
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol. , 20 September 2022
Sec. Stroke
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.966190
 Ronda Lun1,2,3*
Ronda Lun1,2,3* Danielle Carole Roy2
Danielle Carole Roy2 Yu Hao4
Yu Hao4 Rishi Deka5,6,7
Rishi Deka5,6,7 Wen-Kuan Huang8
Wen-Kuan Huang8 Babak B. Navi9,10
Babak B. Navi9,10 Deborah M. Siegal3,11
Deborah M. Siegal3,11 Tim Ramsay2,3
Tim Ramsay2,3 Dean Fergusson2,3
Dean Fergusson2,3 Risa Shorr12
Risa Shorr12 Dar Dowlatshahi1,2,3
Dar Dowlatshahi1,2,3Background: Patients newly diagnosed with cancer represent a population at highest risk for stroke. The objective of this systematic review and meta-analysis was to estimate the incidence of stroke in the first year following a new diagnosis of cancer.
Methods: We searched MEDLINE and EMBASE from January 1980 to June 2021 for observational studies that enrolled adults with a new diagnosis of all cancers excluding non-melanoma skin cancer, and that reported the incidence of stroke at 1 year. PRISMA guidelines for meta-analyses were followed. Two reviewers independently extracted data and appraised risk of bias. We used the Dersimonian and Laird random effects method to pool cumulative incidences after logit transformation, and reported pooled proportions as percentages. Statistical heterogeneity was assessed using the I2 statistic.
Results: A total of 12,083 studies were screened; 41 studies were included for analysis. Data from 2,552,121 subjects with cancer were analyzed. The cumulative incidence of total stroke at 1 year was 1.4% (95% CI 0.9–2.2%), while the pooled incidence of ischemic stroke was 1.3% (95% CI 1.0–1.8%) and 0.3% (95% CI 0.1–0.9%) for spontaneous intracerebral hemorrhage (ICH), with consistently high statistical heterogeneity (>99% I2).
Conclusion: The estimated incidence of stroke during the first year after a new diagnosis of cancer is 1.4%, with a higher risk for ischemic stroke than ICH. Cancer patients should be educated on the risk of stroke at the time of diagnosis. Future studies should evaluate optimal primary prevention strategies in this high-risk group of patients.
Systematic review registration: https://osf.io/ucwy9/.
Cancer is a well-known risk factor for stroke (1), and both conditions are associated with a high degree of morbidity and mortality. There appears to be an increased risk for arterial thromboembolic events in the months preceding a diagnosis of cancer (2–4). Many studies have found that the risk for stroke increases around the time of cancer diagnosis and that this elevated risk may persist for up to 10 years after diagnosis (1). While this risk attenuates over time, it is hypothesized that the first year after a new diagnosis of cancer represents the period during which cancer patients have the highest stroke risk (2). From a clinical care standpoint, identifying patients with a new diagnosis of cancer who have not yet experienced a stroke represents an opportunity to study and implement primary prevention strategies in order to maximize quality of life in cancer patients. Thus, the objective of this systematic review and meta-analysis was to examine the risk of stroke in the first year following a new diagnosis of cancer.
The data underlying this article will be shared on reasonable request to the corresponding author.
The protocol for this study was registered at the Open Science Framework (osf.io/ucwy9) and published in a peer-reviewed journal (1). This study was conducted based on the guidelines of the Cochrane Handbook of Systematic Reviews (2). It was reported using the updated guidelines for Preferred Reporting Items for Systematic Reviews (PRISMA) (3).
Our systematic literature search was limited to studies in adults with a new diagnosis of cancer, encompassing all cancer subtypes except non-melanoma skin cancers (i.e., basal cell and squamous cell carcinoma) due to their favorable prognosis and treatment with local measures not requiring systemic therapy, resulting in inaccuracies in administrative diagnostic coding. Studies must report the incidence of ischemic stroke and/or spontaneous intracerebral hemorrhage (ICH) during the first year after cancer diagnosis. If a graphical representation of the data was provided without a corresponding numerical value, the incidence at 1 year was extracted using the Engauge Digitizer software (4, 5). As the primary objective of this systematic review was to synthesize the natural history (i.e., incidence) of stroke in the cancer population, our search was limited to only observational studies and we excluded any interventional studies, as they represent a different population—it is estimated that <5% of adult cancer patients enroll in clinical trials (6), and this population is comparatively much healthier and younger than the general cancer population (7). Further details on our inclusion/exclusion criteria can be found in our published protocol (1).
Our search strategy was conducted with the assistance of a health science librarian with expertise in systematic reviews (R.S.). We searched MEDLINE and EMBASE via OVID and PubMed and included all relevant studies from January 1980 to June 2021. A sample search strategy can be found in Supplementary Table 1; detailed search strategies can be found in our published protocol (1). We further searched the abstract databases from both the International Conference of Stroke and the European Stroke Organization Conference for the same time period. Our search was restricted to human adult subjects, and the language was limited to English language only.
Two reviewers (R.L. and D.C.R.) independently completed two-level screening for articles using the Covidence Systematic Review software. Any discrepancies were resolved by discussion with a third senior author (D.D.). Findings from the screening process were summarized using a flow diagram (Figure 1).
A standardized data extraction form was created a priori and piloted independently by the two reviewers. The data extraction form was separated into bibliographic information [i.e., study ID, authors, title, funding status, journal, country and year of publication, study type, and name of databased used (if applicable)], subject information (i.e., number of participants total, number of participants with cancer, sex, age, cancer type, stage, and prevalence of comorbidities), and outcomes (i.e., incidence of ischemic stroke and/or ICH at 1 month, 3 months, 6 months, and 1 year). Wherever information had to be extracted using the Engauge Digitizer, only information at 1 year was extracted.
One reviewer (R.L.) performed primary data extraction from the included studies and a second reviewer (D.C.R.) peer reviewed the extracted information. Disagreements were resolved via discussion. For studies that met inclusion criteria but did not report the specific outcome we required, the corresponding author of the study was contacted for the data a minimum of two times.
Risk of bias (ROB) of individual studies was assessed using the Newcastle-Ottawa Scale (NOS) for cohort studies (8). Two raters (R.L. and D.C.R.) independently implemented the tool for all included studies and any disagreements in the rating were resolved by discussion. The following thresholds for converting the NOS to the Agency for Healthcare Research and Quality (AHRQ) standards are:
• Good quality: 3 or 4 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome/exposure domain.
• Fair quality: 2 stars in selection domain AND 1 or 2 stars in comparability domain AND 2 or 3 stars in outcome/exposure domain.
• Poor quality: 0 or 1 star in selection domain OR 0 stars in comparability domain OR 0 or 1 stars in outcome/exposure domain.
Our primary outcome was the cumulative incidence of stroke (total, ischemic, and hemorrhagic) at 1 year after a new diagnosis of cancer, and defined as the proportion of events divided by the sample size. Given the low number of studies that reported the incidence of stroke at 1, 3, and 6 months, we only reported the incidence of total stroke at these timepoints after pooling ischemic and hemorrhagic stroke. The transformed incidences were pooled using the Dersimonian and Laird random effects method (9). Between-study heterogeneity was assessed by the I2 statistic. To explore potential sources of heterogeneity, we caried out pre-specified subgroup analyses, including stratification based on ROB assessments, year of publication, location of primary cancer for studies that studied only one subtype of cancer, presence of atrial fibrillation, study design (i.e., prospective vs. retrospective cohorts), and nature of study (i.e., population-based or hospital-based). Univariate meta-regression analyses were carried out to test the influence of subgroup effects on heterogeneity. Sensitivity analyses were performed with the leave-one-out method to assess the influence of each study on the overall effect-size estimate. As this is a meta-analysis of pooled proportions from single cohorts (i.e., does not assess the effect of an intervention), publication bias was not assessed (2).
All statistical analyses were performed using the OpenMeta-Analyst software (10, 11).
Our search identified 12,083 studies across all databases searched. After removing duplicates, we screened 10,378 titles and abstracts and found 485 potentially eligible titles. During the second stage of full-text screening, we further excluded 444 studies (Figure 1), resulting in 41 full-text articles for data extraction and synthesis.
The characteristics of included studies are listed in Table 1. The median year of publication was 2018 [interquartile range (IQR) 2015–2019]. Of the 41 studies, 18 were from Asia, 12 were from the United States, and 11 were from Europe. In total, they contributed 10,637,699 subjects for analysis, and 2,552,121 subjects had a new diagnosis of cancer. The sample size of cancer patients in the included studies ranged from 48 to 820,491, with a median sample size of 7,479 (IQR 893–22,737). Overall, 46% of the patients were women. Three studies were prospective cohort studies (12, 13), and the remaining were retrospective cohort studies. There were 29 studies that examined a single type of cancer (i.e., lung cancer), and the remaining studies included multiple types of cancer (Table 1).
Of the included 41 studies, 22 reported the incidence of combined ischemic stroke and spontaneous ICH at 1 year (12, 14–34). For studies that reported the incidence of ischemic stroke and ICH separately, total stroke events were calculated by the sum of events. As shown in Figure 2, the pooled incidence of ischemic stroke and ICH at 1 year was 1.4% (95% CI 0.9–2.2%) with a high degree of statistical heterogeneity present (I2 = 99.92%).
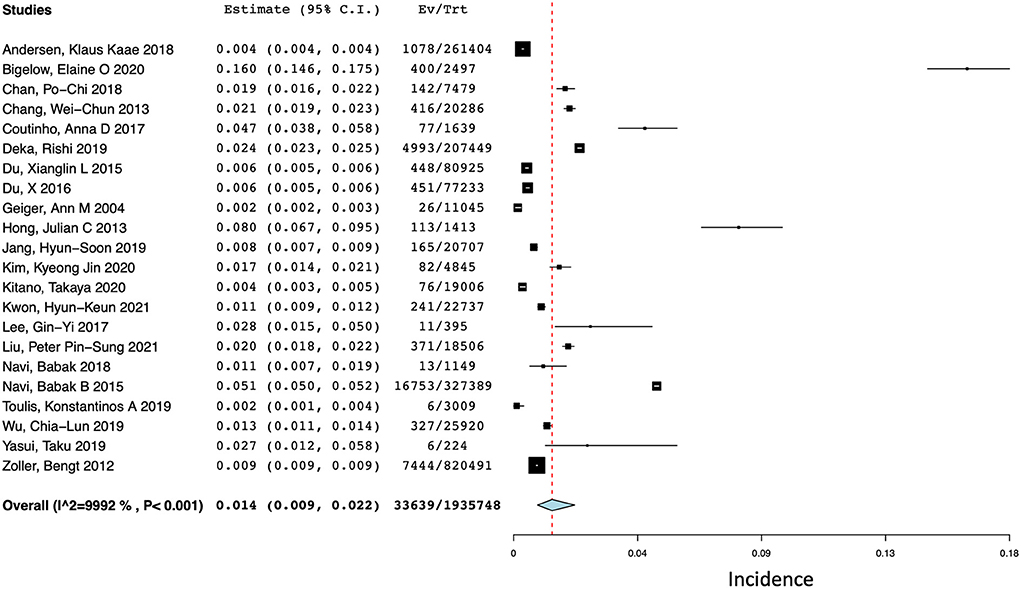
Figure 2. Forest plot of the pooled incidence of total stroke (ischemic and hemorrhagic) at 1 year after a diagnosis of cancer.
Due to the low number of studies that reported the incidence of ischemic stroke and ICH at 1, 3, and 6 months, we only evaluated the cumulative incidence of total stroke (i.e., sum of events) at these timepoints. Five studies reported the incidence of stroke at 1 month (12, 16, 35–37), four reported the incidence of stroke at 3 months (12, 31, 35, 36), and eight reported the incidence of stroke at 6 months (12, 14, 16, 17, 31, 35, 36, 38). Their respective pooled incidences at 1, 3, and 6 months are 1.6% (95% CI 0.2–10.8%; I2 = 99.78%), 1.0% (95% CI 0.3–2.8%; I2 = 99.95%), and 1.3% (95% CI 0.6–2.9%; I2 = 99.96%) (Supplementary Figure 1).
Of the 41 studies, 23 (N = 1,972,059) reported the incidence of ischemic stroke events at 1 year after a diagnosis of cancer (13–15, 17, 18, 20, 25, 26, 30, 34–36, 39–49). The pooled incidence of ischemic stroke events during the first year after a new diagnosis of cancer (see Figure 3) was 1.3% (95% CI 1.0–1.8%) with a high degree of statistical heterogeneity present (I2 = 99.72%).
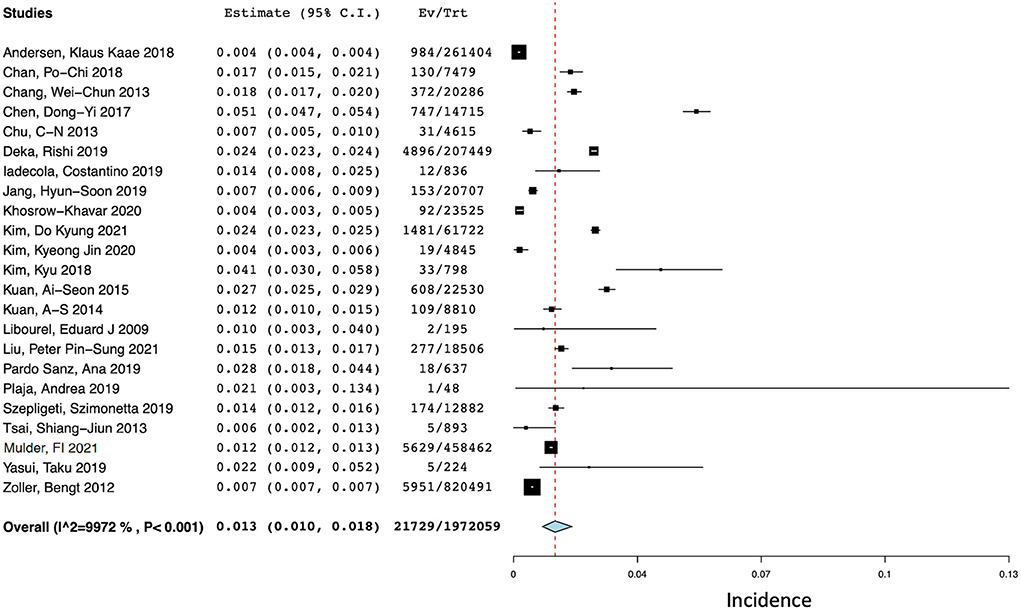
Figure 3. Forest plot of the pooled incidence of ischemic stroke at 1 year after a diagnosis of cancer.
There were 11 studies (N = 1,361,817) that reported the incidence of ICH at 1 year after a diagnosis of cancer (14, 15, 17, 18, 20, 25, 26, 30, 34, 50, 51). The pooled incidences of ICH at 1 year after diagnosis was 0.3% (95% CI 0.1–0.9%) with a high degree of statistical heterogeneity present (I2 = 99.46%) (Figure 4).
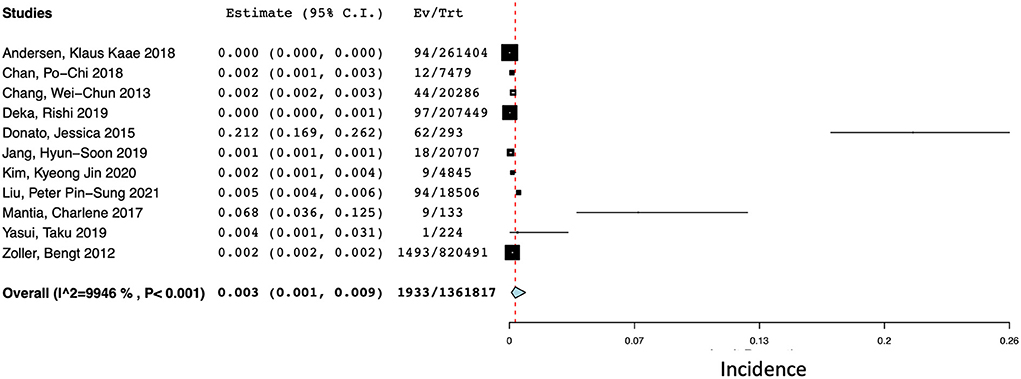
Figure 4. Forest plot of the pooled incidence of spontaneous ICH at 1 year after a diagnosis of cancer.
Three studies exclusively included cancer patients with atrial fibrillation (34, 43, 46). Two of these studies specifically examined the use of anticoagulation in these patients (34, 43). while the third study reported approximately that 85% of their cohort was on anticoagulation (46). In a subgroup analysis of studies that only included cancer patients with atrial fibrillation, the incidence of ischemic stroke was 3.3% at 1 year (95% CI 2.4–4.6%) with relatively low heterogeneity (I2 = 29.70%), which was significantly higher than the incidence of ischemic stroke in 20 other studies [1.2% (95% CI 0.9–1.6%), I2 = 99.76%] (Supplementary Figure 2).
Out of 22 studies that reported the combined incidence of ischemic and hemorrhagic stroke at 1 year, eight were cohort studies that included multiple types of cancer (Supplementary Figure 3) (12, 14, 15, 25, 27, 31, 33, 34). The remaining 14 studies were limited to specific cancer subtypes including head and neck cancer (n = 3) (16, 24, 28), hematologic malignancies (n = 2) (19, 29), breast cancer (n = 2) (21, 23), and thyroid cancer (n = 2) (26, 32). There was only one study for each of the following subtypes of cancer: pancreatic, cervical, prostate, colorectal, and hepatic (17, 18, 20, 22, 30). Subgroup analyses based on cancer type found that the pooled incidence of stroke for the eight studies that enrolled multiple types of cancer had a similar incidence to our overall pooled incidence: 1.1% (95% CI 0.5–2.7%), with significant heterogeneity (I2 = 99.97%). Studies that enrolled head and neck cancer patients exclusively appeared to have a higher incidence of stroke [5.3% (95% CI 0.8–27.5%, I2 = 99.83%)], although this difference was not statistically significant. Studies that enrolled hematologic cancer patients also reported a higher incidence of stroke: 3.9% (95% CI 2.4–6.3%), with moderate heterogeneity, I2 = 63.64% (Supplementary Figure 3). Thyroid cancer appeared to have a non-significant lower risk of stroke: 0.6% (95% CI 0.1–4.8%), while breast cancer patients reported a lower risk of stroke that was statistically significant: 0.4% (95% CI 0.2–0.9%, I2 = 95.10%). Based on results from a single study each, it appeared that pancreatic cancer, cervical cancer, prostate cancer, and hepatic cancer all had elevated risk of stroke compared to the overall pooled incidence of stroke (Supplementary Figure 3).
Out of the 22 studies that reported 1-year incidence of total stroke, there were 5/22 that used a hospital-based sample, and 17/22 that used a population-based sample. There was no statistical difference between the two groups in terms of the overall incidence: 1.6% (95% CI 0.9–2.8%) for population-based studies and 1.4% (95% CI 0.9–2.2%) for hospital-based studies (Supplementary Figure 7). We also performed subgroup analyses stratified by retrospective vs. prospective design and did not find a significant difference in 1-year incidence between these two groups (Supplementary Figure 8): 1.5% (95% CI 0.9–2.3%) for retrospective studies and 1.4 (95% CI 0.7–1.9%) for prospective. It is also important to note that in the analysis for total stroke, only 1/22 studies were prospective and the remaining 21 were retrospective.
Sensitivity analyses were performed with the leave-one-out method to examine the stability of the results (Supplementary Figure 4). We found that no single study significantly altered the pooled effect measure—cumulative incidences ranged from 1.3% (95% CI 0.8–2.0%) to 1.6% (95% CI 0.010–0.025), which was not statistically different from the pooled estimate of 1.4% (95% CI 0.9–2.2%). Subgroup analysis based on the geographic region of enrolled patients found that the risk of stroke appeared to be highest in cohorts from the United States (4.1%, 95% CI 2.8–5.3%) compared to cohorts from Asia (1.5%, 95% CI 1.8–2.7%) or Europe (0.5%, 95% CI 0.1–0.9%) (Supplementary Figure 5). Between-study heterogeneity was statistically quantified with univariate meta-regression analyses, which identified that substantial heterogeneity originated from differences in cancer type (p = 0.014), but not from year of publication, risk of bias, presence of atrial fibrillation, or study design (i.e., retrospective vs. prospective).
Using the NOS risk of bias tool, we found that 6/41 studies were “poor quality,” one was “fair quality,” and the remaining 34 were “good quality” (Supplementary Table 2). Subgroup analysis stratified by the ROB ratings found that 20 of 22 studies that reported 1-year stroke outcomes were deemed “good quality” (12, 14–28, 30–33), while one received a “poor” rating (34) and one received a “fair” rating (29). The studies that received a “good” rating had a similar pooled incidence of stroke at 1 year compared to the primary analysis (Supplementary Figure 6): 1.4% (95% CI 0.9–2.2%, I2 = 99.92%). The study with a “poor” rating reported a slightly higher cumulative incidence of stroke at 1 year [2.7% (95% CI 1.2–5.8%)], although this difference was not statistically significant. The single study that received a “fair” rating similarly reported a slightly higher incidence of stroke at 1 year: 2.8% (95% CI 1.5–5.0%).
Through our systematic literature search, we identified that the cumulative incidence of any stroke during the first year after a new diagnosis of cancer was 1.4%. The risk of ischemic stroke was similar (1.3%), but the risk for spontaneous ICH was much lower comparatively, at 0.3%. The risk for ischemic stroke was particularly high in patients with cancer and atrial fibrillation, and in those with head and neck cancer and hematologic cancers. For context, the incidence of stroke in those aged 55 years or older has been reported to be ~5.3 per 1,000 person-years, which translates to a cumulative incidence of ~0.53% per year for a single individual (52). This suggests that compared to the general population, there is approximately a 2.6-fold increase in the risk for stroke during the first year after a new cancer diagnosis.
There are two previously published systematic reviews that examined the risk of stroke in cancer survivors compared to cancer-free populations (53, 54). Zhang et al. found that the relative risk for stroke was 1.66 times higher (95% CI 1.35–2.04) for cancer survivors compared to the cancer-free population over an unspecified follow-up period (53). Turner et al. reported a hazard ratio of 1.22 (95% CI 1.12–1.33) across all stroke subtypes when examining adult cancer survivors. The slight differences between their estimates and ours is likely a reflection of differences in study population, follow-up time, and statistical pooling methods –by evaluating cancer survivors, both studies would have excluded malignancies with poor prognoses, and may have also excluded those who suffered a stroke already with consequent mortality. Zhang et al. also included those with childhood cancers and used relative risk to approximate and pool all effect measures (i.e., relative risk, hazard ratio, incidence rate ratio, standardized incidence ratios), which may have reduced accuracy, particularly when dealing with relatively rare outcomes (53). Additionally, our population likely represents one that is at highest risk for stroke, given the risk for stroke in cancer patients is not constant over time—previous studies have found that stroke risk increases during the months leading up to a diagnosis of cancer, and peaks around the time of diagnosis (55, 56). Turner et al. similarly reported that the incidence of stroke may be highest immediately after a diagnosis of new cancer (i.e., first 6 months) (54).
Our meta-analysis identified that the combined risk of stroke varied by geographic location: the risk of stroke was significantly higher in cohorts from the United States compared to the Asia and Europe. This is different than previously reported numbers of stroke risk by geographic region in the general population: East Asia has been reported to have the highest lifetime risk of stroke, followed by Central and Eastern Europe (57). The discrepancy reported in our study may reflect the heterogeneity that is present in baseline patient characteristics and cancer subtypes across studies. Using visual inspection of the forest plot, it is evident that the higher incidence of stroke in the United States cohort may be largely driven by three studies: Bigelow (study ID 420), Hong (study ID 2599) and B. Navi (study ID: 2397) (Supplementary Figure 5) (16, 24, 31). Two of these studies (Hong and Bigelow) were specifically investigating the risk of stroke in elderly patients with glottic and oropharyngeal cancer—the respective median/mean ages of cancer subjects include in these two cohort studies were 72 (IQR 69–77) and 73.8 (no standard deviation reported) (16, 24). The population from the 2015 study by Navi selectively included patients with breast, colorectal, lung, pancreatic, or prostate cancer from the Surveillance Epidemiology and End-Results (SEER) Medicare database—the authors chose these cancer types a priori because pancreatic cancer is the cancer type most commonly reported in association with thromboembolic events, and the remaining four represent the most common cancers reported in the United States population (31). This may therefore represent a highly selected group of patients, as gastric, lung and pancreatic cancers are particularly associated with a higher risk for stroke (53, 54, 58). These cancers are also more likely to be at advanced stages at the time of diagnosis, which is an independent risk factor to increase the risk for thromboembolic events (31). Unfortunately information regarding cancer stage was not available for this meta-analysis. Furthermore, the SEER-Medicare database restricts the study population to patients 65 and older so the higher rates of stroke may be partly driven by the older population—this is supported by the high rates of stroke seen in the matched control patients without cancer (31).
Our subgroup analysis found that in patients with cancer and atrial fibrillation, there was an elevated risk for ischemic stroke compared to the general cancer cohort: 3.3 vs. 1.3%, respectively. This is similar to what is reported in the literature—a large national registry-based cohort study reported that the annual incidence rate of ischemic stroke was 3.44% in those with atrial fibrillation and active or history of cancer (59). This risk is stratified by the presence of comorbidities, including older age, and the presence of other vascular risk factors (60, 61). While current risk stratification tools for atrial fibrillation (i.e., CHADS2 or CHADS2-VASC) do not take cancer into account, when applied in a population of cancer patients with atrial fibrillation, they were still found to be predictive of ischemic stroke (62). There is a higher prevalence of atrial fibrillation in patients with cancer compared to those without cancer—a relationship that persists regardless of surgery or cancer therapy (63). The mechanism by which this occurs is postulated to be multifactorial, but may be related to higher incidences of post-operative atrial fibrillation, chemotherapy-related adverse effects, cancer-associated systemic inflammation which promotes atrial re-structuring, and autonomic dysregulation in patients with malignancy (63–66). Thus, the co-occurrence of atrial fibrillation and cancer represents a highly susceptible population with the highest risk for ischemic stroke; these patients may require anticoagulation for primary prevention.
There are no randomized studies that evaluate the efficacy and safety of antithrombotic therapies specifically for the primary prevention of stroke in patients with new/active cancer. Initiation of antithrombotics in this population is challenging for multiple reasons. First, currently there is no risk stratification tool for accurately identifying patients with cancer at the highest risk for stroke. This represents the biggest challenge, as patients with cancer on antithrombotic therapy are also at increased risk for major bleeding compared to non-cancer patients, and therefore accurate patient selection is crucial (67). Second, the choice of antithrombotic for primary prevention should be highly individualized. For example, anticoagulation is recommended for the prevention of stroke in patients with atrial fibrillation, and it is generally accepted that direct oral anticoagulants (DOAC) are preferred to warfarin (68). While patients with cancer were excluded from the large DOAC trials comparing rivaroxaban, apixaban, dabigatran, and edoxaban to warfarin (69–72), recent observational data suggests that DOAC is comparable to warfarin in terms of efficacy and safety profiles, and the Canadian Cardiovascular Society recommends the use of DOAC as first line anticoagulation therapy in patients with atrial fibrillation and active cancer [weak recommendation; low-quality evidence] (68). However, many DOACs are mainly metabolized via cytochrome P450 3A4 in the liver, and concomitant use of DOACs with drugs that regulate this pathway may be contraindicated, as many chemotherapeutic agents fall in this category (73). This example highlights that the choice of antithrombotic therapy in cancer patients should be highly individualized due to multiple patient and treatment considerations, and therefore should be chosen under the guidance of an expert multidisciplinary team (68).
In contrast, there is high-level evidence to support the use of reduced-dose DOACs in the setting of primary prevention for venous thromboembolism (VTE) in ambulatory cancer patients starting chemotherapy at intermediate to high risk for VTE (defined as Khorana Score ≥2) (74, 75). In two large randomized controlled trials, reduced-dose apixaban and rivaroxaban lowered the risk for VTE compared to placebo with an acceptable risk of bleeding (74, 75). Although this indirect evidence generally supports the use of antithrombotic treatments to prevent cardiovascular events among newly diagnosed cancer patients, the low number of cerebrovascular events in both trials may limit the generalizability of these results (74, 75).
Our study reported a very high I2 value, indicative of substantial between-study heterogeneity. Significant heterogeneity is a well-recognized statistical phenomenon for meta-analyses of single proportions, and interpretation of I2 values in this context is challenging (53, 76). Extremely large sample sizes of single cohort studies result in very narrow confidence intervals, thereby I2 for pooled estimates can be extremely high even in the presence of modest inconsistency between studies (77). Moreover, despite the extreme values of heterogeneity, we feel these results are still valid and clinically informative, as the primary objective of this study was to provide a global estimate of the risk for stroke in newly diagnosed cancer patients—a heterogeneous population to begin with. Therefore, it may be reasonable to sacrifice statistical homogeneity for broad inclusion criteria encompassing different cancer subtypes, treatments, and settings to increase the generalizability of our results and statistical power (78). Using univariate meta-regression analyses, we found that substantial heterogeneity could be attributed to differences in cancer type, which is known to influence the risk of stroke (15). Residual heterogeneity likely also originates from differences in baseline patient characteristics which cannot be quantified in an aggregate-level meta-analysis (i.e., patient age, sex, presence of vascular comorbidities, differences in cancer treatment, and use of antithrombotics). For example, Pardo Sanz et al. examined a cohort of patients with breast cancer and atrial fibrillation, where 84.9% of patients were anticoagulated (46). However, as not everyone in the study was anticoagulated, this study could not be included in our subgroup analysis looking at the use of anticoagulation. Therefore, significant heterogeneity remains despite our attempts at quantification of the sources of heterogeneity.
Our meta-analysis provides significant clinical implications for the risk of stroke in patients newly diagnosed with cancer. Our study confirms that the risk of ischemic stroke is high compared to the general population during the first year after a cancer diagnosis, and these patients should be considered for primary prevention strategies. At present, there isn't enough evidence to support the use of antithrombotic therapy empirically in these patients. However, patients should at least be educated that their stroke risk is high during this time period, and patient education regarding healthy lifestyle habits (i.e., healthy diet, regular exercise, smoking cessation, alcohol consumption reduction) should be provided. Furthermore, patient education around what to look for in terms of signs or symptoms of stroke should also be emphasized to minimize delays in access to acute treatments.
Our study has several strengths to note. This meta-analysis produced one of the largest pooled cohorts of newly diagnosed patients with cancer in which to characterize the risk of stroke. We believe our pooled results will provide patients and clinicians with an accurate estimation of the cumulative incidence of stroke during a high-risk period. Our statistical methodology was robust, with the publication of an a priori, peer-reviewed study protocol and a comprehensive search strategy encompassing multiple databases (1). However, our study is not without limitations. First, our pooled results reported significant heterogeneity among the included studies, which is likely due to differences in baseline patient characteristics (i.e., cancer subtype, age, sex, presence of vascular risk factors, ethnicity, treatment and prognoses), as discussed above. However, we believe that the increased statistical power we report due to our large sample size is also a strength of our study and may result in increased generalizability of these results. Unfortunately due to the lack of access to individual patient data and significant variations in how age was summarized and reported across studies, we could not perform subgroup analyses stratified by age. Next, our meta-analysis only included observational cohort studies; we may have missed potential data from randomized/interventional studies and gray literature. This was intentional during the design of our study protocol, as we wanted to focus on the natural history of disease so the results are applicable to all cancer patients, and the clinical trial population is often highly selected in oncology literature. As this meta-analysis was conducted exclusively in the English language, we may have missed literature published in other languages, which might have introduced language bias, though previous studies found little to no effect on summary measures excluding non-English studies (79). Lastly, medical surveillance bias should be considered when interpreting studies reporting risk for other diseases in the cancer population, as cancer patients may be followed more closely than non-cancer patients given the complexities of treatment.
We found that the estimated cumulative incidence of stroke during the first year after a new diagnosis of cancer is 1.4%, and the risk for ischemic stroke is higher than the risk for spontaneous ICH. Patients newly diagnosed with cancer require education around the risk of stroke at the time of their diagnosis, as well as signs and symptoms of stroke to watch out for to minimize delays in access to acute treatments. Healthcare providers should advocate for conservative primary prevention strategies in this group of high-risk patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
RL: conceptualization, data curation, formal analysis, investigation, methodology, software, writing—original draft, reviewing, and editing. DR: data curation, investigation, methodology, reviewing, and editing. YH, RD, W-KH, and BN: data curation, investigation, reviewing, and editing. DS: conceptualization, methodology, supervision, reviewing, and editing. TR: conceptualization, data curation, methodology, supervision, reviewing, and editing. DF: data curation, formal analysis, investigation, methodology, resources, software, supervision, and reviewing and editing. RS: data curation, methodology, and reviewing and editing. DD: conceptualization, data curation, formal analysis, investigation, methodology, resources, supervision, and reviewing and editing. All authors contributed to the article and approved the submitted version.
We would like to acknowledge the collaborators who shared their data with us for this systematic review and meta-analysis. This manuscript was a collaborative effort and could not have been possible without the willingness of each of you. RL would like to recognize the CIHR and Ontario Graduate Student for supporting her with a Masters student scholarship during the time period this research was conducted.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer Y-CW declared a shared affiliation with the author W-KH to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.966190/full#supplementary-material
1. Lun R, Roy DC, Ramsay T, Siegal D, Shorr R, Fergusson D, et al. Incidence of stroke in the first year after diagnosis of cancer-a protocol for systematic review and meta-analysis. PLoS ONE. (2021) 16:e0256825. doi: 10.1371/journal.pone.0256825
2. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane (2020). Available online at: www.training.cochrane.org/handbook
3. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
4. Shi X, Chen Q, Wang F. Mesenchymal stem cells for the treatment of ulcerative colitis: a systematic review and meta-analysis of experimental and clinical studies. Stem Cell Res Ther. (2019) 10:266. doi: 10.1186/s13287-019-1336-4
5. Huang C, Lin X, He J, Liu N. Enrichment and detection method for the prognostic value of circulating tumor cells in ovarian cancer: a meta-analysis. Gynecol Oncol. (2021) 161:613–20. doi: 10.1016/j.ygyno.2021.02.024
6. Unger JM, Cook E, Tai E, Bleyer A. Role of clinical trial participation in cancer research: barriers, evidence, and strategies. Am Soc Clin Oncol Educ Book Am Soc Clin Oncol Meet. (2016) 35:185–98. doi: 10.1200/EDBK_156686
7. Sedrak MS, Freedman RA, Cohen HJ, Muss HB, Jatoi A, Klepin HD, et al. Older adult participation in cancer clinical trials: a systematic review of barriers and interventions. CA Cancer J Clin. (2021) 71:78–92. doi: 10.3322/caac.21638
8. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-Analyses. The Ottawa Hospital Research Institute. Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed February 26, 2021).
9. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
10. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. (2010) 36:1–48. doi: 10.18637/jss.v036.i03
11. Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw. (2012) 49:1–15. doi: 10.18637/jss.v049.i05
12. Navi BB, Howard G, Howard VJ, Zhao H, Judd SE, Elkind MSV, et al. New diagnosis of cancer and the risk of subsequent cerebrovascular events. Neurology. (2018) 90:e2025–33. doi: 10.1212/WNL.0000000000005636
13. Libourel EJ, Sonneveld P, van der Holt B, de Maat MPM, Leebeek FWG. High incidence of arterial thrombosis in young patients treated for multiple myeloma: results of a prospective cohort study. Blood. (2010) 116:22–6. doi: 10.1182/blood-2009-12-257519
14. Zöller B, Ji J, Sundquist J, Sundquist K. Risk of haemorrhagic and ischaemic stroke in patients with cancer: a nationwide follow-up study from Sweden. Eur J Cancer Oxf Engl 1990. (2012) 48:1875–83. doi: 10.1016/j.ejca.2012.01.005
15. Andersen KK, Olsen TS. Risk of ischemic and hemorrhagic strokes in occult and manifest cancers. Stroke. (2018) 49:1585–92. doi: 10.1161/STROKEAHA.118.021373
16. Bigelow EO, Blackford AL, Eytan DF, Eisele DW, Fakhry C. Burden of comorbidities is higher among elderly survivors of oropharyngeal cancer compared with controls. Cancer. (2020) 126:1793–803. doi: 10.1002/cncr.32703
17. Chan PC, Chang WL, Hsu MH, Yeh CH, Muo CH, Chang KS, et al. Higher stroke incidence in the patients with pancreatic cancer: a nation-based cohort study in Taiwan. Medicine. (2018) 97:e0133. doi: 10.1097/MD.0000000000010133
18. Chang WC, Muo CH, Chang SN, Sung FC, Chang YJ, Kao CH, et al. nationwide population-based retrospective cohort study: decreased risk of stroke in cervical cancer patients after receiving treatment. Arch Gynecol Obstet. (2013) 288:867–71. doi: 10.1007/s00404-013-2827-7
19. Coutinho AD, Makenbaeva D, Farrelly E, Landsman-Blumberg PB, Lenihan D. Elevated cardiovascular disease risk in patients with chronic myelogenous leukemia seen in community-based oncology practices in the United States. Clin Lymphoma Myeloma Leuk. (2017) 17:676–83. doi: 10.1016/j.clml.2017.06.011
20. Deka R, Simpson DR, Panizzon MS, Hauger RL, Riviere P, Nalawade V, et al. Stroke and thromboembolic events in men with prostate cancer treated with definitive radiation therapy with or without androgen deprivation therapy. Prostate Cancer Prostatic Dis. (2019) 22:600–8. doi: 10.1038/s41391-019-0150-5
21. Du XL, Zhang Y, Hardy D. Associations between hematopoietic growth factors and risks of venous thromboembolism, stroke, ischemic heart disease and myelodysplastic syndrome: findings from a large population-based cohort of women with breast cancer. Cancer Causes Control. (2016) 27:695–707. doi: 10.1007/s10552-016-0742-5
22. Du XL, Zhang Y. Risks of venous thromboembolism, stroke, heart disease, and myelodysplastic syndrome associated with hematopoietic growth factors in a large population-based cohort of patients with colorectal cancer. Clin Colorectal Cancer. (2015) 14:e21–31. doi: 10.1016/j.clcc.2015.05.007
23. Geiger AM, Fischberg GM, Chen W, Bernstein L. Stroke risk and tamoxifen therapy for breast cancer. J Natl Cancer Inst. (2004) 96:1528–36. doi: 10.1093/jnci/djh285
24. Hong JC, Kruser TJ, Gondi V, Mohindra P, Cannon DM, Harari PM, et al. Risk of cerebrovascular events in elderly patients after radiation therapy versus surgery for early-stage glottic cancer. Int J Radiat Oncol Biol Phys. (2013) 87:290–6. doi: 10.1016/j.ijrobp.2013.06.009
25. Jang HS, Choi J, Shin J, Chung JW, Bang OY, Kim GM, et al. The long-term effect of cancer on incident stroke: a nationwide population-based cohort study in Korea. Front Neurol. (2019) 10:52. doi: 10.3389/fneur.2019.00052
26. Kim KJ, Song JE, Kim JY, Bae JH, Kim NH, Yoo HJ, et al. Effects of radioactive iodine treatment on cardiovascular disease in thyroid cancer patients: a nationwide cohort study. Ann Transl Med. (2020) 8:1235. doi: 10.21037/atm-20-5222
27. Kitano T, Sasaki T, Gon Y, Todo K, Okazaki S, Kitamura T, et al. The effect of chemotherapy on stroke risk in cancer patients. Thromb Haemost. (2020) 120:714–23. doi: 10.1055/s-0040-1708484
28. Kwon HK, Han KD, Cheon YI, Shin SC, Lee M, Sung ES, et al. The incidence of myocardial infarction and stroke in head and neck cancer patients. Sci Rep. (2021) 11:4174. doi: 10.1038/s41598-021-83665-4
29. Lee GY, Lee YT, Yeh CM, Hsu P, Lin TW, Gau JP, et al. Risk of stroke in patients with newly diagnosed multiple myeloma: a retrospective cohort study. Hematol Oncol. (2017) 35:726–33. doi: 10.1002/hon.2340
30. Hsu JY, Liu PPS, Liu AB, Huang HK, Loh CH. High 1-year risk of stroke in patients with hepatocellular carcinoma: a nationwide registry-based cohort study. Sci Rep. (2021) 11:10444. doi: 10.1038/s41598-021-89867-0
31. Navi BB, Reiner AS, Kamel H, Iadecola C, Elkind MSV, Panageas KS, et al. Association between incident cancer and subsequent stroke. Ann Neurol. (2015) 77:291–300. doi: 10.1002/ana.24325
32. Toulis KA, Viola D, Gkoutos G, Keerthy D, Boelaert K, Nirantharakumar K. Risk of incident circulatory disease in patients treated for differentiated thyroid carcinoma with no history of cardiovascular disease. Clin Endocrinol. (2019) 91:323–30. doi: 10.1111/cen.13990
33. Wu VCC, Wang CL, Huang YT, Lan WC, Wu M, Kuo CF, et al. Novel oral anticoagulant versus warfarin in cancer patients with atrial fibrillation: an 8-year population-based cohort study. J Cancer. (2020) 11:92–9. doi: 10.7150/jca.36468
34. Yasui T, Shioyama W, Oboshi M, Oka T, Fujita M. Oral anticoagulants in Japanese patients with atrial fibrillation and active cancer. Intern Med. (2019) 58:1845–9. doi: 10.2169/internalmedicine.2415-18
35. Navi BB, Howard G, Howard VJ, Zhao H, Judd SE, Elkind MSV, et al. The risk of arterial thromboembolic events after cancer diagnosis. Res Pract Thromb Haemost. (2019) 3:639–51. doi: 10.1002/rth2.12223
36. Mulder FI, Horváth-Puhó E, van Es N, Pedersen L, Büller HR, Bøtker HE, et al. Arterial thromboembolism in cancer patients: a danish population-based cohort study. JACC CardioOncology. (2021) 3:205–18. doi: 10.1016/j.jaccao.2021.02.007
37. Gurnari C, Breccia M, Di Giuliano F, Scalzulli E, Divona M, Piciocchi A, et al. Early intracranial haemorrhages in acute promyelocytic leukaemia: analysis of neuroradiological and clinico-biological parameters. Br J Haematol. (2021) 193:129–32. doi: 10.1111/bjh.17018
38. van Herk-Sukel MPP, Shantakumar S., Penning-van Beest FJA, Kamphuisen PW, Majoor CJ, Overbeek LIH, et al. Pulmonary embolism, myocardial infarction, and ischemic stroke in lung cancer patients: results from a longitudinal study. Lung. (2013) 191:501–9. doi: 10.1007/s00408-013-9485-1
39. Chen DY, See LC, Liu JR, Chuang CK, Pang ST, Hsieh IC, et al. Risk of cardiovascular ischemic events after surgical castration and gonadotropin-releasing hormone agonist therapy for prostate cancer: a nationwide cohort study. J Clin Oncol. (2017) 35:3697–705. doi: 10.1200/JCO.2016.71.4204
40. Chu CN, Chen PC, Bai LY, Muo CH, Sung FC, Chen SW. Young nasopharyngeal cancer patients with radiotherapy and chemotherapy are most prone to ischaemic risk of stroke: a national database, controlled cohort study. Clin Otolaryngol. (2013) 38:39–47. doi: 10.1111/coa.12064
41. Khosrow-Khavar F, Filion KB, Bouganim N, Suissa S, Azoulay L. Aromatase inhibitors and the risk of cardiovascular outcomes in women with breast cancer: a population-based cohort study. Circulation. (2020) 141:549–59. doi: 10.1161/CIRCULATIONAHA.119.044750
42. Kim DK, Lee HS, Park JY, Kim JW, Ha JS, Kim JH, et al. Does androgen-deprivation therapy increase the risk of ischemic cardiovascular and cerebrovascular diseases in patients with prostate cancer? A nationwide population-based cohort study. J Cancer Res Clin Oncol. (2021) 147:1217–26. doi: 10.1007/s00432-020-03412-6
43. Kim K, Lee YJ, Kim TH, Uhm JS, Pak HN, Lee MH, et al. Effect of non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients with newly diagnosed cancer. Korean Circ J. (2018) 48:406–17. doi: 10.4070/kcj.2017.0328
44. Kuan AS, Teng CJ, Wu HH, Su VYF, Chen YT, Chien SH, et al. Risk of ischemic stroke in patients with ovarian cancer: a nationwide population-based study. BMC Med. (2014) 12:53. doi: 10.1186/1741-7015-12-53
45. Kuan AS, Chen SC, Yeh CM, Hung MH, Hung YP, Chen TJ, et al. Risk of ischemic stroke in patients with gastric cancer: a nationwide population-based cohort study. Medicine. (2015) 94:e1336. doi: 10.1097/MD.0000000000001336
46. Pardo Sanz A, Rincón LM, Guedes Ramallo P, Belarte Tornero LC, de Lara Delgado G, Tamayo Obregon A, et al. Current status of anticoagulation in patients with breast cancer and atrial fibrillation. Breast Edinb Scotl. (2019) 46:163–9. doi: 10.1016/j.breast.2019.05.017
47. Plaja A, Berastegui E, Nieto-Moragas J, Sarrate E, Gual-Capllonch F, Quiroga V, et al. Thromboembolism and bleeding in patients with cancer and mechanical heart valves. J Thromb Thrombolysis. (2019) 47:454–61. doi: 10.1007/s11239-018-1790-3
48. Frederiksen H, Szépligeti S, Bak M, Ghanima W, Hasselbalch HC, Christiansen CF. Vascular diseases in patients with chronic myeloproliferative neoplasms - impact of comorbidity. Clin Epidemiol. (2019) 11:955–67. doi: 10.2147/CLEP.S216787
49. Tsai SJ, Huang YS, Tung CH, Lee CC, Lee MS, Chiou WY, et al. Increased risk of ischemic stroke in cervical cancer patients: a nationwide population-based study. Radiat Oncol Lond Engl. (2013) 8:41. doi: 10.1186/1748-717X-8-41
50. Donato J, Campigotto F, Uhlmann EJ, Coletti E, Neuberg D, Weber GM, et al. Intracranial hemorrhage in patients with brain metastases treated with therapeutic enoxaparin: a matched cohort study. Blood. (2015) 126:494–9. doi: 10.1182/blood-2015-02-626788
51. Mantia C, Uhlmann EJ, Puligandla M, Weber GM, Neuberg D, Zwicker JI. Predicting the higher rate of intracranial hemorrhage in glioma patients receiving therapeutic enoxaparin. Blood. (2017) 129:3379–85. doi: 10.1182/blood-2017-02-767285
52. Carandang R, Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Kannel WB, et al. Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. JAMA. (2006) 296:2939–46. doi: 10.1001/jama.296.24.2939
53. Zhang F, Wang K, Du P, Yang W, He Y, Li T, et al. Risk of stroke in cancer survivors: a meta-analysis of population-based cohort studies. Neurology. (2021) 96:e513–26. doi: 10.1212/WNL.0000000000011264
54. Turner M, Murchie P, Derby S, Ong AY, Walji L, McLernon D, et al. Is stroke incidence increased in survivors of adult cancers? A systematic review and meta-analysis. J Cancer Surviv. (2021) 379:2429–437. doi: 10.1007/s11764-021-01122-7
55. Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Tagawa ST, et al. Arterial thromboembolic events preceding the diagnosis of cancer in older persons. Blood. (2019) 133:781–9. doi: 10.1182/blood-2018-06-860874
56. Wei YC, Chen KF, Wu CL, Lee TW, Liu CH, Shyu YC, et al. Stroke rate increases around the time of cancer diagnosis. Front Neurol. (2019) 10:579. doi: 10.3389/fneur.2019.00579
57. Global R. Country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. (2018) 379:2429–37. doi: 10.1056/NEJMoa1804492
58. Navi BB, Iadecola C. Ischemic stroke in cancer patients: a review of an underappreciated pathology. Ann Neurol. (2018) 83:873–83. doi: 10.1002/ana.25227
59. Aspberg S, Yu L, Gigante B, Smedby KE, Singer DE. Risk of ischemic stroke and major bleeding in patients with atrial fibrillation and cancer. J Stroke Cerebrovasc Dis. (2020) 29:104560. doi: 10.1016/j.jstrokecerebrovasdis.2019.104560
60. Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ. Validation of clinical classification schemes for predicting strokeresults from the national registry of atrial fibrillation. JAMA. (2001) 285:2864–70. doi: 10.1001/jama.285.22.2864
61. Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. (2010) 137:263–72. doi: 10.1378/chest.09-1584
62. Gutierrez A, Patell R, Rybicki L, Khorana AA. Predicting outcomes in patients with cancer and atrial fibrillation. Ther Adv Cardiovasc Dis. (2019) 13:1753944719860676. doi: 10.1177/1753944719860676
63. Fitzpatrick T, Carrier M, Le Gal G. Cancer, atrial fibrillation, and stroke. Thromb Res. (2017) 155:101–5. doi: 10.1016/j.thromres.2017.05.006
64. Guzzetti S, Costantino G, Vernocchi A, Sada S, Fundarò C. First diagnosis of colorectal or breast cancer and prevalence of atrial fibrillation. Intern Emerg Med. (2008) 3:227–31. doi: 10.1007/s11739-008-0124-4
65. Cheng WL, Kao YH, Chen SA, Chen YJ. Pathophysiology of cancer therapy-provoked atrial fibrillation. Int J Cardiol. (2016) 219:186–94. doi: 10.1016/j.ijcard.2016.06.009
66. Cardinale D, Sandri MT, Colombo A, Salvatici M, Tedeschi I, Bacchiani G, et al. Prevention of atrial fibrillation in high-risk patients undergoing lung cancer surgery. Ann Surg. (2016) 264:244–51. doi: 10.1097/SLA.0000000000001626
67. Angelini DE, Radivoyevitch T, McCrae KR, Khorana AA. Bleeding incidence and risk factors among cancer patients treated with anticoagulation. Am J Hematol. (2019) 94:780–5. doi: 10.1002/ajh.25494
68. Andrade JG, Aguilar M, Atzema C, Bell A, Cairns JA, Cheung CC, et al. The 2020 Canadian cardiovascular society/Canadian heart rhythm society comprehensive guidelines for the management of atrial fibrillation. Can J Cardiol. (2020) 36:1847–948. doi: 10.1016/j.cjca.2020.09.001
69. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. (2013) 369:2093–104. doi: 10.1056/NEJMoa1310907
70. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. (2009) 361:1139–51. doi: 10.1056/NEJMoa0905561
71. Granger CB, Alexander JH, McMurray JJV, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. (2011) 365:981–92. doi: 10.1056/NEJMoa1107039
72. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. (2011) 365:883–91. doi: 10.1056/NEJMoa1009638
73. Short NJ, Connors JM. New oral anticoagulants and the cancer patient. Oncologist. (2014) 19:82–93. doi: 10.1634/theoncologist.2013-0239
74. Khorana AA, Soff GA, Kakkar AK, Vadhan-Raj S, Riess H, Wun T, et al. Rivaroxaban for thromboprophylaxis in high-risk ambulatory patients with cancer. N Engl J Med. (2019) 380:720–8. doi: 10.1056/NEJMoa1814630
75. Carrier M, Abou-Nassar K, Mallick R, Tagalakis V, Shivakumar S, Schattner A, et al. Apixaban to prevent venous thromboembolism in patients with cancer. N Engl J Med. (2019) 380:711–9. doi: 10.1056/NEJMoa1814468
76. Schwarzer G, Rücker G. Meta-analysis of proportions. Methods Mol Biol Clifton NJ. (2022) 2345:159–72. doi: 10.1007/978-1-0716-1566-9_10
77. Iorio A, Spencer FA, Falavigna M, Alba C, Lang E, Burnand B, et al. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ. (2015) 350:h870. doi: 10.1136/bmj.h870
78. Gøtzsche PC. Why we need a broad perspective on meta-analysis. BMJ. (2000) 321:585–6. doi: 10.1136/bmj.321.7261.585
Keywords: stroke, cancer, population health, epidemiology, meta-analysis, systematic review, incidence, risk
Citation: Lun R, Roy DC, Hao Y, Deka R, Huang W-K, Navi BB, Siegal DM, Ramsay T, Fergusson D, Shorr R and Dowlatshahi D (2022) Incidence of stroke in the first year after diagnosis of cancer—A systematic review and meta-analysis. Front. Neurol. 13:966190. doi: 10.3389/fneur.2022.966190
Received: 10 June 2022; Accepted: 26 August 2022;
Published: 20 September 2022.
Edited by:
Ayrton R. Massaro, Hospital Sirio Libanes, BrazilReviewed by:
Yi-Chia Wei, Chang Gung Memorial Hospital, TaiwanCopyright © 2022 Lun, Roy, Hao, Deka, Huang, Navi, Siegal, Ramsay, Fergusson, Shorr and Dowlatshahi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ronda Lun, cmx1bkB1Y2FsZ2FyeS5jYQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.