacerbation in temporal association with viral infections, especially upper respiratory tract infections caused by influenza A virus and Epstein Barr virus (EBV) (1). EBV has also been proposed as a causal agent in the onset of MS (2, 3). Likewise, preceding infections have been proposed as a possible trigger for the induction of pathogenic mechanisms leading to the development of NMOSD and MOGAD (4–10).
During the past 2 years, neurological complications associated with SARS-CoV-2 infection, the aetiologic agent of the coronavirus disease 2019 (COVID-19), have been reported. Some of these complications are thought to be caused by direct damage to the nervous system as a result of direct viral invasion (11). However, in most cases, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) CSF RNA test is negative, and an immune-mediated mechanism is postulated (12–15). In this latter category, reports of MS, NMOSD, and MOGAD cases presenting either as new diagnoses or disease relapses in temporal association with COVID-19 have been accumulating.
This systematic review aims to summarize the available data regarding the occurrence of new disease onset and disease exacerbation of MS, NMOSD, and MOGAD associated with SARS-CoV-2 infection.
This systematic literature review was performed utilizing PRISMA guidelines. Electronic searches for published literature were conducted by a medical librarian using Ovid MEDLINE (1946 to present), Embase.com (1947 to present), and Web of Science (1900 to present). The searches were run in December 2021. A search update was run in May 2022.
The search strategy incorporated controlled vocabulary and free-text synonyms for the concepts of multiple sclerosis (MS), neuromyelitis optica spectrum disorder (NMOSD), myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD), relapse, new diagnosis, and COVID-19. The full database search strategies are documented in Appendix 1. No restrictions on language or any other search filters were applied. All identified studies were combined and de-duplicated in a single reference manager (EndNote). The citations were then uploaded into Covidence systematic review software.
The full reference list of all selected papers was screened for additional relevant sources. Publications meeting the purpose of the review that were not identified through the initial electronic search were added manually to the final review. The paper selection and data extraction process were carried out independently by two authors (IL and SN), with a third author available in case of disagreements.
To ensure maximal coverage of the currently available data pertinent for the topic of this review, we included all available case reports, case series, and cohort studies that met the pre-defined case selection criteria, presented either as manuscripts in peer-reviewed scientific journals or as posters or oral presentations in a scientific congress.
Descriptive statistics was used to present the data from reported cases, using a case analysis approach. Cases with missing data points were excluded from the analysis of the missing variable.
We included patients of any age with confirmed COVID-19 and case description consistent with a new diagnosis or a relapse of MS, NMOSD, or MOGAD, in accordance with the 2017 revised McDonald criteria for MS (16), the 2015 international consensus diagnostic criteria for NMOSD (17), and the international recommendations on the diagnosis of MOGAD (18), respectively. Patients fulfilling a diagnosis of clinically isolated syndrome (CIS), considered as having a high likelihood of MS, were included as well. A relapse was defined as a clinical episode reflecting a focal or multifocal CNS demyelinating event lasting at least 24 h, in the absence of fever or active infection (16). When such an event was reported during an acute febrile state related to COVID-19, it was regarded as a pseudo-relapse, even when considered a relapse in the original publication.
COVID-19 cases were included if meeting one of the following criteria, as defined by the United States Centers for Disease Control and Prevention and the Infectious Diseases Society of America: (1) clinical symptoms consistent with COVID-19 without laboratory confirmation in the absence of an alternative explanation, (2) nasopharyngeal swab positive for COVID-19 PCR with or without symptoms, or (3) positive COVID-19 serologies with or without symptoms (19, 20).
No assumptions were made regarding the duration between COVID-19 and the onset of neurological manifestations. Missing data was noted as not available.
Cases describing clinical manifestations consistent with demyelinating events of the CNS (i.e., optic neuritis, transverse myelitis, acute disseminated encephalomyelitis, etc.) not fulfilling the diagnostic criteria for MS, NMOSD, or MOGAD as described above, were excluded from this review. Papers reporting a suspected diagnosis of COVID-19 that do not fulfill the diagnostic criteria described above, and papers not available for full-text review were also excluded.
Sixty-seven articles were included in the final review. Twelve articles describe post-COVID-19 NMOSD (21–32), 25 describe post-COVID-19 MOGAD (33–56), and 29 describe post-COVID-19 MS (57–85). One paper describes three patients with post-COVID-19 demyelinating events, of which one is NMOSD, one- MOGAD, and one- clinically isolated syndrome (CIS) (86). Another paper describes various CNS inflammatory diseases, of which three were MOGAD and one—NMOSD (87). A PRISMA flow chart illustrating the article selection process is presented in Figure 1.
Cases of post-COVID-19 NMOSD are summarized in Table 1.
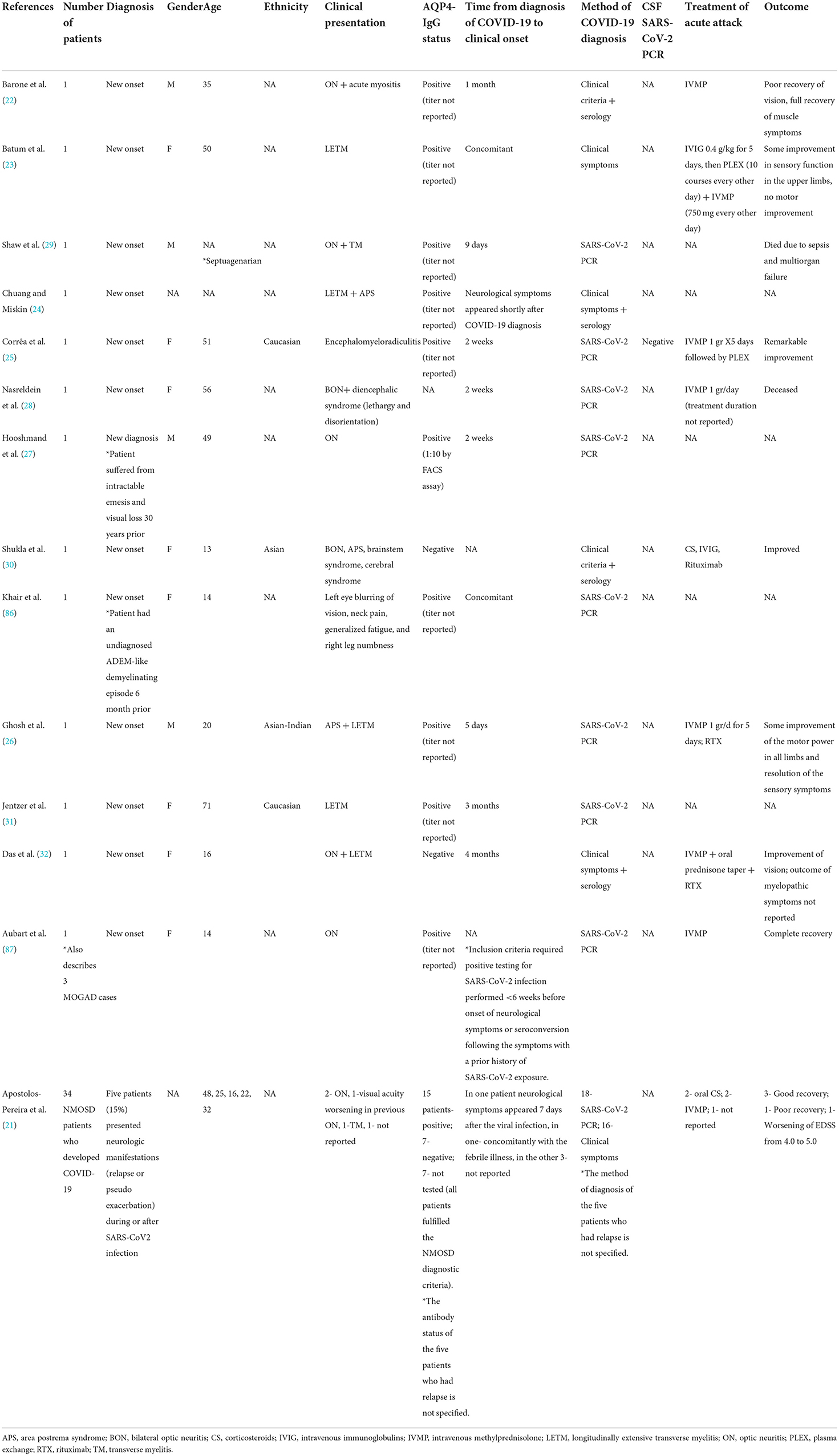
Table 1. COVID-19 and NMOSD: Cases of para- and post-infectious disease development, relapse or pseudo-relapse.
Collectively, 13 case reports and one case series describe the occurrence of 18 NMOSD-related clinical events in the context of COVID-19 (21–32, 86, 87). Eight patients were females, four were males, and in six cases the patients' sex was not reported. The mean age was 33.24 ± 18.5 years.
Ten case reports describe the onset of newly diagnosed NMOSD in people without previous neurological disease (22–26, 28–30, 32, 87). Two case reports describe people with previously undiagnosed neurological disease who then presented with a second clinical manifestation in temporal association to COVID-19, leading to an NMOSD diagnosis (27, 86). In one case, the aquaporin-4 antibodies (AQP4 Abs) were retrospectively found to be positive in a stored serum sample drawn 11 months before SARS-CoV-2 infection and more than a year before the clinical onset of NMOSD (31). Apostolos-Pereira et al. report a series of 34 NMOSD patients who developed COVID-19. Five of these patients (15%) developed neurological manifestations that were regarded as relapse or pseudo-relapse during or after SARS-CoV2 infection (21).
In 10 case reports, AQP4-IgG Abs were positive (22–27, 29, 31, 86, 87). In two case reports the AQP4 abs were negative (30, 32) and each fulfilled the diagnostic criteria for seronegative NMOSD. In one report, AQ4 serostatus was not reported (28). In the case series by Apostolos-Pereira et al., 15 patients tested positive for the AQP4-IgG Abs, 7 tested negative, and in 7 the antibody testing was not available (all patients fulfilled the NMOSD diagnostic criteria). The AQP4 antibody status of the five patients who had a relapse was not specified (21).
Neurological symptoms appeared after a median of 11.5 days (range 0–90 days) from COVID-19 diagnosis. In all cases, COVID-19 symptoms preceded the occurrence of neurological symptoms.
Treatment consisted of corticosteroid (CS) monotherapy in seven cases (21, 22, 28, 87), CS + rituximab in two cases (26, 32), intravenous methylprednisolone (IVMP)+ intravenous immunoglobulin (IVIG)+plasma exchange (PLEX) in one case (23), CS +IVIG+ rituximab in one case (30), and CS + PLEX in one case (25). In the remaining six cases, the treatment regimen was not reported (21, 24, 27, 29, 86). A favorable outcome (i.e., improvement of neurological symptoms) was reported in eight patients (21, 25, 26, 30, 32, 87), while poor neurological outcome (i.e., worsening of neurological disability) was reported in four patients (21–23). Two patients deceased due to systemic complications (28, 29). The clinical outcome was not reported for the remaining four patients (24, 27, 31, 86).
Post-COVID-19 MOGAD cases are summarized in Table 2.
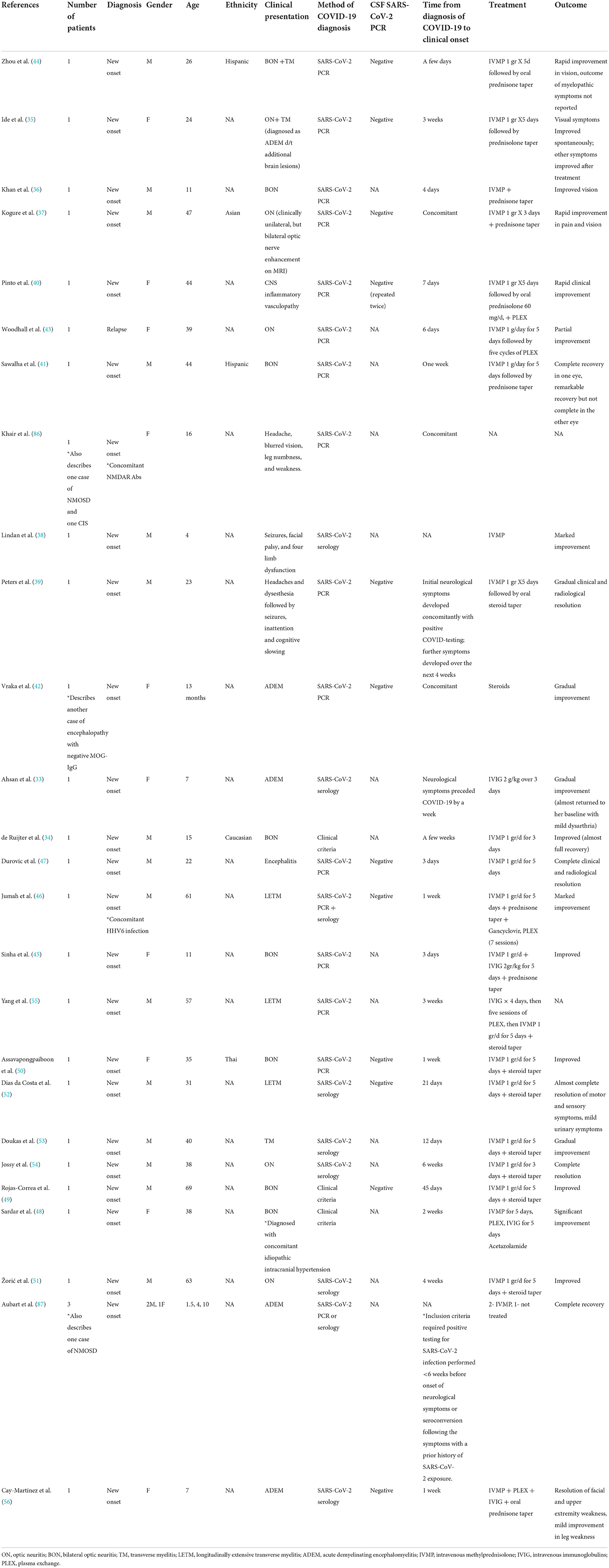
Table 2. COVID-19 and MOGAD: Cases of para- and post-infectious disease development or exacerbation.
A total of 28 cases of MOGAD occurring in temporal relation to COVID-19 have been described (33–47, 56, 86, 87). Seventeen were males, and 11 were females. The mean age was 28.1 ± 20.3 years (range 1–69 years; 10 patients <18 years old). The median time between COVID-19 and neurological symptoms was 6 days (range−7-+45 days). In one case, the neurological symptoms preceded the diagnosis of COVID-19 by 1 week (33). In four cases, neurological symptoms developed concomitantly with COVID-19 (37, 39, 42, 86), and in the remaining 23, COVID-19 diagnosis preceded the onset of neurological symptoms. In 27 (96.5%), a new diagnosis of MOGAD was made in people without prior neurological disease. In one case, a relapse occurred in a patient with known MOGAD (43). The MOG-IgG antibodies were positive in all cases. In one case, NMDAR antibodies and MOG-IgG antibodies were detected concomitantly (86). In another case, human herpes virus 6 (HHV6) PCR was also positive (46). Eighteen patients were treated with CS alone (intravenous methylprednisolone followed by oral prednisone taper, n = 15; IVMP alone, n = 2; details of steroid regimen were not described, n = 1) (42). One patient was treated with intravenous immunoglobulins (IVIG) alone (33). One patient received IVMP+ IVIG (45), three received IVMP + PLEX (40, 43, 46), and three received IVMP+ PLEX+ IVIG (48, 55, 56). One patient was not treated (87). The treatment regimen was not described for one patient (38). Clinical improvement was reported for 26 patients (93%).
Table 3 illustrates MS cases occurring in the context of COVID-19.
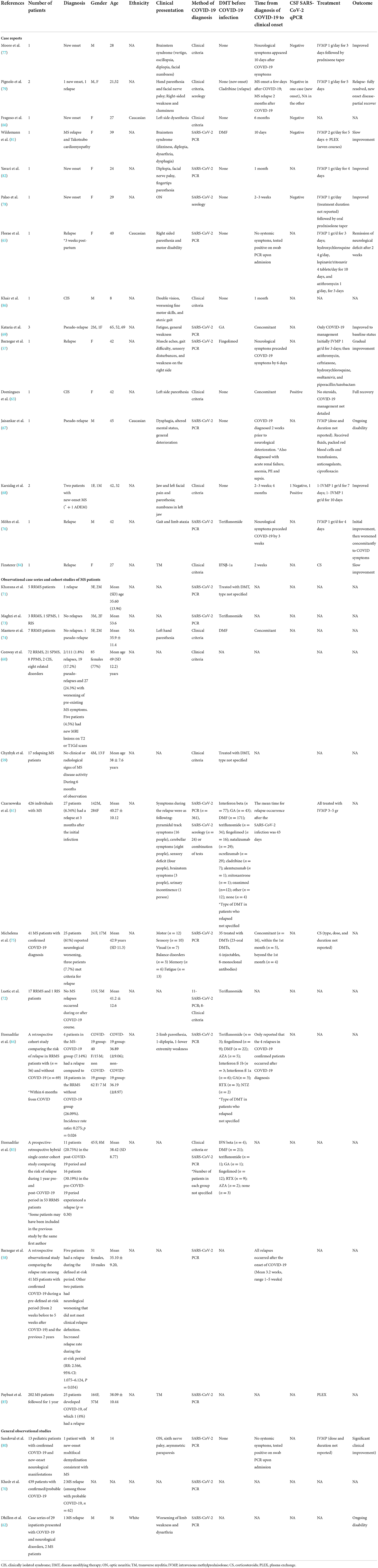
Table 3. COVID-19 and MS: Cases of para- and post-infectious disease development, relapse or pseudo-relapse.
Fifteen case reports and case series reported the occurrence of MS relapse/pseudo-relapse or the onset of a first demyelinating event consistent with MS or CIS in 19 patients (57, 63, 65–69, 76–79, 81, 82, 84, 86). Twelve observational case series and cohort studies documented the occurrence of relapses or pseudo-relapses among patients with a known diagnosis of MS and confirmed diagnosis of COVID-19 (58–61, 64, 71–75, 83, 85). Collectively, 54 relapses and 20 pseudo-relapses were reported in 804 patients (6.7 and 2.5%, respectively).
Three observational cohort studies of COVID-19 patients with various neurological manifestations reported MS cases (62, 70, 80). Overall, one case of multifocal demyelination consistent with MS and three MS relapses were reported among 481 patients (0.8%).
Considering all the reported cases, a total of 73 demyelinating events consistent with CIS/MS (10 new diagnoses and 63 relapses) and 22 events defined as pseudo-relapse were reported in 1,305 people (5.6 and 1.7%, respectively). Of these 73 events, 11 were in females, 10 in males, and sex was not reported in the remaining 64 cases. The mean age was 38.45 ± 15.93 years. Most relapses or first demyelinating events consistent with MS/CIS occurred after the onset of COVID-19. However, in two cases neurological symptoms preceded the diagnosis of COVID-19 by 6 and 21 days, respectively (57, 76). The median time from COVID-19 diagnosis to demyelinating event onset was 13.5 days (range−21-180 days).
Nine hundred eighty-six people with a known MS and COVID-19 diagnosis were reported. Of these, 624 (63.3%) were treated with various disease-modifying treatments (DMTs), 14 (1.5%) were not treated, and for 165 (16.7%), the information on DMTs was not reported. Twenty-one MS patients treated with DMTs (3.4%) had a relapse in temporal association with COVID-19. Five MS patients treated with DMTs (0.8%) had a pseudo relapse, and 144 (23.1%) did not have neurological worsening. The remaining 455 MS patients treated with DMTs (73%) were reported in larger cohorts in which some people were not treated. The information regarding relapses in these cohorts was not stratified between treated and untreated patients (61, 75).
Most MS/CIS cases received treatment with IVMP 1 gram for 3–5 days (57, 61, 65, 68, 76, 77, 79, 80, 82) and had a favorable outcome (57, 65, 68, 77–82). Treatment of pseudo-relapses was primarily focused on COVID-19 management, with return to baseline neurological status upon infection recovery (63, 69).
This systematic review summarizes the currently available data on the occurrence of demyelinating CNS events in the context of COVID-19. As noted, the vast majority of NMOSD and MOGAD cases represent newly diagnosed cases presenting with the typical clinical, radiological, and laboratory findings associated with these two disorders. In contrast, the MS cases described vary between newly diagnosed cases, relapses, and pseudo-relapses. The patients' age of diagnosis in the three disease groups was relatively similar to the age of diagnosis reported in the literature for non-COVID-19 related cases. The clinical presentations and treatment approach were also similar to non-COVID-19 related cases (for further details, please see Tables 1–3).
Several mechanisms involved in the pathogenesis of demyelinating events in the context of SARS-CoV-2 infection have been proposed. These may be related to either direct viral neurotropism or induction of aberrant immune response. The neurotrophic features of the Coronavirus family have been previously reported for the Middle East respiratory syndrome coronavirus (MERS-COV) and SARS-COV-1, and similar evidence has been occasionally reported for SARS-CoV-2 (88–91). However, the fact that the SARS-CoV-2 PCR test in the CSF was negative in many of the reported cases (25, 35, 37, 39, 40, 42, 44, 66, 68, 77–79, 81) would argue against this mechanism of direct pathogenicity. Conversely, some evidence favors the theory of para-infectious or post-infectious immune-mediated etiology. In fact, SARS-CoV-2 infection leads to hyperactivation of pro-inflammatory T cells resulting in increased levels of inflammatory cytokines and chemokines (92) and decreased regulatory T cells to impair immune response (93). The resulting pro-inflammatory hyperimmune state may activate specific immune-mediated mechanisms resulting in CNS inflammation and damage. The favorable response to immunotherapy in the majority of the reported cases appears to support this theory.
The distinction between relapse and pseudo-relapse may not always be straightforward. According to the 2017 McDonald criteria, a relapse should be defined in the absence of fever or acute infection; hence, new or worsening neurological symptoms developed during a febrile illness or in the presence of acute infection in a patient with a known diagnosis of MS should not be defined as true relapse, but rather regarded as a pseudo-relapse. However, there may be situations where the diagnosis of true relapse should still be considered even in the context of acute infection. For example, a true relapse should be considered if the onset of new symptoms is associated with clinical signs that can be attributed to a specific anatomical localization that has not been previously described or correlated with the presence of a new symptomatic MRI lesion. Following this rationale, a few of the described clinical worsening in MS cases were felt to be better classified as pseudo-relapses (67, 69).
Prior studies propose that MS relapses in temporal association with viral infections occur between 1 and 2 weeks before infection to 3–5 weeks after (94–98). Andersen et al. and Correale et al. reported that the highest frequency of relapses and infection-related MS attacks occurred during the first 2 weeks after infection onset (94). In the series reported by Sibley et al., the median time between the onset of infection and occurrence of MS exacerbation was 8 days (95). Buljevac et al. reported a mean duration of 9.5 days between the onset of infection and clinical MS exacerbation. Both Buljevac et al. and Correale et al. also compared the relapse rate ratio during different time intervals and found that the highest rate ratio was observed from weeks 1 to 4, while the exacerbation rate ratio for weeks 3–5 was lower and not statistically significant compared to the non-at risk period (97, 98). Considering these data, MS relapses occurring more than 4–5 weeks from an infection are probably not related to the prior infectious insult. Therefore, MS cases that occurred >6 weeks from COVID-19 (64, 66, 68, 79, 83), although included in this review in order to provide a comprehensive review of available data, are thought to be more likely coincidental and not related to the preceding infectious insult. Likewise, the relation between SARS-CoV-2 infection and the NMOSD and MOGAD cases developing >6 weeks after the infection (31, 32, 49, 54) remains uncertain. The case of MOGAD occurring in temporal association to both HHV6 and COVID-19 infection (46) may also confound the association between COVID-19 and MOGAD.
The use of disease-modifying therapies (DMTs) may be associated with an increased risk of viral and bacterial infections. Early in the course of the COVID-19 pandemic, this notion led to significant concerns regarding COVID-19 outcomes for people with neuroimmunological diseases. While some reports described a less favorable COVID-19 course in people treated with B-cell depleting agents, the use of other DMTs does not seem to be associated with such an increased risk (99–101). Another aspect of interest is whether the efficacy of DMTs is maintained during the pandemic. However, the currently available data is not sufficient to answer this question. While relapses were reported in only 3.4% of MS patients treated with DMTs, information about DMTs use and relapses was available for a relatively small proportion of patients (169/624, 27.1%). The fact that the majority of NMOSD and MOGAD cases reported are of newly diagnoses rather than relapses of previously diagnosed disease, may suggest that the efficacy of immunotherapy during the pandemic is maintained. In the series reported by Apostolos-Pereira et al., 97% of NMOSD patients (33/34) continued their prescribed immunotherapy during the pandemic. The relatively low incidence of neurological exacerbation reported by the authors (5/34, 15%) may further support this theory (21). Still, prospective studies comparing the rate of relapse between COVID-19 patients treated with DMTs and untreated patients are required to answer this question.
The current literature pertaining to the occurrence of demyelinating events in temporal association with COVID-19 is primarily composed of case reports, case series, and relatively small cohort studies. Therefore, while the rate of such events appears low based upon this review, especially considering the high prevalence of SARS-CoV-2 infection, the available data does not permit the determination of whether the rate of CNS demyelinating events (either new onset or true relapse) differs among people with confirmed COVID-19 compared to those who do not contract the infection. Additional questions that remain unanswered at this point are whether there are differences in the severity of demyelinating attacks and the response to acute treatments between demyelinating events occurring in association with COVID-19 and those not associated with the infection.
In conclusion, the rate of CNS demyelinating events occurring in the context of SARS-CoV-2 infection is relatively low given the global prevalence of infection. The clinical outcomes of new-onset or relapsing MS, NMOSD, or MOGAD associated with antecedent or concurrent SARS-CoV-2 infection is mostly favorable. Larger prospective epidemiological studies are needed to better characterize the impact of COVID-19 on CNS demyelinating diseases.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
IL, SN, GM, and MiL contributed to the conception and design of the study. IL and MiL organized the database. IL and SN performed the data collection. IL wrote the first draft of the manuscript. MeL conducted the literature search. All authors contributed to manuscript revision, read, and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.970383/full#supplementary-material
1. Oikonen M, Laaksonen M, Aalto V, Ilonen J, Salonen R, Erälinna JP, et al. Temporal relationship between environmental influenza A and Epstein-Barr viral infections and high multiple sclerosis relapse occurrence. Mult Scler. (2011) 17:672–80. doi: 10.1177/1352458510394397
2. Ramagopalan SV, Valdar W, Dyment DA, DeLuca GC, Yee IM, Giovannoni G, et al. Association of infectious mononucleosis with multiple sclerosis. A population-based study. Neuroepidemiology. (2009) 32:257–62. doi: 10.1159/000201564
3. Marrodan M, Alessandro L, Farez MF, Correale J. The role of infections in multiple sclerosis. Mult Scler. (2019) 25:891–901. doi: 10.1177/1352458518823940
4. Graber DJ, Levy M, Kerr D, Wade WF. Neuromyelitis optica pathogenesis and aquaporin 4. J Neuroinflammation. (2008) 5:22. doi: 10.1186/1742-2094-5-22
5. Zhong X, Zhou Y, Lu T, Wang Z, Fang L, Peng L, et al. Infections in neuromyelitis optica spectrum disorder. J Clin Neurosci. (2018) 47:14–9. doi: 10.1016/j.jocn.2017.10.005
6. Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. (2016) 13:280. doi: 10.1186/s12974-016-0718-0
7. Nakajima H, Motomura M, Tanaka K, Fujikawa A, Nakata R, Maeda Y, et al. Antibodies to myelin oligodendrocyte glycoprotein in idiopathic optic neuritis. BMJ Open. (2015) 5:7766. doi: 10.1136/bmjopen-2015-007766
8. Ramanathan S, Reddel SW, Henderson A, Parratt JD, Barnett M, Gatt PN, et al. Antibodies to myelin oligodendrocyte glycoprotein in bilateral and recurrent optic neuritis. Neurol Neuroimmunol Neuroinflamm. (2014) 1:e40. doi: 10.1212/NXI.0000000000000040
9. Cobo-Calvo A, Ruiz A, Maillart E, Audoin B, Zephir H, Bourre B, et al. Clinical spectrum and prognostic value of CNS MOG autoimmunity in adults: The MOGADOR study. Neurology. (2018) 90:e1858–e69. doi: 10.1212/WNL.0000000000005560
10. Koga M, Takahashi T, Kawai M, Fujihara K, Kanda T. A serological analysis of viral and bacterial infections associated with neuromyelitis optica. J Neurol Sci. (2011) 300:19–22. doi: 10.1016/j.jns.2010.10.013
11. Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. (2020) 94:55–8. doi: 10.1016/j.ijid.2020.03.062
12. Gutiérrez-Ortiz C, Méndez-Guerrero A, Rodrigo-Rey S, San Pedro-Murillo E, Bermejo-Guerrero L, Gordo-Mañas R, et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. (2020) 95:e601–e5. doi: 10.1212/WNL.0000000000009619
13. Goel K, Kumar A, Diwan S, Kohli S, Sachdeva HC, Ganapathy U, et al. Neurological manifestations of COVID-19: a series of seven cases. Indian J Crit Care Med. (2021) 25:219–23. doi: 10.5005/jp-journals-10071-23723
14. Rahimi K. Guillain-Barre syndrome during COVID-19 pandemic: an overview of the reports. Neurol Sci. (2020) 41:3149–56. doi: 10.1007/s10072-020-04693-y
15. Paterson RW, Brown RL, Benjamin L, Nortley R, Wiethoff S, Bharucha T, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. (2020) 143:3104–20. doi: 10.1093/brain/awaa240
16. Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. (2018) 17:162–73. doi: 10.1016/S1474-4422(17)30470-2
17. Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. (2015) 85:177–89. doi: 10.1212/WNL.0000000000001729
18. Jarius S, Paul F, Aktas O, Asgari N, Dale RC, de Seze J, et al. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J Neuroinflammation. (2018) 15:134. doi: 10.1186/s12974-018-1144-2
19. Hanson KE, Caliendo AM, Arias CA, Hayden MK, Englund JA, Lee MJ, et al. The infectious diseases society of America Guidelines on the Diagnosis of COVID-19: molecular diagnostic testing. Clin Infect Dis. (2021) 2021:ciab048. doi: 10.1093/cid/ciab048
20. Prevention CfDCa. Coronavirus Disease 2019 (COVID-19) 2020 Interim Case Definition. (2020). Available online at: https://ndc.services.cdc.gov/case-definitions/coronavirus-disease-2019-2020-08-05/ (accessed August 5, 2020).
21. Apostolos-Pereira SL, Campos Ferreira L, Boaventura M, de Carvalho Sousa NA, Joca Martins G, d'Almeida JA, et al. Clinical features of COVID-19 on patients with neuromyelitis optica spectrum disorders. Neurol Neuroimmunol Neuroinflamm. (2021) 8:1060. doi: 10.1212/NXI.0000000000001060
22. Barone S, Rapisarda L, Manzo L, Mechelli A, Pascarella A, Bruno P, et al. A case of neuromyelitis optica spectrum disorder (NMOSD) and acute myositis following SARS-CoV-2 infection. J Neurol Sci. (2021) 429:119862. doi: 10.1016/j.jns.2021.119862
23. Batum M, Kisabay Ak A, Mavioglu H. Covid-19 infection-induced neuromyelitis optica: a case report. Int J Neurosci. (2020) 2020:1–7. doi: 10.1080/00207454.2020.1860036
24. Chuang T-Y, Miskin D. SARS-CoV-2 Associated Neuromyelitis optica (2590). Philadelphia, PA: AAN Enterprises (2021).
25. Corrêa DG, de Souza Lima FC, da Cruz Bezerra D, Coutinho AC, Hygino da Cruz LC. COVID-19 associated with encephalomyeloradiculitis and positive anti-aquaporin-4 antibodies: cause or coincidence? Multiple Scler J. (2021) 27:973–6. doi: 10.1177/1352458520949988
26. Ghosh R, De K, Roy D, Mandal A, Biswas S, Biswas S, et al. A case of area postrema variant of neuromyelitis optica spectrum disorder following SARS-CoV-2 infection. J Neuroimmunol. (2020) 350:577439. doi: 10.1016/j.jneuroim.2020.577439
27. Hooshmand S, Obeidat A, Brod S. Reactivation of Latent Neuromyelitis Optic Secondary to COVID-19 Infection: A Case Report (4562). Philadelphia, PA: AAN Enterprises (2021).
28. Nasreldein A, Ibrahem H, Bahie A. Neuromyelitis optica spectrum disorders (NMOSD) attack triggered by COVID-19 infection (a case report). Multiple Scler J. (2020) 2020:77–8.
29. Shaw VC, Chander G, Puttanna A. Neuromyelitis optica spectrum disorder secondary to COVID-19. Br J Hosp Med. (2020) 81:1–3. doi: 10.12968/hmed.2020.0401
30. Shukla V, Singh VA, Singh VR, Maharaj P, Fernandes M, Cadan S, et al. 542 Seronegative NMOSD–A Post SARS-CoV-2 Neurological Complication Associated With Paediatric Multisystem Inflammatory Syndrome (PIMS)? London: BMJ Publishing Group Ltd (2021). doi: 10.1136/archdischild-2021-rcpch.59
31. Jentzer A, Carra-Dallière C, Lozano C, Riviere S, Darmon O, Ayrignac X, et al. Neuromyelitis optica spectrum disorder following COVID-19 infection with increase in pre-existing anti-aquaporin-4 antibodies. J Neurol. (2022) 269:2850–3. doi: 10.1007/s00415-022-10972-9
32. Das D, Bhattacharjee H, Rehman O, Deori N, Magdalene D, Bharali G, et al. Neuromyelitis optica spectrum disorder post-COVID-19 infection: a rare case report from Northeast India. Indian J Ophthalmol. (2022) 70:1833–6. doi: 10.4103/ijo.IJO_61_22
33. Ahsan N, Jafarpour S, Santoro JD. Myelin oligodendrocyte glycoprotein antibody encephalitis following severe acute respiratory syndrome coronavirus 2 in a pediatric patient. Clin Exp Pediatr. (2021) 64:310–2. doi: 10.3345/cep.2020.01963
34. de Ruijter NS, Kramer G, Gons RAR, Hengstman GJD. Neuromyelitis optica spectrum disorder after presumed coronavirus (COVID-19) infection: a case report. Mult Scler Relat Disord. (2020) 46:102474. doi: 10.1016/j.msard.2020.102474
35. Ide T, Kawanami T, Eriguchi M, Hara H. SARS-CoV-2-related myelin oligodendrocyte glycoprotein antibody-associated disease: a case report and literature review. Intern Med. (2022) 2022:21. doi: 10.2169/internalmedicine.8709-21
36. Khan A, Panwala H, Ramadoss D, Khubchandani R. Myelin Oligodendrocyte Glycoprotein (MOG) antibody disease in a 11 year old with COVID-19 infection. Indian J Pediatr. (2021) 88:488–9. doi: 10.1007/s12098-020-03656-7
37. Kogure C, Kikushima W, Fukuda Y, Hasebe Y, Takahashi T, Shibuya T, et al. Myelin oligodendrocyte glycoprotein antibody-associated optic neuritis in a COVID-19 patient: a case report. Medicine. (2021) 100:e25865. doi: 10.1097/MD.0000000000025865
38. Lindan CE, Mankad K, Ram D, Kociolek LK, Silvera VM, Boddaert N, et al. Neuroimaging manifestations in children with SARS-CoV-2 infection: a multinational, multicentre collaborative study. Lancet Child Adolesc Health. (2021) 5:167–77. doi: 10.1016/S2352-4642(20)30362-X
39. Peters J, Alhasan S, Vogels CBF, Grubaugh ND, Farhadian S, Longbrake EE. MOG-associated encephalitis following SARS-CoV-2 infection. Mult Scler Relat Disord. (2021) 50:102857. doi: 10.1016/j.msard.2021.102857
40. Pinto AA, Carroll LS, Nar V, Varatharaj A, Galea I. CNS inflammatory vasculopathy with antimyelin oligodendrocyte glycoprotein antibodies in COVID-19. Neurol Neuroimmunol Neuroinflamm. (2020) 7:813. doi: 10.1212/NXI.0000000000000813
41. Sawalha K, Adeodokun S, Kamoga GR. COVID-19-induced acute bilateral optic neuritis. J Investig Med High Impact Case Rep. (2020) 8:2324709620976018. doi: 10.1177/2324709620976018
42. Vraka K, Ram D, West S, Chia WYE, Kurup P, Subramanian G, et al. Two paediatric patients with encephalopathy and concurrent COVID-19 infection: two sides of the same coin? Case Rep Neurol Med. (2021) 2021:6658000. doi: 10.1155/2021/6658000
43. Woodhall M, Mitchell JW, Gibbons E, Healy S, Waters P, Huda S. Case report: myelin oligodendrocyte glycoprotein antibody-associated relapse with COVID-19. Front Neurol. (2020) 11:598531. doi: 10.3389/fneur.2020.598531
44. Zhou S, Jones-Lopez EC, Soneji DJ, Azevedo CJ, Patel VR. Myelin oligodendrocyte glycoprotein antibody-associated optic neuritis and myelitis in COVID-19. J Neuroophthalmol. (2020) 40:398–402. doi: 10.1097/WNO.0000000000001049
45. Sinha R, Wander A, Kapoor A, Yadav R, Kumar A, Gulati S. Acute Demyelinating Syndrome (MOG Antibody Positive) associated with COVID-19 infection: a widening spectrum. Clin Pediatr. (2021) 60:501–3. doi: 10.1177/00099228211037210
46. Jumah M, Rahman F, Figgie M, Prasad A, Zampino A, Fadhil A, et al. COVID-19, HHV6 and MOG antibody: a perfect storm. J Neuroimmunol. (2021) 353:577521. doi: 10.1016/j.jneuroim.2021.577521
47. Durovic E, Bien C, Bien CG, Isenmann S. MOG antibody-associated encephalitis secondary to Covid-19: case report. BMC Neurol. (2021) 21:414. doi: 10.1186/s12883-021-02449-5
48. Sardar S, Safan A, Okar L, Sadik N, Adeli G. The diagnostic dilemma of bilateral optic neuritis and idiopathic intracranial hypertension coexistence in a patient with recent COVID-19 infection. Clin Case Rep. (2021) 9:e04347. doi: 10.1002/ccr3.4347
49. Rojas-Correa DX, Reche-Sainz JA, Insausti-García A, Calleja-García C, Ferro-Osuna M. Post COVID-19 myelin oligodendrocyte glycoprotein antibody-associated optic neuritis. Neuroophthalmology. (2022) 46:115–21. doi: 10.1080/01658107.2021.1916044
50. Assavapongpaiboon B, Apinyawasisuk S, Jariyakosol S. Myelin oligodendrocyte glycoprotein antibody-associated optic neuritis with COVID-19 infection: a case report and literature review. Am J Ophthalmol Case Rep. (2022) 26:101491. doi: 10.1016/j.ajoc.2022.101491
51. Žorić L, Rajović-Mrkić I, Colak E, Mirić D, Kisić B. Optic neuritis in a patient with seropositive myelin oligodendrocyte glycoprotein antibody during the post-COVID-19 period. Int Med Case Rep J. (2021) 14:349–55. doi: 10.2147/IMCRJ.S315103
52. Dias da Costa M, Leal Rato M, Cruz D, Valadas A, Antunes AP, Albuquerque L. Longitudinally extensive transverse myelitis with anti-myelin oligodendrocyte glycoprotein antibodies following SARS-CoV-2 infection. J Neuroimmunol. (2021) 361:577739. doi: 10.1016/j.jneuroim.2021.577739
53. Doukas SG, Santos AP, Mir W, Daud S, Zivin-Tutela TH. A rare case of myelin oligodendrocyte glycoprotein antibody-associated transverse myelitis in a 40-year-old patient with COVID-19. Cureus. (2022) 14:e23877. doi: 10.7759/cureus.23877
54. Jossy A, Jacob N, Sarkar S, Gokhale T, Kaliaperumal S, Deb AK. COVID-19-associated optic neuritis - a case series and review of literature. Indian J Ophthalmol. (2022) 70:310–6. doi: 10.4103/ijo.IJO_2235_21
55. Yang E, Husein A, Martinez-Perez J, Li T. Post-COVID-19 longitudinally extensive transverse myelitis with myelin oligodendrocyte glycoprotein antibodies. Case Rep Neurol Med. (2022) 2022:1068227. doi: 10.1155/2022/1068227
56. Cay-Martínez KC, Shen MY, Silver WG, Vargas WS. Postinfectious encephalomyelitis associated with myelin oligodendrocyte glycoprotein antibody in a pediatric patient with COVID-19. Pediatr Neurol. (2021) 124:40–1. doi: 10.1016/j.pediatrneurol.2021.08.001
57. Barzegar M, Mirmosayyeb O, Nehzat N, Sarrafi R, Khorvash F, Maghzi AH, et al. COVID-19 infection in a patient with multiple sclerosis treated with fingolimod. Neurol Neuroimmunol Neuroinflamm. (2020) 7:753. doi: 10.1212/NXI.0000000000000753
58. Barzegar M, Vaheb S, Mirmosayyeb O, Afshari-Safavi A, Nehzat N, Shaygannejad V. Can coronavirus disease 2019 (COVID-19) trigger exacerbation of multiple sclerosis? A retrospective study. Mult Scler Relat Disord. (2021) 52:102947. doi: 10.1016/j.msard.2021.102947
59. Chyzhyk V, Sialitski M, Boika A, Bahamaz V, Mazurenka K, Ponomarev V. Is SARS-CoV-2 important for multiple sclerosis? Eur J Neurol. (2021) 2021:492.
60. Conway S, Healy B, Zurawski J, Severson C, Kaplan T, Stazzone L, et al. COVID-19 and neurologic outcomes in multiple sclerosis and related disorders. Multiple Scler J. (2021) 2021:765.
61. Czarnowska A, Kapica-Topczewska K, Zajkowska O, Adamczyk-Sowa M, Kubicka-Baczyk K, Niedziela N, et al. Symptoms after COVID-19 infection in individuals with multiple sclerosis in Poland. J Clin Med. (2021) 10:225225. doi: 10.3390/jcm10225225
62. Dhillon PS, Dineen RA, Morris H, Tanasescu R, Nikfekr E, Evans J, et al. Neurological disorders associated with COVID-19 hospital admissions: experience of a single tertiary healthcare center. Front Neurol. (2021) 12:640017. doi: 10.3389/fneur.2021.640017
63. Domingues RB, Mendes-Correa MC, de Moura Leite FBV, Sabino EC, Salarini DZ, Claro I, et al. First case of SARS-CoV-2 sequencing in cerebrospinal fluid of a patient with suspected demyelinating disease. J Neurol. (2020) 267:3154–6. doi: 10.1007/s00415-020-09996-w
64. Etemadifar M, Sedaghat N, Aghababaee A, Kargaran PK, Maracy MR, Ganjalikhani-Hakemi M, et al. COVID-19 and the risk of relapse in multiple sclerosis patients: a fight with no bystander effect? Mult Scler Relat Disord. (2021) 51:102915. doi: 10.1016/j.msard.2021.102915
65. Florea AA, Sirbu CA, Ghinescu MC, Plesa CF, Sirbu AM, Mitrica M, et al. SARS-CoV-2, multiple sclerosis, and focal deficit in a postpartum woman: a case report. Exp Ther Med. (2021) 21:92. doi: 10.3892/etm.2020.9524
66. Fragoso YD, Pacheco FAS, Silveira GL, Oliveira RA, Carvalho VM, Martimbianco ALC. COVID-19 in a temporal relation to the onset of multiple sclerosis. Mult Scler Relat Disord. (2021) 50:102863. doi: 10.1016/j.msard.2021.102863
67. Jaisankar PJ, Kucera A, Lomiguen CM, Chin J. Complications of COVID-19 pneumonia and multiple sclerosis exacerbation. Cureus. (2021) 13:e17506. doi: 10.7759/cureus.17506
68. Karsidag S, Sahin S, Ates MF, Cinar N, Kendirli S. Demyelinating disease of the central nervous system concurrent with COVID-19. Cureus. (2021) 13:e17297. doi: 10.7759/cureus.17297
69. Kataria S, Tandon M, Melnic V, Sriwastava S. A case series and literature review of multiple sclerosis and COVID-19: clinical characteristics, outcomes and a brief review of immunotherapies. eNeurologicalSci. (2020) 21:100287. doi: 10.1016/j.ensci.2020.100287
70. Khedr EM, Abo-Elfetoh N, Deaf E, Hassan HM, Amin MT, Soliman RK, et al. Surveillance study of acute neurological manifestations among 439 Egyptian patients with COVID-19 in Assiut and Aswan University Hospitals. Neuroepidemiology. (2021) 55:109–18. doi: 10.1159/000513647
71. Khurana D, Kaur G, Chellapa R. COVID-19 in multiple sclerosis: experience from North India. J Neurol Sci. (2021) 429:118164. doi: 10.1016/j.jns.2021.118164
72. Luetic G, Menichini ML, Burgos M, Alonso R, Carnero Contentti E, Carrá A, et al. COVID-19 in Argentine teriflunomide-treated multiple sclerosis patients: first national case series. Mult Scler Relat Disord. (2021) 53:103049. doi: 10.1016/j.msard.2021.103049
73. Maghzi AH, Houtchens MK, Preziosa P, Ionete C, Beretich BD, Stankiewicz JM, et al. COVID-19 in teriflunomide-treated patients with multiple sclerosis. J Neurol. (2020) 267:2790–6. doi: 10.1007/s00415-020-09944-8
74. Mantero V, Abate L, Basilico P, Balgera R, Salmaggi A, Nourbakhsh B, et al. COVID-19 in dimethyl fumarate-treated patients with multiple sclerosis. J Neurol. (2021) 268:2023–5. doi: 10.1007/s00415-020-10015-1
75. Michelena G, Casas M, Eizaguirre MB, Pita MC, Cohen L, Alonso R, et al. Can COVID-19 exacerbate multiple sclerosis symptoms? A case series analysis. Mult Scler Relat Disord. (2022) 57:103368. doi: 10.1016/j.msard.2021.103368
76. Möhn N, Saker F, Bonda V, Respondek G, Bachmann M, Stoll M, et al. Mild COVID-19 symptoms despite treatment with teriflunomide and high-dose methylprednisolone due to multiple sclerosis relapse. J Neurol. (2020) 267:2803–5. doi: 10.1007/s00415-020-09921-1
77. Moore L, Ghannam M, Manousakis G. A first presentation of multiple sclerosis with concurrent COVID-19 infection. eNeurologicalSci. (2021) 22:100299. doi: 10.1016/j.ensci.2020.100299
78. Palao M, Fernández-Díaz E, Gracia-Gil J, Romero-Sánchez CM, Díaz-Maroto I, Segura T. Multiple sclerosis following SARS-CoV-2 infection. Mult Scler Relat Disord. (2020) 45:102377. doi: 10.1016/j.msard.2020.102377
79. Pignolo A, Aprile M, Gagliardo C, Giammanco GM, D'Amelio M, Aridon P, et al. Clinical onset and multiple sclerosis relapse after SARS-CoV-2 infection. Neurol Int. (2021) 13:695–700. doi: 10.3390/neurolint13040066
80. Sandoval F, Julio K, Méndez G, Valderas C, Echeverría AC, Perinetti MJ, et al. Neurologic features associated with SARS-CoV-2 infection in children: a case series report. J Child Neurol. (2021) 36:853–66. doi: 10.1177/0883073821989164
81. Wildemann B, Jarius S, Lehmann LH, André F, Frey N, Schnitzler P, et al. COVID-19-related severe MS exacerbation with life-threatening Takotsubo cardiomyopathy in a previously stable patient and interference of MS therapy with long-term immunity against SARS-CoV-2. J Neurol. (2022) 269:1138–41. doi: 10.1007/s00415-021-10779-0
82. Yavari F, Raji S, Moradi F, Saeidi M. Demyelinating changes alike to multiple sclerosis: a case report of rare manifestations of COVID-19. Case Rep Neurol Med. (2020) 2020:6682251. doi: 10.1155/2020/6682251
83. Etemadifar M, Abhari AP, Nouri H, Salari M, Maleki S, Amin A, et al. Does COVID-19 increase the long-term relapsing-remitting multiple sclerosis clinical activity? A cohort study. BMC Neurol. (2022) 22:64. doi: 10.1186/s12883-022-02590-9
84. Finsterer J. SARS-CoV-2 triggered relapse of multiple sclerosis. Clin Neurol Neurosurg. (2022) 215:107210. doi: 10.1016/j.clineuro.2022.107210
85. Paybast S, Hejazi SA, Molavi P, Habibi MA, Naser Moghadasi A. A one year follow of patients with multiple sclerosis during COVID-19 pandemic: a cross-sectional study in Qom province, Iran. Mult Scler Relat Disord. (2022) 60:103712. doi: 10.1016/j.msard.2022.103712
86. Khair A, Husain S, Ortiz M, Kaur G, Bean S. Para and post-COVID-19 acute demyelinating disorders in children, expanding the spectrum of clinical and radiological characteristics. Ann Neurol. (2021) 2021:S113–S4. doi: 10.7759/cureus.23405
87. Aubart M, Roux CJ, Durrleman C, Gins C, Hully M, Kossorotoff M, et al. Neuro-inflammatory disease following SARS-CoV-2 infection in children. J Pediatr. (2022) 247:22–8.e2. doi: 10.1016/j.jpeds.2022.05.018
88. Zhou Z, Kang H, Li S, Zhao X. Understanding the neurotropic characteristics of SARS-CoV-2: from neurological manifestations of COVID-19 to potential neurotropic mechanisms. J Neurol. (2020) 267:2179–84. doi: 10.1007/s00415-020-09929-7
89. Verstrepen K, Baisier L, De Cauwer H. Neurological manifestations of COVID-19, SARS and MERS. Acta Neurol Belg. (2020) 120:1051–60. doi: 10.1007/s13760-020-01412-4
90. Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dubé M, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. (2019) 12:14. doi: 10.3390/v12010014
91. Wu Y, Xu X, Chen Z, Duan J, Hashimoto K, Yang L, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. (2020) 87:18–22. doi: 10.1016/j.bbi.2020.03.031
92. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
93. Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. (2020) 71:762–8. doi: 10.1093/cid/ciaa248
94. Andersen O, Lygner PE, Bergström T, Andersson M, Vahlne A. Viral infections trigger multiple sclerosis relapses: a prospective seroepidemiological study. J Neurol. (1993) 240:417–22. doi: 10.1007/BF00867354
95. Sibley WA, Bamford CR, Clark K. Clinical viral infections and multiple sclerosis. Lancet. (1985) 1:1313–5. doi: 10.1016/S0140-6736(85)92801-6
96. Panitch HS. Influence of infection on exacerbations of multiple sclerosis. Ann Neurol. (1994) 36(Suppl.):S25–8. doi: 10.1002/ana.410360709
97. Buljevac D, Flach HZ, Hop WC, Hijdra D, Laman JD, Savelkoul HF, et al. Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain. (2002) 125:952–60. doi: 10.1093/brain/awf098
98. Correale J, Fiol M, Gilmore W. The risk of relapses in multiple sclerosis during systemic infections. Neurology. (2006) 67:652–9. doi: 10.1212/01.wnl.0000233834.09743.3b
99. Simpson-Yap S, De Brouwer E, Kalincik T, Rijke N, Hillert JA, Walton C, et al. Associations of disease-modifying therapies with COVID-19 severity in multiple sclerosis. Neurology. (2021) 97:e1870–e85. doi: 10.1212/WNL.0000000000012753
100. Sormani MP, De Rossi N, Schiavetti I, Carmisciano L, Cordioli C, Moiola L, et al. Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. (2021) 89:780–9. doi: 10.1002/ana.26028
Keywords: COVID-19, multiple sclerosis (MS), neuromyelitis optica spectrum disorder (NMOSD), myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD), diagnosis, relapse, exacerbation
Citation: Lotan I, Nishiyama S, Manzano GS, Lydston M and Levy M (2022) COVID-19 and the risk of CNS demyelinating diseases: A systematic review. Front. Neurol. 13:970383. doi: 10.3389/fneur.2022.970383
Received: 15 June 2022; Accepted: 01 September 2022;
Published: 20 September 2022.
Edited by:
Hans-Peter Hartung, Heinrich Heine University of Düsseldorf, GermanyReviewed by:
Kelli M. Money, University of Colorado Anschutz Medical Campus, United StatesCopyright © 2022 Lotan, Nishiyama, Manzano, Lydston and Levy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Itay Lotan, bG90YW4uaXRheTFAZ21haWwuY29t; aWxvdGFuQG1naC5oYXJ2YXJkLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.