- Department of Intensive Care, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
Background: To investigate the value of serum Cyclophilin A(Cyp A) in evaluating the prognosis of patients with different severity of craniocerebral injury.
Methods: The clinical data of patients with craniocerebral injury treated in the Department of Emergency from July 2014 to August 2017 were collected. The patients were divided into survival group and death group, good neurological function group and poor neurological function group with 28-day prognosis and were divided into mild (13–15) group, moderate (9–12) group, and severe (3–8) group with Glasgow Coma Scale (GCS) score. Clinical parameters such as Cyp A and mortality in groups and the relationship between Cyp A and GCS score were compared and its predictive value for prognosis was analyzed with Binary Logistics regression, Cox proportional hazards model and kaplan-meier survival curve.
Results: In a single-center retrospective study, 503 patients were enrolled, including 365 males and 138 females; serum Cyp A in the survival group was significantly smaller than the death group [18.7 (10.1, 51.5) ng/mL vs. 149.8 (79.5, 194.4) ng/mL, P < 0.005]. There were significant differences in mortality and Cyp A levels between patients with different severity of craniocerebral injury (P < 0.001). Serum Cyp A levels were negatively correlated with GCS scores in all patients with craniocerebral injury, mild, moderate, or severe craniocerebral injury (r = −0.844, r = −0.256, r = −0.540, r = −0.531, P < 0.001). Predictive value of Serum Cyp A level for all patients with craniocerebral injury, mild, moderate, and severe craniocerebral injury is 0.890, 0.789, 0.806, and 0.833, respectively. Logistics regression analysis showed that lactate (OR = 1.260, 95%CI: 1.023–1.551) and Cyp A (OR = 1.021, 95%CI: 1.011–1.031) were positively correlated with death (P < 0.05), Lactic acid (HR 1.115; 95%CI:1.001–1.243; P = 0.048), GCS score (HR 0.888; 95% CI: 0.794–0.993; P = 0.038), Cyp A levels (HR 1.009; 95% CI: 1.004–1.013; P < 0.001) had a significant effect on short-term mortality. Similar results were seen when neurologic function was used as the outcome. Kaplan-meier survival curve analysis found survival rate of patients with Cyp A level below the cut-off value was significantly higher.
Conclusion: Serum Cyp A has a certain predictive value for the prognosis of patients with different severity of craniocerebral injury. Among them, patients with severe craniocerebral injury have the highest predictive value and mild craniocerebral injury patients have the least.
Introduction
Craniocerebral injury is one of the most common acute and critical illnesses in emergency departments. In recent years, with the rapid development of modern construction industry and transportation industry, the number of patients with craniocerebral injury has also increased year by year, resulting in increased intracranial pressure and Decreased cerebral blood perfusion, causing ischemic necrosis of brain cells, affecting the function of the central nervous system in the brain. In severe cases, it can lead to death or disability of the injured (1). However, most of the current clinical examination methods for evaluating craniocerebral injury are limited to imaging, such as head CT, MRI, etc., which are time-consuming and labor-intensive, and the examination cost is high, which is not conducive to the early evaluation of the prognosis of the injured. Some scholars found that the secretion of Cyclophilin A(Cyp A) in brain tissue of rats with traumatic brain injury increased (2, 3). Serum Cyp A level is closely related to the disease severity and 30-day adverse neurological outcome of patients with subarachnoid hemorrhage (4), indicating that Cyp A may be an effective indicator for evaluating the prognosis of patients with craniocerebral injury. However, there are few reports on the prediction of the prognosis of patients with mild, moderate and severe craniocerebral injury by serum Cyp A levels. Therefore, this paper analyzed the clinical data of 503 patients with craniocerebral injury to explore the role of serum Cyp A levels in evaluating craniocerebral injury. The prognostic value of patients with brain injury and its relationship with the severity of brain injury aims to provide more rapid, simple and effective indicators for the assessment and treatment of patients with brain injury. The report is as follows.
Methods
Study design
The data of 986 injured patients admitted to the emergency department of our hospital from July 2014 to August 2017 were collected. After excluding those patients presentation with did not go to the emergency department of our hospital within 24 h after trauma (n = 104), with severe injury to other parts (n = 325), with age <18 years old (n = 9), with heart and brain, liver, kidney and other important organ basic diseases (n = 34), with use of anticoagulant drugs within 6 months before injury or with coagulation dysfunction (n = 11). Finally, 503 patients with craniocerebral injury were included. The study protocols were approved by the Ethics Committee of The First Affiliated Hospital, Zhejiang University School of Medicine.
Data collection
A total of 503 patients were finally included. The gender, age, cause of trauma, Venous blood was collected from the cubital vein within 3 h after admission. Clinical severity of head trauma was assessed by Glasgow Coma Scale (GCS) score on admission by 2 experienced emergency physicians. Serum Cyp A was detected by the American BIORAD Coda automatic enzyme immunoassay analyzer. Laboratory data were obtained from the Clinical Laboratory Department of The First Affiliated Hospital, Zhejiang University School of Medicine. All patients immediately took life-saving rescue interventions, including oxygen inhalation, debridement, drug administration, tracheal intubation, tracheotomy, cerebral resuscitation, and anti-shock treatments. The prognosis of the patients was followed up by telephone after 28 days. Neurologic outcome was assessed using the Glasgow-Pittsburgh Cerebral Performance Category (CPC) and was dichotomized as either good (CPC 1 and 2) or poor (CPC 3 to 5) (5). The primary outcome was 28-day mortality. The secondary outcome was 28-day neurologic outcome.
Statistical analysis
SPSS 22.0 statistical software was used for analysis. The Kolmogorov-Smirnov test was used to test the normality of the quantitative data. Since all the data were non-normally distributed, they were expressed as the median (quartile) M(IQR). With the 28-day prognosis as the observation end point, the 503 patients included in the study were divided into survival group (459 cases) and death group (44 cases). The differences in clinical parameters such as serum Cyp A level and GCS score were compared between the two groups by the two-sample Mann-Whitney U. According to the Glasgow Coma Scale (GCS), the patients with traumatic brain injury were divided into mild (GCS 13–15) groups, medium (GCS 9–12) groups, and severe (GCS 3–8) groups, and the mortality and serum Cyp A levels of patients were compared between the three groups by Kruskal-Wallis univariate ANOVA (k samples) test. Count data were expressed by the number of cases and percentages, and comparison between groups was performed by X2 test. Cochran-Armitage trend test was used to compare the trend of the mortality rate in mild groups, medium groups, and severe groups. Spearman rank correlation analysis was used to explore the correlation between serum Cyp A level and GCS score and the receiver operating characteristic (ROC) curve was drawn to predict the prognosis of patients with different severity of craniocerebral injury. Based on the optimal threshold values of Cyp A, we divided craniocerebral trauma patients into Cyp A ≤ 68.9 ng/ml groups and Cyp A > 68.9 ng/ml groups, we plotted Kaplan–Meier survival curves by use of 28-day mortality data, and compared groups by the log-rank test, and then multiple logistic regression analyses and cox proportional hazards model analysis with a forward stepwise were conducted. The p-value of P < 0.05 was considered statistically significant.
Results
Clinic information of patients
A total of 503 patients were finally included, including 365 (72.6%) males and 138 (27.4%) females. The age was 49.0 (34.0, 61.0) years and there was no statistical difference in gender and cause of trauma. The GCS score of the survival group was significantly higher than that of the death group, which was 15 (14, 15) points vs. 6.5 (4.0, 11.8) points, and the serum Cyp A level was significantly lower than that of the death group, which was 18.7 (10.1, 51.5) ng/mL vs. 149.8 (79.5, 194.4) ng/mL. Similar results were observed when neurological outcome was used as the outcome (Table 1).
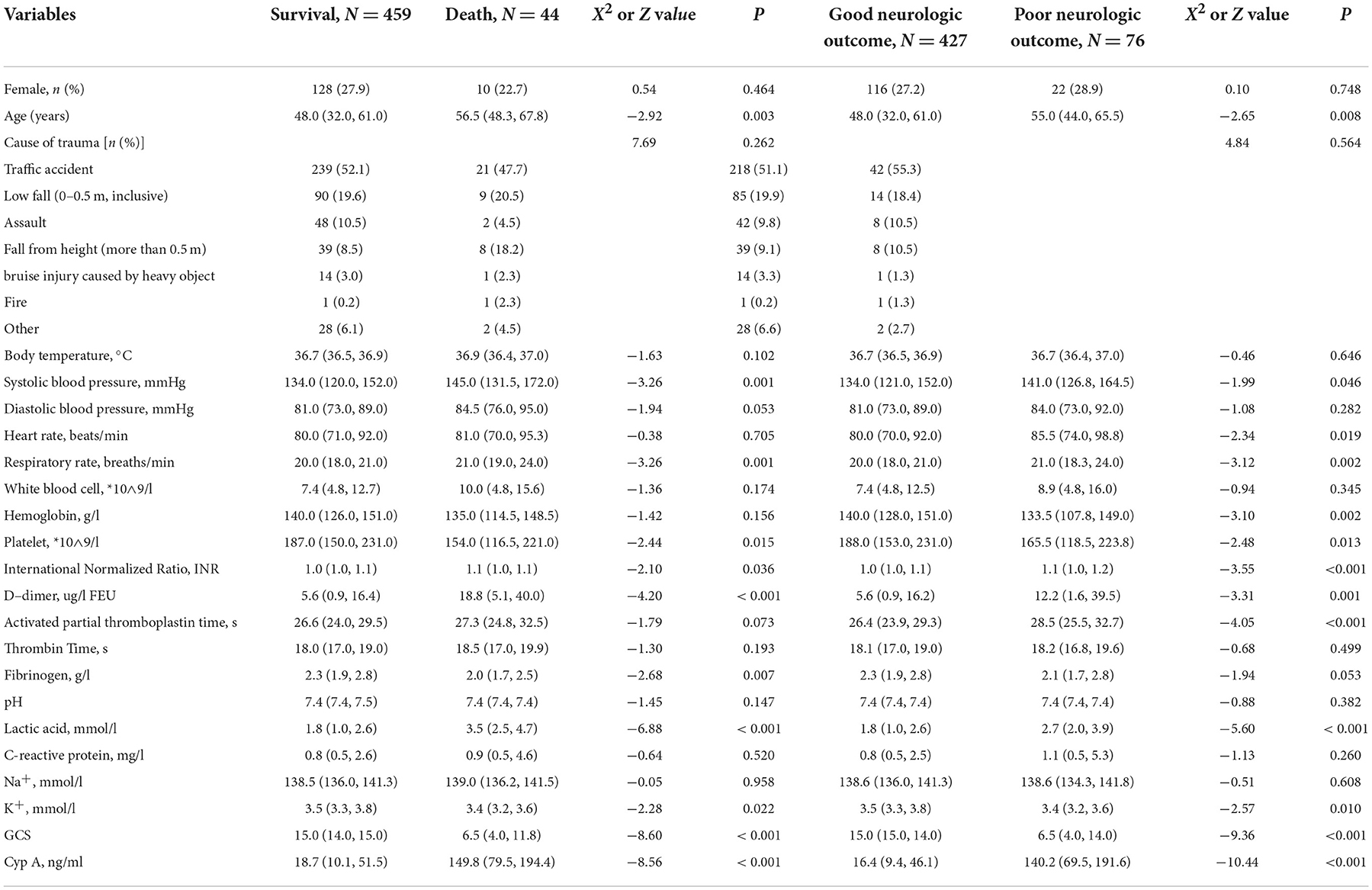
Table 1. Clinic information of the study population stratified by survival status and neurologic outcome.
Comparison of mortality and Cyp A in patients with different severity of craniocerebral injury
There were significant differences in the mortality and Cyp A levels among patients with different severity of craniocerebral injury (P < 0.001). And the Cochran-Armitage trend test showed that with the severity of craniocerebral injury, the mortality of patients had an increasing trend (Ptrend < 0.001), (see Table 2).
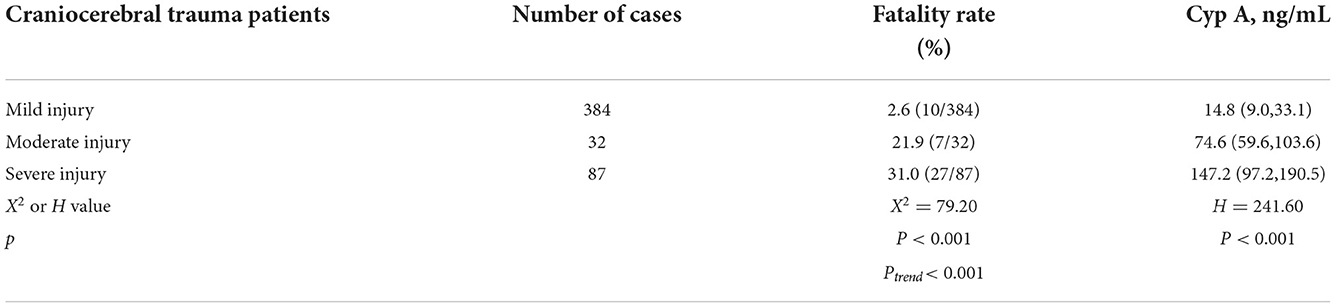
Table 2. Comparison of mortality and Cyp A levels in patients with different severity of craniocerebral injury.
Correlation between serum Cyp A value and GCS score
Spearman rank correlation analysis showed that the serum Cyp A level of all patients with craniocerebral injury was negatively correlated with GCS score (r = −0.844, P < 0.001). Based on GCS score, all patients were divided into three groups: mild (GCS 13–15) groups, medium (GCS 9–12) groups, and severe (GCS 3–8) groups and then serum Cyp A values of above three groups were closely related to the GCS score respectively (r = −0.256, r = −0.540, r = −0.531, P < 0.001). The more severe the injury in patients with craniocerebral trauma, the higher the correlation between serum Cyp A level and GCS score (Figure 1).
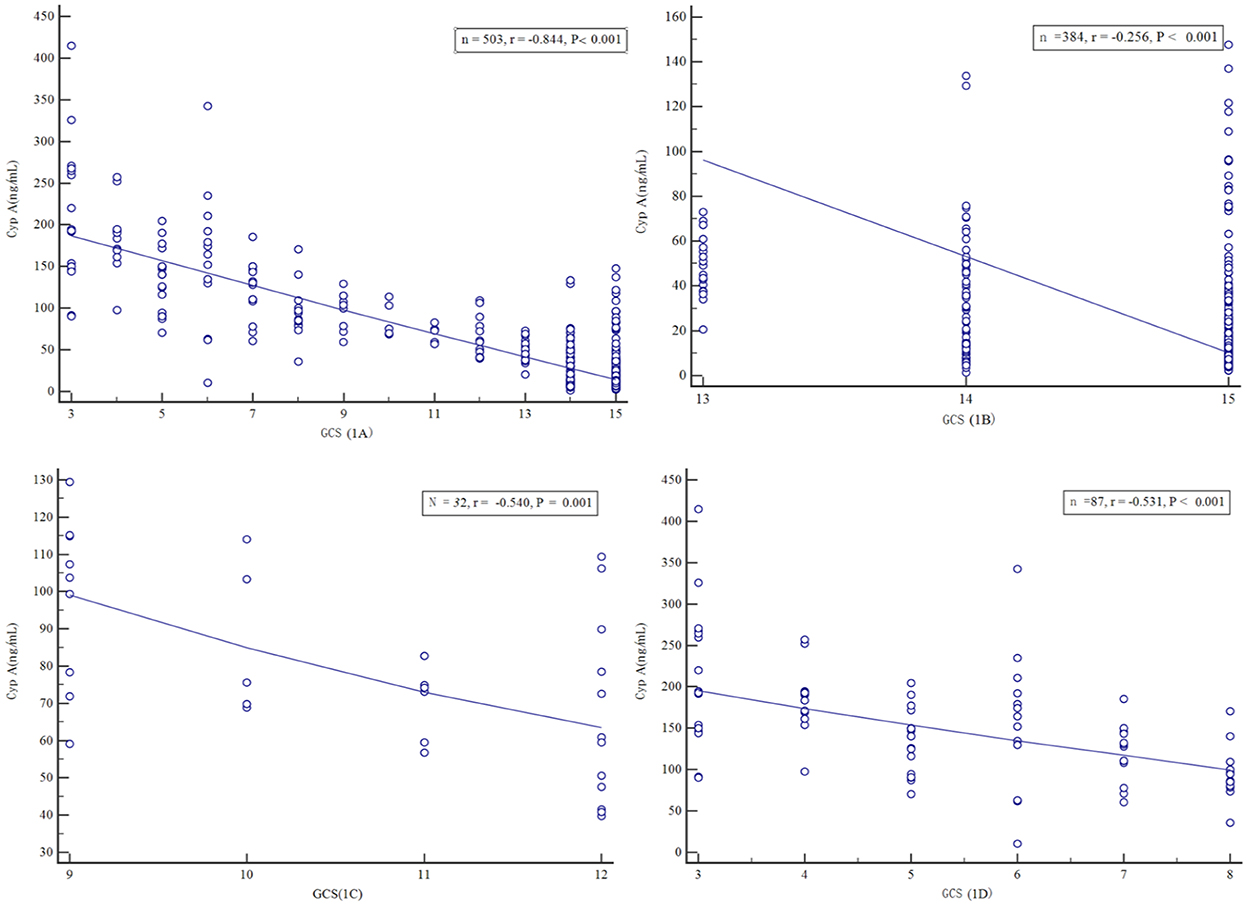
Figure 1. Scatter plot of serum Cyclophilin A level versus Glasgow Coma Scale score, in all craniocerebral trauma patients (A), in mild craniocerebral trauma patients (B), in moderate craniocerebral trauma patients (C), in severe craniocerebral trauma patients (D).
Predictive value of serum Cyp A level on prognosis of patients with craniocerebral injury
The ROC curve analysis showed that the serum Cyp A level had a certain predictive value for the mortality of all patients with craniocerebral injury, mild craniocerebral injury, moderate craniocerebral injury, and severe craniocerebral injury (Figure 2), which were 0.890, 0.789, 0.806, and 0.833 respectively (Table 3). The more severe the injury in patients with craniocerebral trauma, the greater the predictive value of serum Cyp A levels.
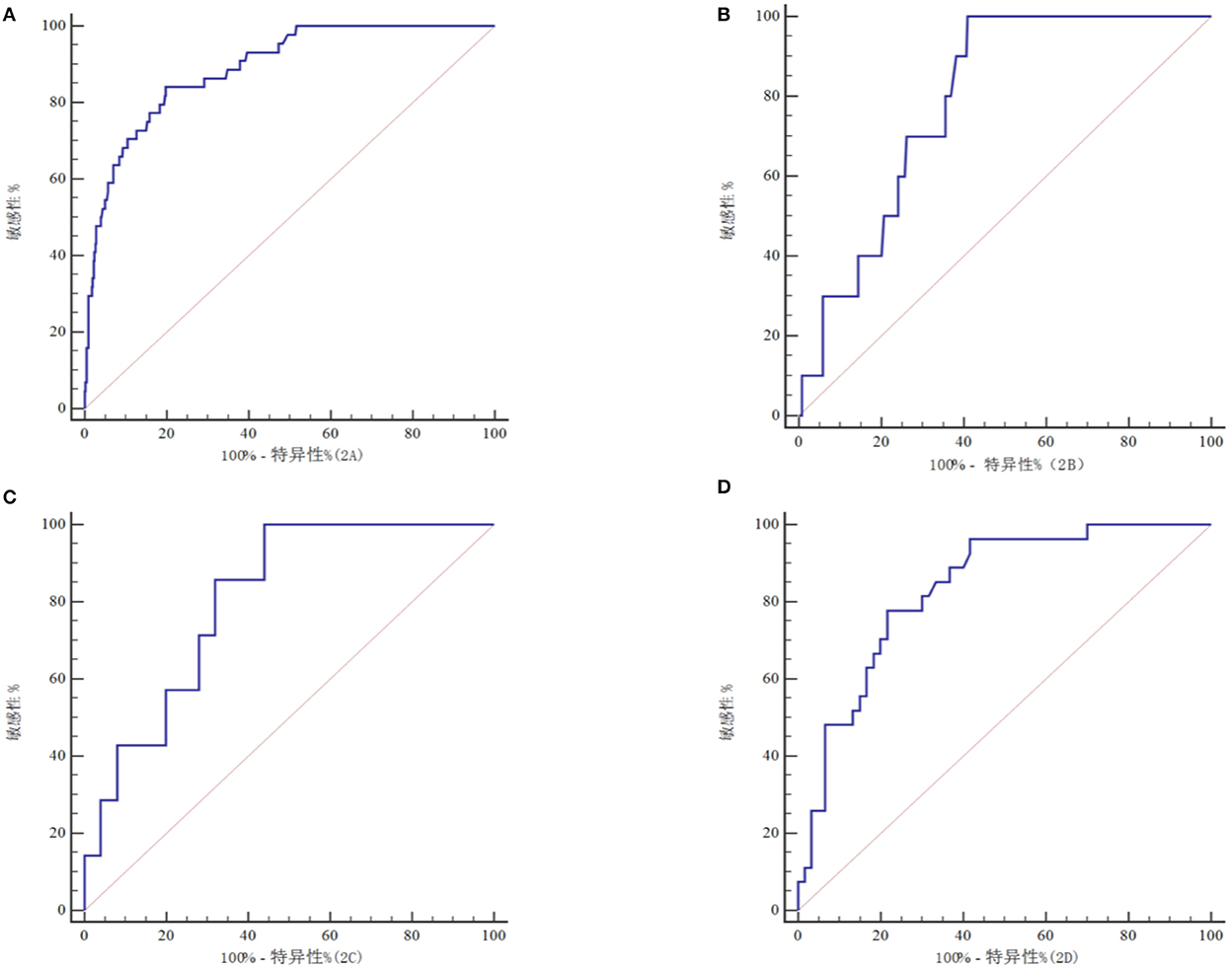
Figure 2. ROC curve of to predict prognosis in all craniocerebral trauma patients (A), in mild craniocerebral trauma patients (B), in moderate craniocerebral trauma patients (C), in severe craniocerebral trauma patients (D).

Table 3. Predictive efficiency of serum Cyclophilin A level for craniocerebral trauma patients' prognosis.
Logistics regression analysis to predict 28-day mortality and poor neurologic outcome
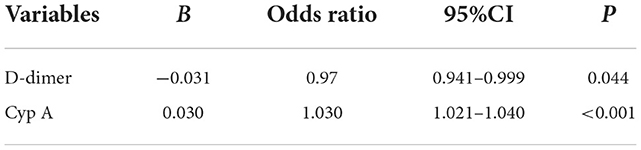
According to the above results, the indicators with statistical significance in the prognosis of patients with craniocerebral trauma were screened. Binary Logistics regression analysis showed that lactate (OR = 1.260, 95%CI: 1.023–1.551) and Cyp A (OR = 1.021, 95%CI: 1.011–1.031) were positively correlated with death (P < 0.05), as shown in Table 4. Based on the prognosis of neurological function, D-dimer (OR = 0.97, 95%CI: 0.941–0.999) correlated negatively with prognosis, while CypA (OR = 1.030, 95%CI: 1.021–1.040) correlated positively (P < 0.05), (see Table 5).
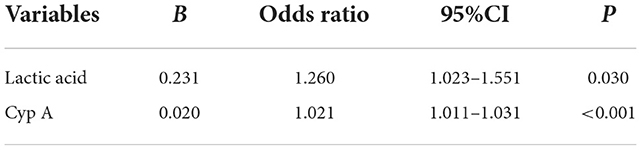
Table 4. Binary logistic regression analysis of the risk factors for death of craniocerebral traumatic patients.
Cox proportional hazards model analysis to predict 28-day mortality
The statistically significant prognostic indicators of patients with craniocerebral trauma were included in the Cox proportional hazards model for analysis, and the results showed that lactic acid (HR 1.115; 95%CI: 1.001–1.243; P = 0.048), GCS score (HR 0.888; 95% CI: 0.794–0.993; P = 0.038), Cyp A levels (HR 1.009; 95% CI: 1.004–1.013; P < 0.001) had a significant effect on short-term mortality in patients with craniocerebral trauma, as shown in Table 6.
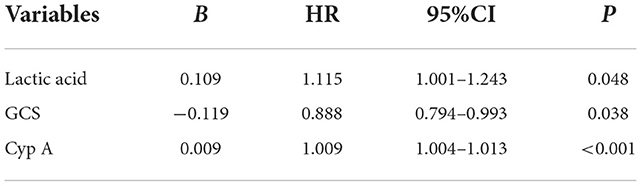
Table 6. Multivariable cox proportional hazard regression analysis of the risk factors for death of craniocerebral traumatic patients.
Kaplan-meier plot showing survival in craniocerebral trauma patients grouped by Cyp A levels
We further compared 28-day mortality risk for craniocerebral trauma patients based on the cutoff value of Cyp A. 37 of 128 craniocerebral trauma patients with high Cyp A(>68.9 ng/ml) died and 368 of 375 patients with low Cyp A survived. Kaplan-meier survival curve analysis was performed and the mortality rate of patients with different groups of Cyp A was compared. The study found that the survival rate of patients with Cyp A level below the cut-off value was significantly higher (P < 0.001) (Figure 3).
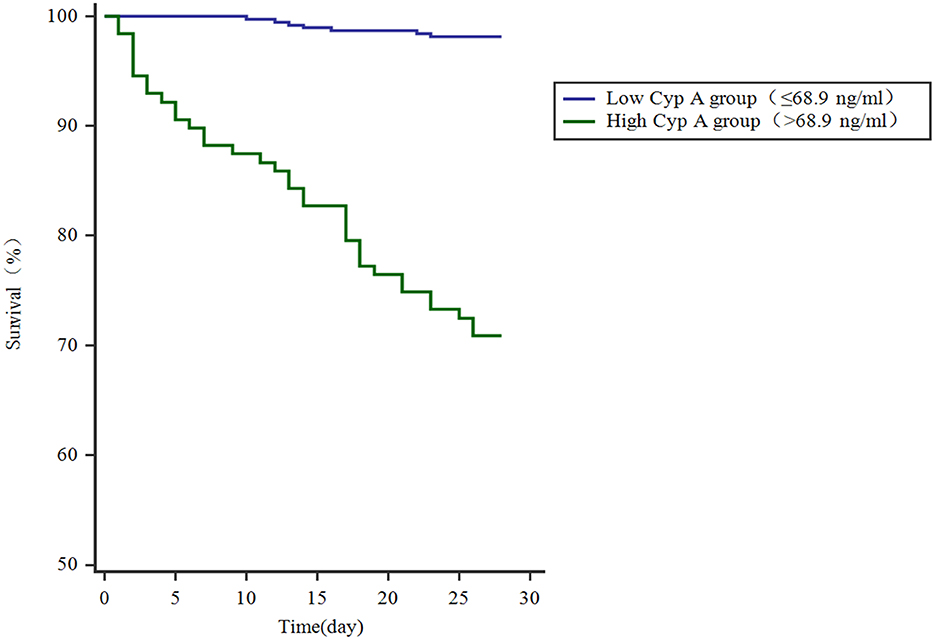
Figure 3. Kaplan-meier plot showing survival in craniocerebral trauma patients grouped by Cyp A levels.
Discussion
Craniocerebral injury is relatively common in clinical practice. The injured often suffer from intracranial hypertension, cerebral edema, and even brain herniation. Severe cases can lead to many complications, poor prognosis and death, which seriously affects the subsequent quality of life of the injured. Therefore, Early diagnosis, timely and accurate assessment of injury severity, and effective control interventions are critical to improving prognosis.
Cyp A is a small-molecule soluble protein with conservative structure and wide distribution. It has prolyl cis-trans isomerase function and cytokine-like activity. It is involved in the regulation of immune function, inflammation, apoptosis, viral infection, cholesterol metabolism, and injury, protein repair, the occurrence and development of malignant tumors (6, 7). In the case of ischemia, hypoxia, inflammation, the body will stimulate the tissue to produce and release Cyp A to increase (8). In inflammatory diseases such as chronic obstructive pulmonary disease, lung cancer, coronary artery disease, chronic kidney disease, acute myocardial infarction, acute intracerebral hemorrhage, the serum Cyp A concentration is significantly increased (9–14). A prospective study by Jin et al. (15) included 105 patients with severe traumatic brain injury (GCS 3–8) and 105 healthy subjects, and found that serum Cyp A levels were often elevated in patients with severe traumatic brain injury, and its level was negatively correlated with GCS scores(r = −0.562, P < 0.001), which was an independent risk factor for death and poor prognosis (Glasgow Outcome Scale score of 1–3) after 90-day follow-up. However, the research objects of serum Cyp A predicting the prognosis of patients with craniocerebral injury are only patients with severe craniocerebral injury, and there are very few research reports on the prognosis of patients with mild and moderate craniocerebral injury. In addition, it is worth noting that it is unknown whether this conclusion applies to all patients with traumatic brain injury. Therefore, all patients with craniocerebral injury were included in our study, which found that serum Cyp A can effectively evaluate the prognosis of all patients with craniocerebral injury. Serum Cyp A can accurately assess the prognosis of patients with different degrees of craniocerebral injury. The more severe the injury in patients with craniocerebral trauma, the greater the predictive value of serum Cyp A levels. Thus, serum Cyp A can be used as a reliable predictor of death in patients with traumatic brain injury. In addition, the correlation analysis of this study found that the relation between serum Cyp A levels and GCS scores in patients with severe craniocerebral injury was the largest, followed by medium cases and the least in mild cases, which indicated that the more severe the injury, the lower the GCS score, the greater the serum Cyp A, and the greater the risk of poor prognosis.
It is worth noting that this study has certain limitations: Firstly, this study only included the yellow race, whether this conclusion is applicable to other populations still needs further related research. Secondly, since the venous blood collected from the cubital vein of patients with craniocerebral injury within 3 h after admission to the hospital for serum Cyp A testing, its accuracy will also be disturbed to a certain extent.
Conclusions
Serum Cyp A is an important indicator for evaluating the severity of injury and the risk of death in patients with craniocerebral injury. The increase of Cyp A in peripheral blood is closely related to the severity and clinical prognosis of patients. The detection of this indicator can help physicians to more comprehensively assess the severity of the patient's injury and clinical prognosis, and provide physicians with the basis for medical decision-making and treatment guidance at an early stage, which has important clinical significance for reducing the patient's disability rate and improving the patient's prognosis.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The study protocols were approved by the Ethics Committee of The First Affiliated Hospital, Zhejiang University School of Medicine. The patients/participants provided their written informed consent to participate in this study.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dang B, Chen W, He W, Chen G. Rehabilitation Treatment and Progress of Traumatic Brain Injury Dysfunction. Neural Plast. (2017) 2017:1582182. doi: 10.1155/2017/1582182
2. Dang B, Li H, Xu X, Shen H, Wang Y, Gao A, et al. Cyclophilin A/Cluster of Differentiation 147 Interactions Participate in Early Brain Injury After Subarachnoid Hemorrhage in Rats. Crit Care Med. (2015) 43:e369–81. doi: 10.1097/CCM.0000000000001146
3. Rodriguez J, Xie C, Li T, Sun Y, Wang Y, Xu Y, et al. Inhibiting the interaction between apoptosis-inducing factor and cyclophilin A prevents brain injury in neonatal mice after hypoxia-ischemia. Neuropharmacology. (2020) 171:108088. doi: 10.1016/j.neuropharm.2020.108088
4. Kao HW, Lee KW, Chen WL, Kuo CL, Huang CS, Tseng WM, et al. Cyclophilin A in Ruptured Intracranial Aneurysm: A Prognostic Biomarker. Medicine (Baltimore). (2015) 94:e1683. doi: 10.1097/MD.0000000000001683
5. Booth CM, Boone RH, Tomlinson G, Detsky AS. Is this patient dead, vegetative, or severely neurologically impaired? Assessing outcome for comatose survivors of cardiac arrest. JAMA. (2004) 291:870–9. doi: 10.1001/jama.291.7.870
6. Dawar FU, Xiong Y, Khattak M, Li J, Lin L, Mei J. Potential role of cyclophilin A in regulating cytokine secretion. J Leukoc Biol. (2017) 102:989–92. doi: 10.1189/jlb.3RU0317-090RR
7. Russo L, Mascanzoni F, Farina B, Dolga AM, Monti A, Caporale A, et al. Design, Optimization, and structural characterization of an apoptosis-inducing factor peptide targeting human cyclophilin a to inhibit apoptosis inducing factor-mediated cell death. J Med Chem. (2021) 64:11445–59. doi: 10.1021/acs.jmedchem.1c00777
8. Satoh K. Cyclophilin A in cardiovascular homeostasis and diseases. Tohoku J Exp Med. (2015) 235:1–15. doi: 10.1620/tjem.235.1
9. Zhang M, Tang J, Yin J, Wang X, Feng X, Yang X, et al. The clinical implication of serum cyclophilin A in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. (2018) 13:357–63. doi: 10.2147/COPD.S152898
10. Ohtsuki T, Satoh K, Omura J, Kikuchi N, Satoh T, Kurosawa R, et al. Prognostic impacts of plasma levels of cyclophilin A in patients with coronary artery disease arterioscler. Thromb Vasc Biol. (2017) 37:685–93. doi: 10.1161/ATVBAHA.116.308986
11. Nakano N, Sakashita S, Matsuoka R, Murata Y, Shiba-Ishii A, Kobayashi N, et al. Cyclophilin A expression and its prognostic significance in lung adenocarcinoma. Pathol Int. (2017) 67:555–63. doi: 10.1111/pin.12593
12. Liu MC, Lee YW, Lee PT, Chang CS, Tai YL, Yu JR, et al. Cyclophilin A is associated with peripheral artery disease and chronic kidney disease in geriatrics: The Tianliao Old People (TOP) study. Sci Rep. (2015) 5:9937. doi: 10.1038/srep09937
13. Huang CH, Chang CC, Kuo CL, Huang CS, Lin CS, Liu CS. Decrease in plasma cyclophilin A concentration at 1 month after myocardial infarction predicts better left ventricular performance and synchronicity at 6 months: a pilot study in patients with ST elevation myocardial infarction. Int J Biol Sci. (2015) 11:38–47. doi: 10.7150/ijbs.10271
14. Chen B, Shen J, Zheng G, Qiu S, Yin H, Mao W, et al. Serum cyclophilin A concentrations and prognosis of acute intracerebral hemorrhage. Clin Chim Acta. (2018) 486:162–7. doi: 10.1016/j.cca.2018.08.002
Keywords: Cyclophilin A, craniocerebral trauma, prognosis, severity, Glasgow Coma Scale (GCS) score
Citation: Li P-F, Zhang J-C, He X-J, Niu J-H, Wu W-F and Li T (2022) The correlation between serum Cyclophilin A level and severity, prognosis of craniocerebral injury. Front. Neurol. 13:968071. doi: 10.3389/fneur.2022.968071
Received: 21 June 2022; Accepted: 25 October 2022;
Published: 28 November 2022.
Edited by:
Wael M. Y. Mohamed, International Islamic University Malaysia, MalaysiaReviewed by:
Xiao-Qiao Dong, Zhejiang Chinese Medical University, ChinaQuan Du, Zhejiang Chinese Medical University, China
Copyright © 2022 Li, Zhang, He, Niu, Wu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tong Li, ZG9jdG9ybHQyMDIyQDEyNi5jb20=
†These authors have contributed equally to this work
 Peng-Fei Li
Peng-Fei Li Jing-Chen Zhang†
Jing-Chen Zhang†