
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 30 September 2022
Sec. Neuroepidemiology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.965031
Background: Q-Motor is a suite of motor tests originally designed to assess motor symptoms in Huntington's disease. Among others, Q-Motor encompasses a finger tapping task and a grasping and lifting task. To date, there are no systematic investigations regarding effects of variables which may affect the performance in specific Q-Motor tests per se, and normative Q-Motor data based on a large population-based sample are not yet available.
Objective: We investigated effects of age and sex on five selected Q-Motor outcomes representing the two core Q-Motor tasks speeded finger tapping and grasping and lifting in a community sample of middle-aged to elderly adults. Furthermore, we explored effects of the potentially mediating variables educational attainment, alcohol consumption, smoking status, and depressive symptoms. Moreover, we explored inter-examiner variability. Finally, we compared the findings to findings for the Purdue Pegboard test.
Methods: Based on a sample of 726 community-dwelling adults and using multiple (Gaussian) regression analysis, we modeled the motor outcomes using age, sex, years in full-time education, depressive symptoms in the past seven days, alcohol consumption in the past seven days, and smoking status as explanatory variables.
Results: With regard to the Q-Motor tests, we found that more advanced age was associated with reduced tapping speed, male sex was associated with increased tapping speed and less irregularity, female sex was associated with less involuntary movement, more years of education were associated with increased tapping speed and less involuntary movement, never smoking was associated with less involuntary movement compared to current smoking, and more alcohol consumed was associated with more involuntary movement.
Conclusion: The present results show specific effects of age and sex on Q-Motor finger tapping and grasping and lifting performance. In addition, besides effects of education, there also were specific effects of smoking status and alcohol consumption. Importantly, the present study provides normative Q-Motor data based on a large population-based sample. Overall, the results are in favor of the feasibility and validity of Q-Motor finger tapping and grasping and lifting for large observational studies. Due to their low task-complexity and lack of placebo effects, Q-Motor tests may generate additional value in particular with regard to clinical conditions such as Huntington's or Parkinson's disease.
Motor performance of the hands is reflected in strength, speed, dexterity, and hand preference. Deficits in motor performance are found in many different (e.g., neurological) conditions. A core set of functional measures, among them finger tapping speed, grip strength, and Grooved and Purdue Pegboard (dexterity, speed, bimanual coordination) has proven useful for identifying manual motor impairment (1).
Q-(“Quantitative”-)Motor is a suite of objective, non-invasive, and easy-to-administer motor tests originally designed to assess motor symptoms in biomarker studies and clinical trials targeting Huntington's disease (HD). Q-Motor was recently developed to provide an alternative to the “Unified Huntington's Disease Rating Scale Total Motor Score” (UHDRS-TMS) to address concerns such as limited sensitivity for dysfunction and change, restriction to manifest stages of disease, intra- and inter-rater variability, subjective error, and rater-induced placebo-effects (2).
The Q-Motor apparatus encompasses pre-calibrated, temperature-independent force (-torque) transducers and 3D position-orientation sensors, enabling standardized measurements across time points and sites. Processing and (pre-) analysis of Q-Motor data can be carried out in a blinded and automated manner (2). The core Q-Motor suite includes six different tests. Two of these tests, which are routinely applied in HD research, are speeded finger tapping (digito-motography) (3, 4) and grasping and lifting (manu-/ choreo-motography) (4–6).
The Q-Motor speeded finger tapping test is one specific variant of the classical and widely used neuropsychological finger tapping test [for overviews, see (1, 7, 8)]. The latter was originally designed to measure self-directed manual motor speed and fine motor control, though performance also depends on kinesthetic and visual abilities as well as motivational status. The finger tapping test has been used with regard to and has proven sensitive to a wide variety of conditions, among them e.g., chronic alcoholism, Korsakoff's syndrome, closed-head injury, chronic pain, Multiple Sclerosis, Parkinson's disease (PD), and HD. While the task itself may appear simple, it actually is a complicated motor sequencing and motor inhibition-excitation task (9, 10). The purpose of the test is to assess subtle motor (and other cognitive) impairments. Noteworthy, interpretation and comparison of test results is complicated by the use of different tapping devices [manual vs. electronic/computerized apparatus vs. smartphone app; e.g., Halstead-Reitan Finger Tapping Test (11), Computerized Finger Tapping Test (12), T3 computer-assisted finger tapping task (13), WPS Electronic Tapping Test (12), CNS Vital Signs (14), potentiometer (15), or Android mobile application (16)] as well as different administration techniques, e.g., with regard to the number of trials or trial duration, both of which may result in subtle performance differences. In the past, mainly the manual apparatus has been used, and tapping speed was operationalized as the mean number of taps across five 10 s trials for which the total number of taps was within a range of five. More recently, further measures were applied, e.g., inter-tap interval (17), inter-tap variability (18), tap initiation time (19), tap down-time (20), or the occurrence of abnormal finger movements (21). All these core measures can be assessed with the Q-Motor digito-motography tapping test (3).
The Q-Motor grasping and lifting test was specifically designed to apply object grasping, lifting, holding, and transport tasks in order to investigate e.g., coordination of prehensile forces during precision grip (22), movement trajectories and force control during object transport (23), and quantity of involuntary movements, e.g., chorea in HD (5, 24) or L-Dopa induced dyskinesia in PD (25). The use of electro-magnetic position-angle sensors thereby enables standardized tracking of object position and orientation in all three planes.
Q-Motor tests and measures have been used in a number of multi-center trials and biomarker studies, among them e.g., TRACK-HD (26), TRACK-ON-HD (27), and PRIDE-HD (28) [see (2) for further trials], which investigate HD course, symptomatic, or disease-modifying treatments. However, what is currently lacking and may be important for the design of future studies employing Q-Motor measures are (i) systematic investigations into variables which may influence the performance in specific Q-Motor tests per se, and (ii) normative data from large population-based samples. For instance, due to lack of normative data, Q-Motor findings in clinical samples are currently sometimes hard to interpret.
Many previous investigations have consistently demonstrated effects of age and sex on finger tapping performance [for overviews, see (1, 7, 8)]. For grasping and lifting performance, such investigations are still pending. With regard to age, previous studies found increased tapping speed with increasing age in children [e.g., (29, 30)] and usually decreased speed [e.g., (9, 17, 31–38)] and increased variability (35) with advancing age in adults, particularly from the fifth decade on, but also starting as early as the third decade (35). With regard to sex, previous studies found increased tapping speed in boys compared to girls (29) and usually in males compared to females [e.g., (9, 11, 12, 18, 33, 35, 39–42)]. Moreover, there are conflicting results indicating that males may either show lower (18) or higher (20) inter-tap variability compared to females. Whether observed sex effects were influenced by body or hand size differences between the sexes remains currently unresolved.
A recent study (30) investigated effects of age and sex on Q-Motor outcomes in a community-dwelling sample (N = 29) of children and adolescents aged 6 to 17 years. The study included measures of speeded finger tapping and grasping and lifting. The results indicated robust age-related trends in both speeded finger tapping and grasping and lifting performance. Regarding finger tapping, for instance there were associations of advanced age with decreased (i.e., better) tap inter-onset interval mean and standard deviation, and with increased (i.e., better) tapping frequency revealing improvements in motor coordination with development. With respect to grasping and lifting, there were e.g., associations of advanced age with decreased (i.e., better) orientation and (particularly) position indexes, synonymous of less motor impersistency or fluctuation, which also supports maturation of motor development and shows that these effects were detectable with sensitive technology in a rather small sample size.
In addition to age and sex, there are other variables which may influence motor performance (and which, at the same time, are influenced by the age and the sex of subjects). Such variables could act as mediators in the analysis of effects of age and sex on motor performance. For instance, effects of alcohol on motor control [e.g., delay of reaction times, adverse effects on cognitive and motor processing, or the transient alleviation of neurological symptoms (e.g., essential tremor)] are well-recognized, and withdrawal of alcohol may trigger movement disorders (43). Moreover, smoking behavior (or nicotine, respectively) may affect motor performance. For instance, acute effects of nicotine and smoking on finger tapping performance have been reported previously [e.g., (44–47)]. Furthermore, depression is known to typically affect psychomotor skills, with either agitation or retardation considered core symptoms [cf. (48), for a review].
The primary objective of the present study was to investigate effects of age and sex on five selected Q-Motor outcomes representing the two core Q-Motor tasks speeded finger tapping and grasping and lifting in a community sample of middle-aged to elderly adults. To learn more about potential effects of age and sex on Q-Motor performance is crucial to improve the interpretation of existing Q-Motor data from clinical samples. Furthermore, the present study explored effects of the potentially mediating variables educational attainment, alcohol consumption, smoking status, and depressive symptoms. Moreover, given that the tasks were explained and supervised by a number of different study nurses, we explored inter-examiner variability to get an impression regarding the robustness of the test results with respect to the executing personnel. Additionally, the present study compared the findings for the Q-Motor outcomes to findings for the Purdue Pegboard test [for overviews, see (1, 7)], a classical neuropsychological test to assess hand and finger dexterity. The Purdue Pegboard is a well-established test that allows to explore the link between Q-Motor measures with different features of motor coordination. Finally, this study provides normative Q-Motor data from a large population-based sample [N = 726; the largest dataset investigated so far included about 120 control subjects in the TRACK-HD study (26)], which is not yet available, and which can be used as a comparative data set in future research studies. For instance, the normative Q-Motor data will enable future studies to compare their data to this dataset and assess the behavior of Q-Motor measures in population-based studies and beyond. Moreover, the normative data will be helpful to inform the design of future research and interventional trials.
The BiDirect Study (49, 50) is an observational, prospective cohort study originally designed to investigate the bidirectional relationship between depression and (subclinical) arteriosclerosis. BiDirect enrolled three cohorts of participants: (i) patients hospitalized due to an acute episode of depression at the time of recruitment, (ii) patients shortly after myocardial infarction or an acute coronary event at the time of recruitment, and (iii) population-based control subjects randomly invited from the registry of the city of Münster, Germany. The BiDirect Study was approved by the ethics committee of the University of Münster and the Westfalian Chamber of Physicians in Münster, North-Rhine-Westfalia, Germany. Written informed consent for participation was obtained from all participants.
The subjects analyzed here were those members of the BiDirect Study control cohort who (i) participated in the second BiDirect visit (N = 800), and who (ii) contributed Q-Motor or Pegboard data at this visit (N = 763), and who (iii) had not to be excluded from Q-Motor or Pegboard data analysis based on plausibility checks of the data (N = 726). Reasons due to which participants were excluded encompassed acute injuries of fingers/ hands/ arms, missing fingers/ parts of fingers, disability, acampsia, hand deformation, rheumatism, diagnosis of Multiple Sclerosis, diagnosis of PD, essential tremor, and vision deficits. The sample size was further reduced in analyses of specific Q-Motor or Pegboard outcomes due to missing data in such outcomes or, if so, due to missing data in explanatory variables used during display or statistical modeling of motor outcomes.
Q-Motor testing was performed during the second and third out of in total four BiDirect examinations, which took place between 2010 and 2020. Purdue Pegboard testing was performed during all four BiDirect examinations.
Q-Motor was executed in a quiet room as the first unit of the BiDirect neuropsychological assessment module. The Purdue Pegboard test was conducted subsequently. In each case, the participants were instructed and assessed by trained and certified examiners based on written standard operation procedures. Participants were not allowed to consume caffeine in the 30 min time period prior to neuropsychological assessment onset.
The BiDirect Study focused on two core Q-Motor tests: (i) speeded finger tapping (digitomotography), and (ii) grasping and lifting (manumotography and choreomotography). Grasping and lifting was performed first, speeded finger tapping was performed subsequently. Both tasks were executed with each hand separately; for each task and hand, three trials were conducted. The signals were recorded by means of a customized software tool (WinSC, Umeå University, Sweden) installed on a personal computer running Windows XP.
Prior to Q-Motor testing, the height of the chair was adjusted such that the forearms were approximately parallel to the floor, with the elbows at ~90° when the tasks were performed.
Regarding Q-Motor grasping and lifting, the task was to grasp a grip device with affixed position sensor (Polhemus Fastrak, VT, USA) and force transducer (Mini-40, ATI Industrial Automation, NC, USA) with a precision grip of thumb and index finger, lift it, and hold it as steady as possible in a certain height and position (indexed by a marker) for 20 s. The elbow of the task-performing arm was not allowed to be placed on the table, but had to be held in the air. The non-task-performing hand was to be placed on the thigh. Start and end of a trial were signaled by acoustic cues. Prior to the first trial, the task was demonstrated by the examiner, and the subjects were supposed to familiarize themselves with the haptics and weight of the grip device. The timing of the three subsequent trials was subject-paced, with breaks of usually a few seconds in between trials. Additional details regarding the apparatus can be found in Reilmann et al. (24). Supplementary Figure 1 displays a typical example of a raw signal recorded during a grasping and lifting trial, together with depictions of outcome measures which can be derived from the raw signal.
In case of grasping and lifting, the outcomes “lifting position-index mean (deg/s)” (LFPIMN; the means of the absolute values of the derivatives of the x, y, and z channels were computed during the static holding phase and summed to create the index) and “lifting orientation-index mean (cm/s)” [LFOIMN; the means of the absolute values of the derivatives of the roll, pitch, and yaw channels were summed and defined as the index (24)] were analyzed; both outcomes are measures of involuntary movement.
Regarding Q-Motor speeded finger tapping, the task was to tap as quickly as possible for 10 s on a force transducer (Mini-40, ATI Industrial Automation, NC, USA) with the elongated index finger. The task-performing hand was placed on a hand rest. The non-task-performing hand was to be placed on the thigh. Start and end of a trial were signaled by acoustic cues. Prior to the first trial, the task was demonstrated by the examiner, and the subjects were supposed to shortly try the task to familiarize themselves with the setup. The timing of the three subsequent trials was subject-paced, with breaks of usually a few seconds in between trials. Additional details regarding the apparatus can be found in Bechtel et al. (3). Supplementary Figure 2 displays a typical example of a raw signal excerpt recorded during a speeded finger tapping trial, together with depictions of outcome measures which can be derived from the raw signal.
In case of speeded finger tapping, the outcomes “tapping frequency mean (Hz)” (TSFRMN-a measure of speed), “tap inter-onset interval mean (s)” (TSIOMN-a measure of speed), and “tap inter-onset interval standard deviation (SD) (s)” (TSIOSD-a measure of irregularity) were analyzed.
As an aside, tapping frequency and mean tap inter-onset interval are related variables that both reflect tapping speed, and there may be no unique extra information in tap inter-onset intervals compared to frequencies. However, tap inter-onset intervals are-like tap durations, tap forces, and inter-tap intervals (which were not reported here)-part of a variable family that describes the gestalt and characteristics of individual taps, while the tapping frequency provides a much more general measure of a complete trial. Frequencies are more comprehensible, while inter-onset intervals potentially yield more sensitivity and insight in individual tapping properties.
Pseudonymized data were transferred to the Q-Motor team at the George-Huntington-Institute Münster, where the data were processed, quality-controlled, and pre-analyzed by trained personnel in the following steps: (i) rejection of compromised trials; (ii) baseline filtering of trials; (iii) averaging across valid trials within hand; (iv) compression of averaged signals into point measures of central tendency and/or variability. During quality control, trials were excluded for example if the data contained sensor errors/artifacts (e.g., gauge saturation), or if trials were not recorded according to the protocol (e.g., participants had touched sensors too late or too early). In about 85% of cases, three valid trials could be averaged. In about 98% of cases, at least two valid trials could be averaged. Further (i.e., statistical) analyses were conducted at the BiDirect Study site.
The Purdue Pegboard test examines the ability to manipulate small pins (“pegs”) between the distal parts of the fingers, requiring quick, precise, and fine inter-digital movements. The Purdue Pegboard test was also performed with each hand separately; for each hand, one trial was conducted. The task was to place as many pins as possible into the board, one below the other, within 30 s. Prior to each trial, the subjects were supposed to practice with three pins. The Pegboard data were analyzed at the BiDirect Study site.
In case of Purdue Pegboard testing, the number of pins placed within 30 s (PEGB) was analyzed.
Each outcome was usually available for left and right hands. The selection of outcomes to be analyzed was exclusively theory-driven; no variable selection algorithms were applied. An overview regarding the motor outcomes is given in Table 1.
The BiDirect Study did not employ a handedness inventory. Rather, participants were asked (at the first and second visits) whether they were right-handed, left-handed, or else. Participants who answered consistently were classified as either right-handers or left-handers, respectively. The handedness of the remaining participants was classified as “unclear.” Most of the results presented in the current article are restricted to the right-handed participants, who constituted by far the largest subgroup.
Explanatory variables of primary interest were age and sex. Moreover, we considered potential mediating effects of educational attainment (number of years in full-time education; continuous), depressive symptoms in the past seven days [Center for Epidemiologic Studies Depression Scale (CES-D) score (51); continuous], alcohol consumption in the past seven days (average consumption in grams/day; continuous), and smoking status (current vs. former vs. never smoker; categorical). Additionally, we investigated possible effects of different examiners.
The data were analyzed with R (52) using RStudio Desktop (RStudio Inc., Boston, MA, USA) in several subsequent steps.
First, we computed univariate multiple linear (Gaussian) regression analyses, modeling the motor outcomes separately. Depending on the distribution of an outcome (Figure 1), we used either non-transformed or log10-transformed data [see e.g., (30)]. Model 1 included the explanatory variables of interest, i.e., age and sex, and the adjusted variable examiner; this model estimated total effects of age and sex. Model 2 included all explanatory variables (i.e., additionally education years, CES-D score, alcohol consumption, and smoking status); this model approximated direct effects of age and sex. The presumed causal relationships among the explanatory and motor outcome variables are illustrated in Supplementary Figure 3.
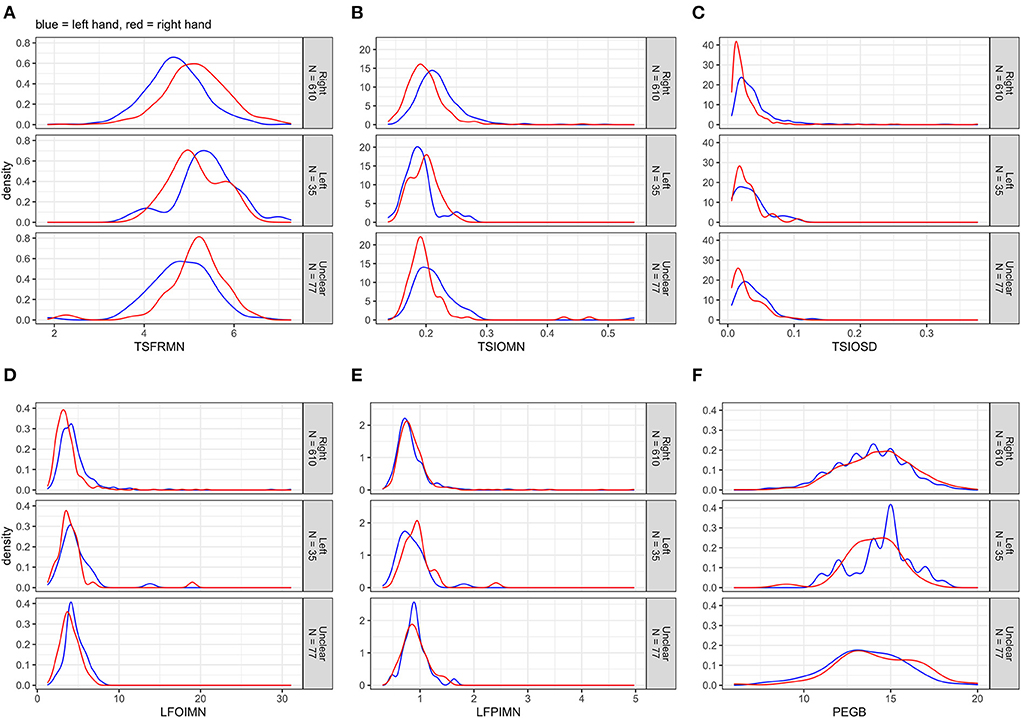
Figure 1. (A–F) Distributions of the non-transformed motor outcomes for left and right hands separately, stratified by handedness.
All statistical analyses were computed on the subgroup of right-handed subjects. The significance threshold was set to alpha = 0.05. All analyses were cross-sectional and should be regarded as exploratory.
Table 2 displays descriptive statistics with respect to explanatory variables used in the present study, stratified by handedness. Supplementary Figure 4 depicts univariate distributions of explanatory variables, stratified by handedness.
The participants were mostly middle-aged to elderly, and the sex ratio was well-balanced with only slightly more women than men. Education years were bi-modally distributed (with peaks around 13 and 18 years of education, respectively), most participants had consumed no or little alcohol and had reported rather few depressive symptoms in the past week and were either never or former smokers. The numbers of examinations conducted by the (overall 12) different examiners varied considerably (minimum = 1, maximum = 261). The vast majority of participants (84.4%) was right-handed.
Figure 1 displays the distributions of the non-transformed motor outcomes for left and right hands separately, stratified by handedness. The tapping frequency mean (TSFRMN) and the Pegboard number of pins placed (PEGB) were normally distributed; the remaining outcomes were right-skewed.
Figure 2 displays the distributions of the outcomes after log10-transformation. In cases of log lifting position-index mean (LFPIMNlog) and log lifting orientation-index mean (LFOIMNlog), the distributions changed noticeably toward normality; this was not the case for log tap inter-onset interval mean (TSIOMNlog) and log tap inter-onset interval SD (TSIOSDlog).
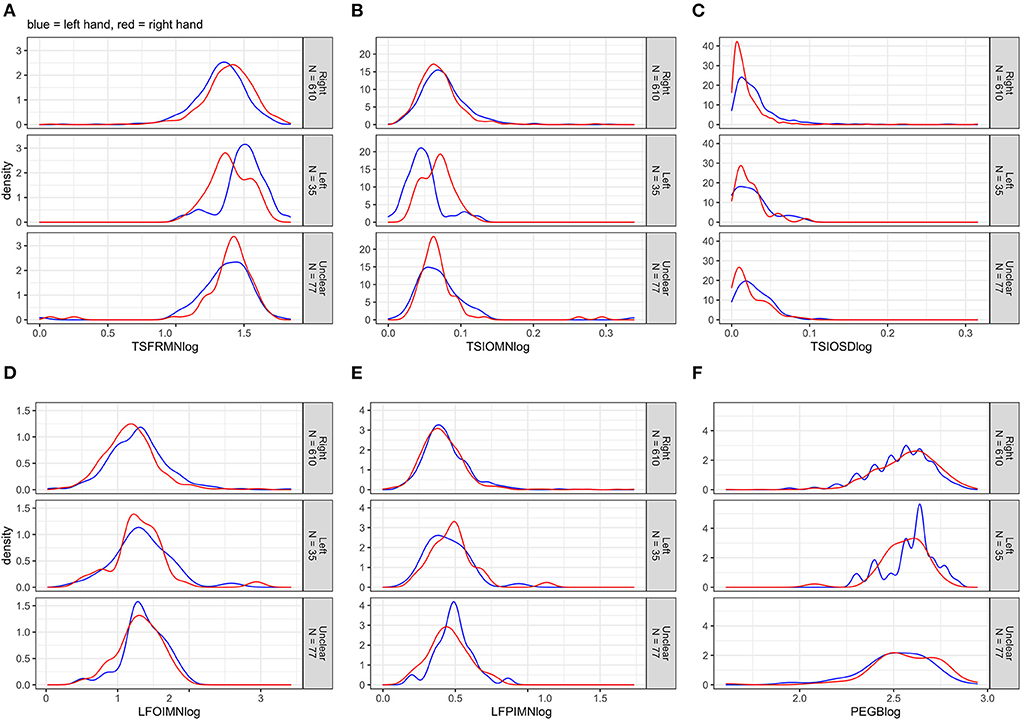
Figure 2. (A–F) Distributions of the log10-transformed motor outcomes for left and right hands separately, stratified by handedness.
Table 3 displays measures of central tendency and variability for the non-transformed outcomes in left and right hands separately, stratified by test, age category, and handedness. Table 4 displays these measures stratified by test, sex, and handedness. In all outcomes and across the age categories and the sexes, right-handers tended to perform better with their right hand, and left-handers tended to perform better with their left hand.
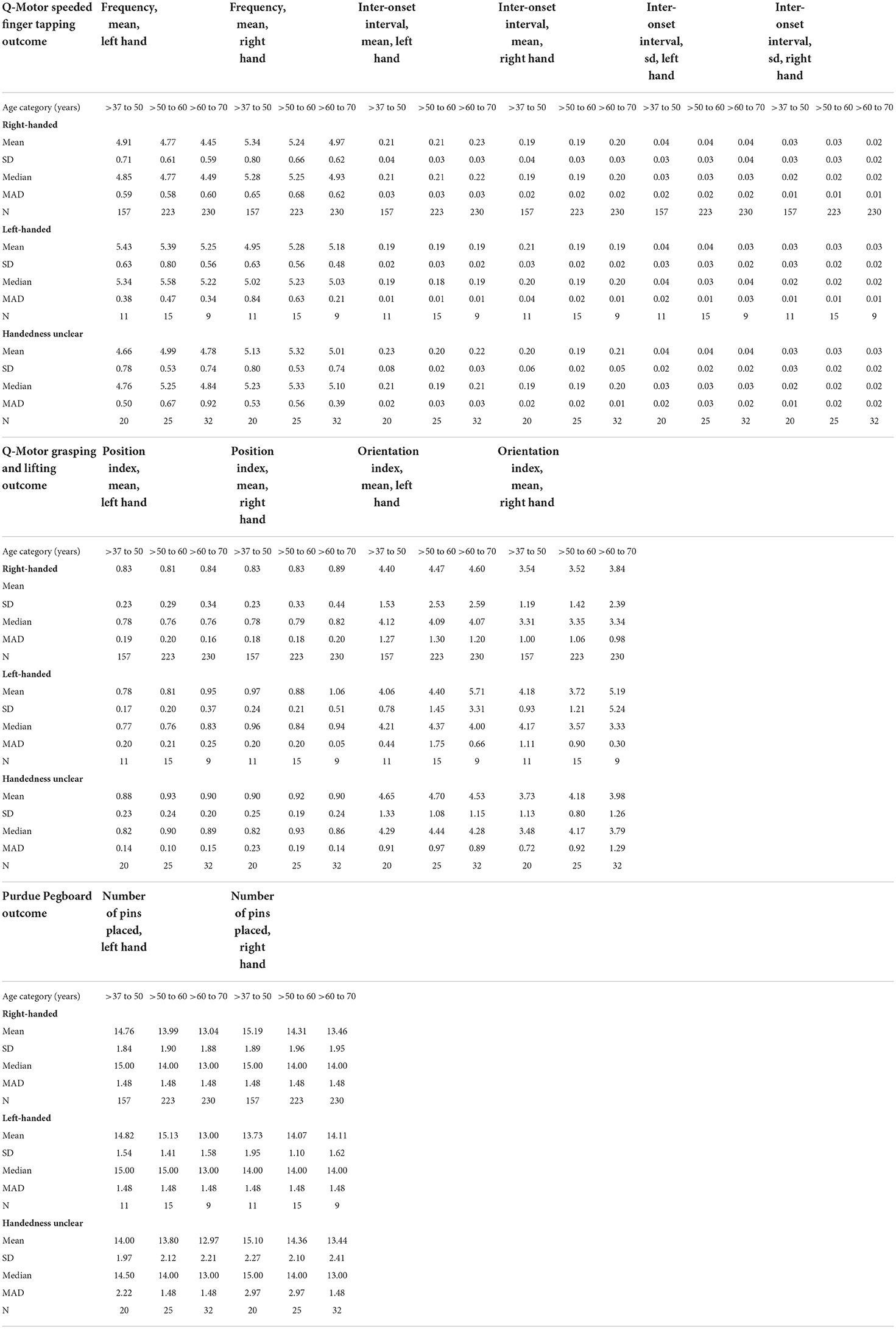
Table 3. Measures of central tendency and variability for the (non-transformed) motor outcomes in left and right hands, stratified by test, age category, and handedness.
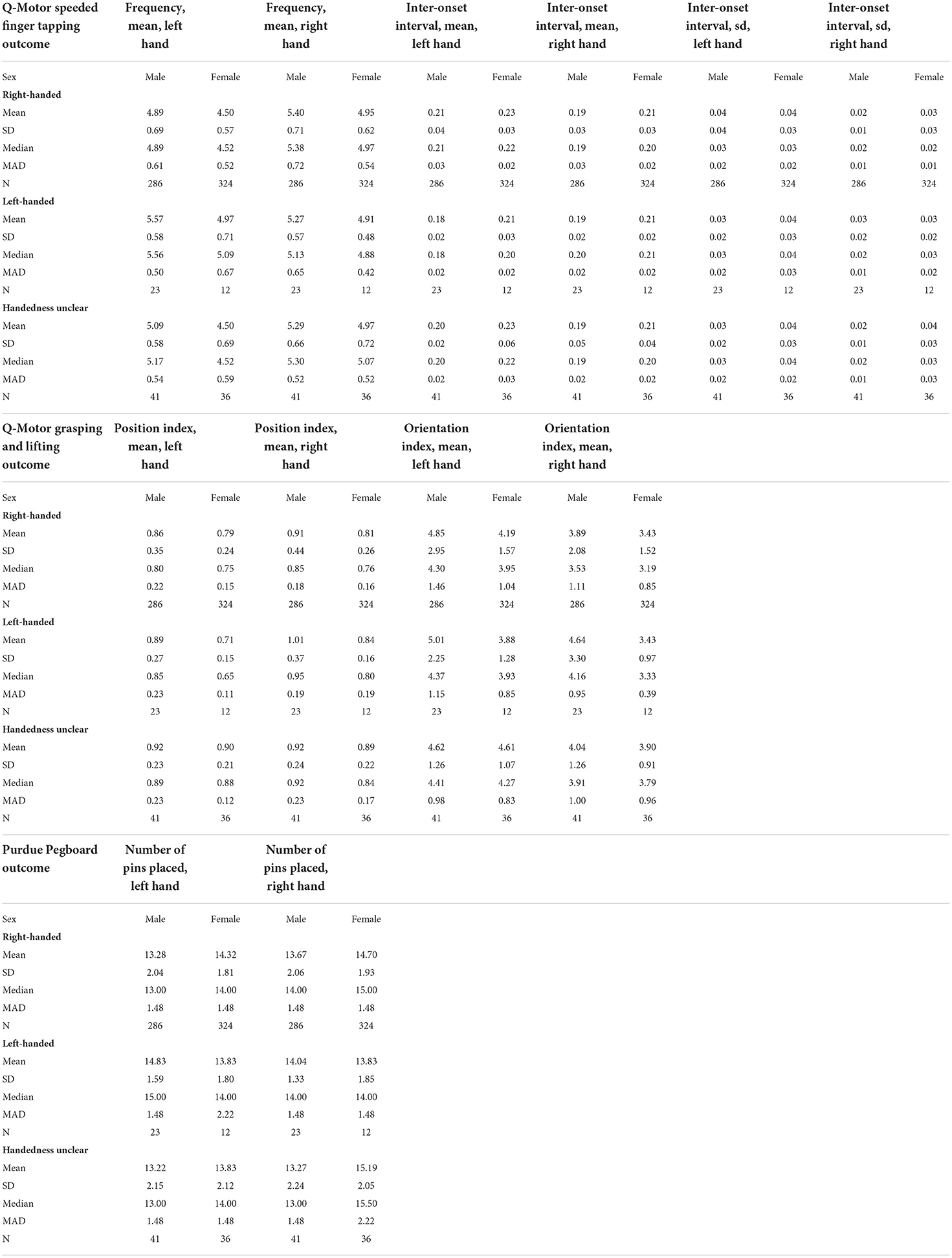
Table 4. Measures of central tendency and variability for the (non-transformed) motor outcomes in left and right hands, stratified by test, sex, and handedness.
Unadjusted, bivariate associations between age or sex and motor outcome variables (either non-transformed or log10-transformed, depending on the original distribution) for the right-handed participants are displayed in Figures 3, 4, respectively. Supplementary Figure 5 through 9 hold analogous displays for the remaining explanatory variables. With regard to age, the visually most noticeable associations were with tapping frequency mean (TSFRMN), log tap inter-onset interval mean (TSIOMNlog), and Pegboard number of pins placed (PEGB). With regard to sex, the visually most noticeable associations were with tapping frequency mean (TSFRMN), log tap inter-onset interval mean (TSIOMNlog), log lifting orientation-index mean (LFOIMNlog), and Pegboard number of pins placed (PEGB).
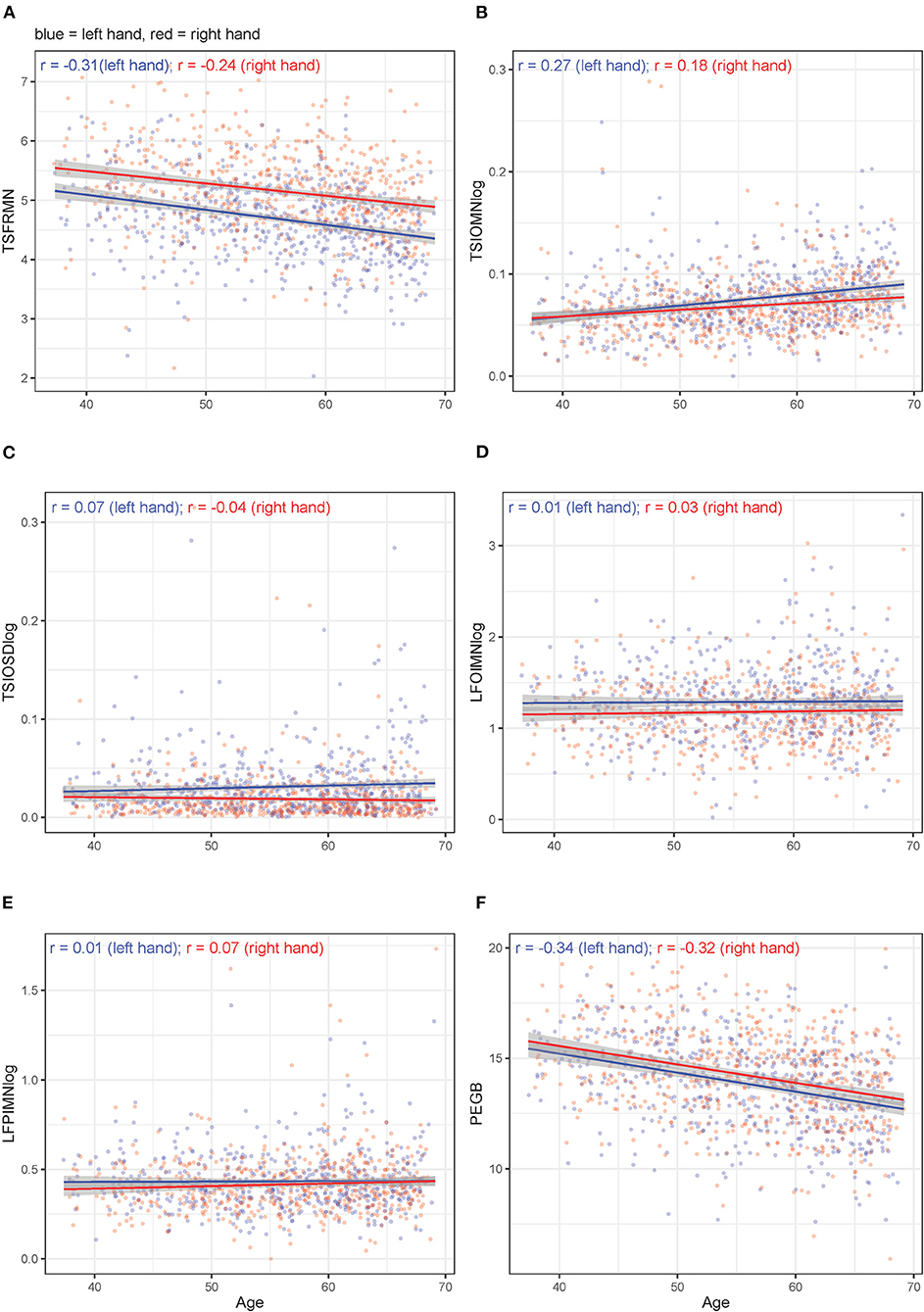
Figure 3. (A–F) Unadjusted, bivariate associations between age and the motor outcomes in right-handed participants for left and right hands separately.
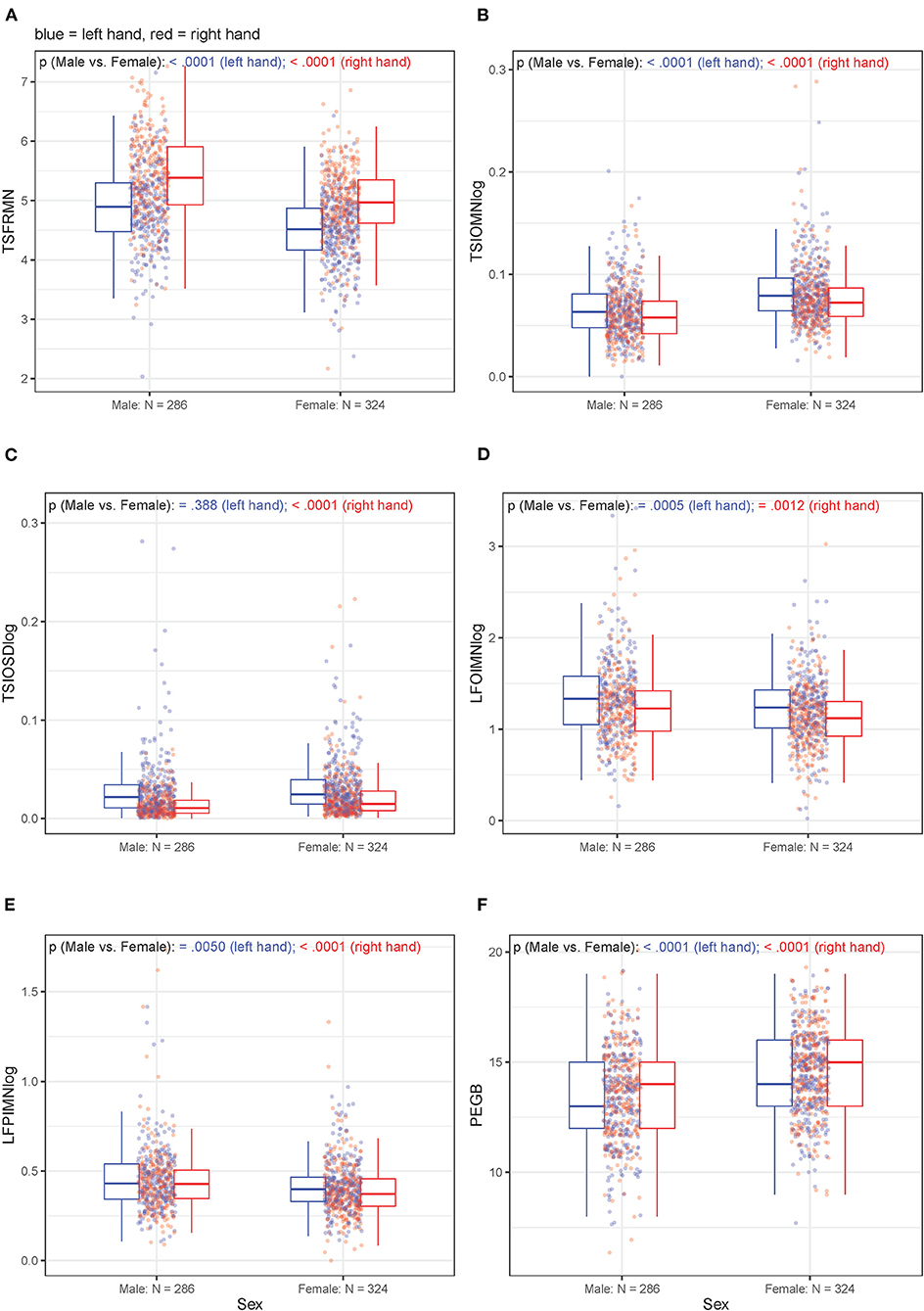
Figure 4. (A–F) Unadjusted, bivariate associations between sex and the motor outcomes in right-handed participants for left and right hands separately.
Table 5 summarizes (Pearson product-moment) correlation coefficients between all motor outcomes (either non-transformed or log10-transformed, depending on the original distribution) for the right-handed subjects, stratified by task-performing hand.
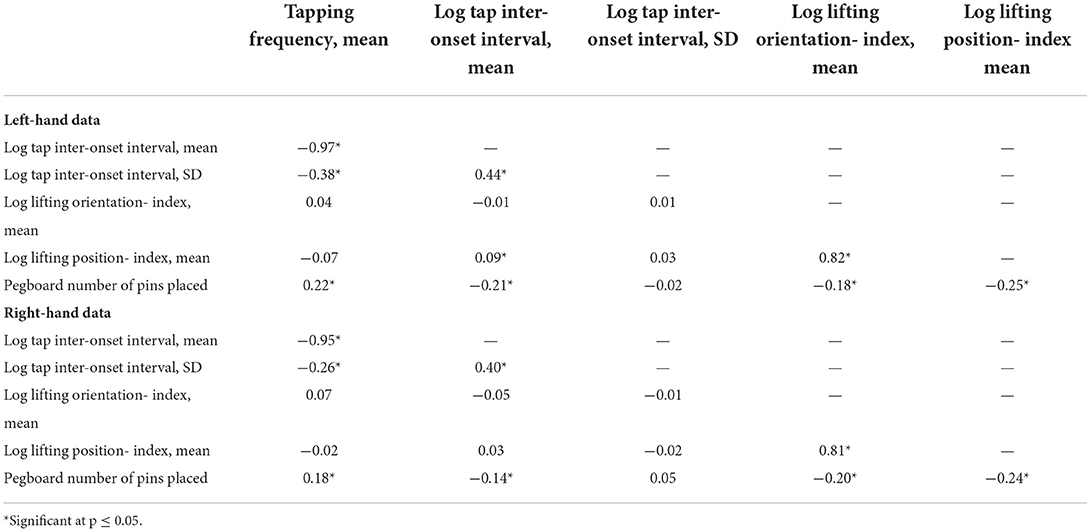
Table 5. Correlations between Q-Motor and Pegboard outcomes for the right-handed participants, stratified by task-performing hand.
Supplementary Figure 10 displays associations between Purdue Pegboard and Q-Motor outcomes (either non-transformed or log10-transformed). All such associations were positive, i.e., better performance in Pegboard was generally associated with better performance in Q-Motor. There was no correlation between Pegboard number of pins placed (PEGB) and tap inter-onset interval SD (TSIOSD). The remaining Pegboard - Q-Motor correlations were only weak.
Except for tap inter-onset SD-left hand and log tap inter-onset SD-left hand (in both models 1 and 2), all multiple linear regression analyses were significant. Adjusted R2 values of significant models were in the range between 3.69% (lifting position-index mean-right hand) and 18.54% (Pegboard number of pins placed-left hand) for model 1 (Table 6); for model 2, the range was from 3.91% (tap inter-onset SD-right hand) to 21.09% (Pegboard number of pins placed-left hand; Table 7).
Moreover, adjusted R2 values (which take the number of terms in the model into account) were usually higher in model 2 compared to model 1, indicating that model 2 tended to be a better fit to the data (Tables 6, 7).
With regard to individual explanatory variables from model 1 (including age, sex, and examiner), the results demonstrated significant effects of age for tapping frequency mean (left + right hands), log tap inter-onset interval mean (left + right hands), and Pegboard number of pins placed (left + right hands). In each case, more advanced age was associated with decreased performance. Significant effects of sex were found for tapping frequency mean (left + right hands; female sex was associated with decreased performance), log tap inter-onset interval mean (left + right hands; decreased performance in females), log tap inter-onset interval SD (right hand; decreased performance in females), log lifting position-index mean (left + right hands; increased performance in females), log lifting orientation-index mean (left + right hands; increased performance in females), and Pegboard number of pins placed (left + right hands; increased performance in females). Significant effects of examiner were found for log lifting position-index mean (left + right hands) and log lifting orientation-index mean (left + right hands; Table 8).
Regarding effects of individual explanatory variables from model 2 (additionally including education, alcohol consumption, smoking status, and depressive symptoms), the results demonstrated significant effects of age for tapping frequency mean (left + right hands), log tap inter-onset interval mean (left + right hands), and Pegboard number of pins placed (left + right hands). In each case, advanced age was associated with decreased performance. Significant effects of sex were found for tapping frequency mean (left + right hands; decreased performance in females), log tap inter-onset interval mean (left + right hands; decreased performance in females), log tap inter-onset interval SD (right hand; decreased performance in females), log lifting position-index mean (left + right hands; increased performance in females), log lifting orientation-index mean (left + right hands; increased performance in females), and Pegboard number of pins placed (left + right hands; increased performance in females). Significant effects of education were found for tapping frequency mean (left + right hands), log tap inter-onset interval mean (left + right hands), log lifting position-index mean (left hand), and Pegboard number of pins placed (left + right hands). In each case, more education years were associated with increased performance. A significant effect for alcohol consumption was found for log lifting orientation-index mean (left hand); more alcohol consumed was associated with decreased performance. Significant effects for smoking status were found for log position-index mean (left + right hands; current smoking was associated with decreased performance compared to never smoking), log lifting orientation index mean (left + right hands; current smoking was associated with decreased performance compared to never smoking), and Pegboard number of pins placed (left + right hands; both current and former smoking were associated with decreased performance compared to never smoking). A significant effect of depressive symptoms was found for Pegboard number of pins placed (left hand; more depressive symptoms were associated with decreased performance). Significant effects of examiner were found for tapping frequency mean (left hand), log lifting position-index mean (left + right hands), and log lifting orientation-index mean (left + right hands; Table 9).
In the right-handed subjects who were analyzed here, as a general pattern, the models tended to perform better in case of left-hand as compared to right-hand data for tapping frequency mean, tap inter-onset interval mean, lifting position-index mean, and Pegboard number of pins placed. For lifting orientation-index mean, the models tended to perform better in case of right-hand data (Tables 6, 7).
Based on observational, cross-sectional data of middle-aged to elderly, community-dwelling adults from the BiDirect Study (49, 50), the aims of the present analyses were to (i) investigate associations of age and sex with five selected outcomes representing the Q-Motor core tests speeded finger tapping (speed, irregularity) and grasping and lifting (involuntary movements). Furthermore, we (ii) explored effects of the (potentially mediating) variables education, alcohol consumption, smoking status, and depressive symptoms, (iii) explored inter-examiner variability, and (iv) compared the findings regarding the Q-Motor outcomes to findings for the Purdue Pegboard test. Although descriptive findings are presented stratified by handedness, the statistical analyses were restricted to right-handed participants, which constituted the by far largest subgroup.
In the present study, significant effects of age were found for finger tapping speed (tapping frequency mean, left and right hands; log tap inter-onset interval mean, left and right hands), but not with finger tapping irregularity. More advanced age was associated with reduced speed.
Significant effects of sex were found for finger tapping speed (tapping frequency mean, left and right hands; log tap inter-onset interval mean, left and right hands) and finger tapping irregularity (log tap inter-onset interval SD, right hand). Male sex was associated with increased speed and less irregularity.
The age and sex effects on finger tapping speed and irregularity observed here with the specific Q-Motor finger tapping apparatus (electric force-transducer, customized software tool to record the signals) and mode of test administration and data analysis (averaging across three consecutive trials of in each case 10 s duration) basically replicated the findings of numerous previous studies employing a number of different devices, modes of test administration, and sample age and sex distributions [see e.g., (35) for an overview]. Thus, the present findings basically argue in favor of the validity of the Q-Motor speeded finger tapping test. This is further supported by the observation that the overall finger tapping rates (Tables 3, 4) were well in the range of the 4.8 to 5.7 taps per second that was usually reported in the literature. Also, tapping rates were about 10% faster in the preferred compared to the non-preferred hand (cf. Tables 3, 4, right-handers), a finding which has been often reported before [see e.g., (35)]. Moreover, the present findings suggest the feasibility of the Q-Motor speeded finger tapping test for the setting of a large observational study; this is not self-evident, as such studies are typically characterized by less controlled conditions, including e.g., less-specifically trained and more frequently alternating personnel, or limited time to complete many different assessments (53).
Notably, sensitivity of Q-Motor finger tapping speed for the age of subjects was also found in a previous experimental study (N = 29) with children and adolescents (30), where younger children tapped slower than older children. However, this study did also find age effects on tapping irregularity (which were not found here in adults), with younger children showing more irregularity than older children. However, the sensitivity of Q-Motor finger tapping speed and irregularity for the sex of subjects found in the present study had not been observed by van der Plas et al. in their study with children. Although it appears generally difficult to compare motor performance in children vs. adults, it is interesting to note such similarities and disparities, albeit we acknowledge that the small sample size of their study may also account for the differences.
In the present study, there were no significant effects of age on involuntary movement.
Significant effects of sex on involuntary movement were found for both position (log lifting position-index mean, left and right hands) and orientation (log lifting orientation-index mean, left and right hands) indexes. Female sex was associated with less involuntary movement.
Age and sex effects on Q-Motor grasping and lifting outcomes had not yet been systematically investigated in community-dwelling adults. Thus, the present results constitute an important dataset for the design of future studies. Notably, the lack of sensitivity of Q-Motor involuntary movement measures for the age of subjects observed here is in contrast to the results of van der Plas et al. (30) in children and adolescents, where younger children were found to show more involuntary movement with regard to both position as well as orientation compared to older children. This may be explained by maturation of motor coordination in children. Interestingly, this study is the first to detect sensitivity of Q-Motor involuntary movement measures for the sex of subjects (not found by van der Plas et al. in children).
Notably, the mean position and orientation indexes observed here (Tables 3, 4) for the right-handers were well in the range of those reported by Reilmann et al. (24) for their sample of healthy, right-handed control subjects (N = 19; 12 females; age range 20 to 63 years).
In the present study, there were significant effects of education on finger tapping speed (tapping frequency mean, left and right hands; log tap inter-onset interval mean, left and right hands), but not on finger tapping irregularity. More years of education were associated with increased speed.
Previous findings regarding effects of IQ and education on finger tapping performance were somewhat mixed, but there are studies which found that tapping speed was higher with increasing IQ [e.g., (36)] and more education years [e.g., (9, 54)], though effects were comparably smaller than age and sex effects. The education effects on finger tapping speed observed here with the Q-Motor apparatus and mode of test administration replicated the findings of several previous studies using different devices or modes of test administration [e.g., (9)] and hence argue in favor of the validity of Q-Motor speeded finger tapping, as well as its feasibility for large observational studies.
Although typically observed (and while it appears important to register them), it remains unresolved why education effects on finger tapping performance arise in the first place [e.g., (9)]. However, it is reasonable to assume that education is a proxy for a number of underlying person characteristics. For instance, one may speculate that such effects would represent e.g., more motivation or effort, better ability to focus, better task comprehension, better performance strategies, possibly more access to motor or fine motor challenges and exercises, or better health of those comparably higher educated.
There were no significant effects of alcohol consumption, smoking status, or depressive symptoms on finger tapping performance.
In the present study, significant effects of education on involuntary movement were found for position (log lifting position-index mean, left hand): more years of education were associated with less involuntary movement. Given that there are no previous studies, this result is a novel contribution. As discussed above, potential explanations for such education effects currently remain speculative.
Significant effects of smoking status were found for position (log lifting position-index mean, left and right hands) and orientation (log lifting orientation-index mean, left and right hands) indexes: never smoking was associated with less involuntary movement compared to current smoking. These effects seem rather robust, as they are detected in both the position and orientation indexes bilaterally. Effects of smoking status on involuntary movement could theoretically materialize in different ways, among them direct effects of nicotine (or nicotine withdrawal) on the central nervous system, or indirect, possibly accumulating effects of smoking duration and/ or intensity on vascular or nervous system health. Noteworthy, previous studies have found acute effects of nicotine and smoking on finger tapping performance [e.g., (44–47)]. A meta-analysis (55) concluded that those studies possibly reflected true nicotine-related performance enhancements in the form of tapping speed increments. Unfortunately, the BiDirect Study did neither record when a currently smoking participant had smoked his last cigarette, nor were there any rules regarding smoking during the waiting periods, so that short-term effects of nicotine on performance could not be quantified. However, given that current smokers tended to perform worse compared to never smokers, while there was no trend for a performance difference between never and former smokers, one may speculate that acute nicotine deprivation may have had an influence. If true, it may be worthwhile to consider or even regulate nicotine consumption/ smoking behavior in the time window preceding a grasping and lifting session. In conclusion, the Q-Motor effects observed seem plausible and may offer an approach to objectively monitoring impact of smoking on motor coordination in population based studies in a sensitive way.
A significant effect of alcohol consumption was found for the orientation index (log lifting orientation-index mean, left hand): more alcohol consumed was associated with more involuntary movement. Similar to effects of smoking status, effects of alcohol consumption on involuntary movement could theoretically materialize in different ways. It appears most likely that the performance disadvantage seen in those who had drunk comparably more alcohol during the past seven days would reflect either cumulative negative effects of regular or extensive drinking on e.g., vascular or nervous system health, or negative effects of acute alcohol deprivation. However, notably these effects were less robust across measures than the effects of nicotine consumption.
There were no significant effects of depressive symptoms on involuntary movement.
In the present study, significant effects of examiner were found for finger tapping speed (tapping frequency mean, left hand) and involuntary movement with regard to position (log lifting position-index mean, left and right hands) as well as orientation (log lifting orientation-index mean, left and right hands).
From our point of view, the results regarding effects of examiner are generally difficult to assess. As a matter of fact, the numbers of examinations carried out by different persons varied considerably (from as few as one to as many as 261). Moreover, the distributions of motor outcomes differed noticeably between different examiners (Supplementary Figure 9), even in cases where persons had conducted comparable numbers of examinations. Furthermore, whether or not a categorical explanatory variable becomes significant in a statistical model depends, amongst others, on the choice of reference category; this choice is somewhat arbitrary and can be data-driven or theoretically motivated. For example, while it appears tempting to use the examiner who had conducted by far the most examinations as the reference, with hindsight there is no way of knowing whether or not this specific examiner administered the tests as intended. In the present study, we decided to use an examiner as the reference who had conducted a “typical” number (i.e., 45) of examinations.
Taken together, the results indicate that, if any, effects of examiner would have affected rather involuntary movement, and not so much tapping speed or irregularity. As a consequence, it may be worthwhile to review and possibly even better standardize Q-Motor grasping and lifting instructions, and to sensitize future examiners for certain critical task specifics prone to insufficiently precise participant briefing.
In the present study, with the exception of log tap inter-onset interval SD, there were positive associations of the Q-Motor outcomes with the Purdue Pegboard outcomes for both hands: better performance in Q-Motor was strictly related with better performance in the Purdue Pegboard (Supplementary Figure 10). The correlation coefficients between Q-Motor and Pegboard were of very similar, but only modest magnitude (Table 5), confirming that these tests partly overlap, but also capture rather different aspects of motor capability. The fact that there was no correlation with speeded finger tapping irregularity is not surprising, as this was the only “variability” measure.
The Purdue Pegboard test is a “classical” neuropsychological test that is characterized by e.g., inexpensiveness, relative simplicity of administration, and simplicity of performance evaluation. In the present study, we found significant and plausible effects of age (reduced performance with advancing age), sex (better performance in females), education (better performance with more years of education), smoking status (better performance in never compared to current as well as former smokers), and examiner with Pegboard performance. Noteworthy, the Pegboard test was the only of the tests analyzed here which was sensitive to depressive symptoms (reduced performance with more depressive symptoms) of the subjects, and it was also the only test which indicated performance differences between never and former smokers. Thus, when it comes to sensitivity for the age, sex, and other variables describing community-dwelling, middle-aged to elderly adults, there appeared to be no advantage of the Q-Motor tests over the Purdue Pegboard test. However, it is quite possible that the Q-Motor tests would demonstrate advantages with respect to clinical samples, for instance and most likely in case of HD or PD. The Pegboard test is clearly the most complex of the tasks analyzed here, and thus it is not too surprising that this task proved sensitive to the largest number of variables analyzed here. However, in clinical samples, the task complexity may present challenges and may limit the applicability and proper execution depending on the stage or severity of disease. Moreover, complex tasks are more likely to be affected by learning and placebo effects (26). Q-Motor, in contrast, repeatedly exhibited lack of placebo effects [e.g., (4, 28)]. Furthermore, the sensitivity of Q-Motor assessments allowed detection of progression of motor changes even in pre-manifest HD gene carriers both cross-sectionally (56) and longitudinally (26). The high level of sensitivity and the rater-independence are important features of the Q-Motor tests, which may open unique opportunities for precise phenotyping of samples in future studies.
The data analyzed here originate from an observational study, and the current analyses were cross-sectional. Thus, the effects of age and sex on the Q-Motor outcomes found here may or may not reflect causal effects. Moreover, the current sample was “selected” to a certain degree, because the subjects had volunteered for participation in the BiDirect Study in the first place, because only a subset of the participants originally enrolled had returned to the second BiDirect visit, and because subjects with certain conditions had been excluded from the current analyses. As a consequence, the current sample and results may not be representative for the general population of a mid-sized, north-western European city. Further, it cannot be excluded that the effects found here may be biased due to residual confounding. Furthermore, the sample included mainly right-handers, and handedness determination was limited to asking subjects which hand they preferred. Thus, the findings should carefully be compared to studies employing different (possibly more sophisticated) hand preference determination. Moreover, average education of the subjects was rather high and bi-modally distributed. Finally, formal performance validity testing was not employed, allowing the possibility that the subjects may not have performed with full motivation or effort.
The present results show specific effects of age and sex on Q-Motor speeded finger tapping and grasping and lifting performance. In addition, besides effects of education, there also were specific effects of smoking status and alcohol consumption. The latter were restricted to grasping and lifting and potentially due to acute substance deprivation. These are important findings, which imply that these variables may confound associations with Q-Motor outcomes potentially found in future (e.g., clinical) studies, and that careful reasoning about potential effects of these variables during the design of Q-Motor studies is vital.
It is also important to emphasize that the present results fill another knowledge gap: this study is the first to provide normative Q-Motor data from a large population-based sample. The normative data presented here can serve to inform the analysis and interpretation of Q-Motor data from future population-based or clinical studies.
Overall, the present results are in favor of the feasibility and validity of Q-Motor speeded finger tapping and grasping and lifting for large observational studies, albeit there were indications that care should be taken with regard to examiner training.
Comparisons of the effects on Q-Motor performance with effects on Purdue Pegboard performance in the setting of a large population-based observational study indicated that the Q-Motor tests may generate additional value in particular with regard to clinical conditions such as Huntington's or Parkinson's disease, where low task-complexity and lack of placebo effects are important preconditions.
The data of the BiDirect Study, including the data used and/or analysed during the current study, are available via the last author (KB) upon reasonable request.
The studies involving human participants were reviewed and approved by Ethik-Kommission der Ärztekammer Westfalen-Lippe und der Westfälischen Wilhelms-Universität Münster, Münster, Germany. The patients/participants provided their written informed consent to participate in this study.
HT and KB conceived of the present study. HT performed the statistical analyses, interpreted the results, and drafted the manuscript. RR provided the Q-Motor apparatus. RS performed the blinded Q-Motor data pre-analyses. HT, KB, RS, and RR participated in the interpretation of the findings, contributed to the writing of the manuscript, and approved the final version of the manuscript. All authors contributed to the article and approved the submitted version.
The BiDirect Study was funded by the German Federal Ministry of Education and Research (Grant Nos: 01ER0816 and 01ER1506 to KB). We acknowledge support from the Open Access Publication Fund of the University of Münster.
We are grateful to all BiDirect Study participants, to the staff of the BiDirect Study, and to the staff of the George-Huntington-Institute Münster.
Author RS is an employee of the George Huntington Institute (GHI), a private research institute focused on clinical and preclinical research in Huntington's disease, and QuantiMedis, a clinical research organization providing Q-Motor (quantitative motor) services in clinical trials and research. Author RR is founding director and owner of the George-Huntington-Institute, a private research institute focused on clinical and preclinical research in Huntington's disease, and QuantiMedis, a clinical research organization providing Q-Motor (quantitative motor) services in clinical trials and research. He also serves as a member of the Task Force on Technology of the International Parkinson and Movement Disorder Society (IPMDS). He has provided consulting services, advisory board functions, clinical trial services, quantitative motor analyses, and/or lectures for Actelion, Alnylam, Amarin, Annexion, AOP Orphan Pharmaceuticals, Cure Huntington Disease Initiative Foundation (CHDI), Desitin, Hoffmann-La Roche, IONIS, Ipsen, Lundbeck, MEDA Pharma, Medivation, Mitoconix, Neurosearch, Novartis, Omeros, Pfizer, Prana Biotechnology, Prilenia, PTC Therapeutics, Raptor, Siena Biotech, Temmler Pharma, Teva, uniQure, Vaccinex, VectorY, Voyager, and Wave Life Sciences.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.965031/full#supplementary-material
1. Strauss E, Sherman EMS, Spreen O, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary, 3rd ed. Oxford New York, NY: University Press, Oxford (2006).
2. Reilmann R, Schubert R. Motor outcome measures in Huntington disease clinical trials. Handb Clin Neurol. (2017) 144:209–25. doi: 10.1016/B978-0-12-801893-4.00018-3
3. Bechtel N, Scahill RI, Rosas HD, Acharya T, van den Bogaard SJA, Jauffret C, et al. Tapping linked to function and structure in premanifest and symptomatic Huntington disease. Neurology. (2010) 75:2150–60. doi: 10.1212/WNL.0b013e3182020123
4. Reilmann R, Rouzade-Dominguez M-L, Saft C, Süssmuth SD, Priller J, Rosser A, et al. A randomized, placebo-controlled trial of AFQ056 for the treatment of chorea in Huntington's disease. Mov Disord. (2015) 30:427–31. doi: 10.1002/mds.26174
5. Reilmann R, Bohlen S, Klopstock T, Bender A, Weindl A, Saemann P, et al. Grasping premanifest Huntington's disease - shaping new endpoints for new trials. Mov Disord. (2010) 25:2858–62. doi: 10.1002/mds.23300
6. Reilmann R, Kirsten F, Quinn L, Henningsen H, Marder K, Gordon AM. Objective assessment of progression in Huntington's disease: a 3-year follow-up study. Neurology. (2001) 57:920–4. doi: 10.1212/WNL.57.5.920
7. Lezak MD. Neuropsychological Assessment, 5th ed. New York, NY: Oxford University Press, Oxford (2012).
8. Mitrushina MN. Handbook of Normative Data for Neuropsychological Assessment, 2nd ed. New York, NY: Oxford University Press (2005).
9. Prigatano GP, Goncalves CWP, Oliveira SB, de Denucci SM, Pereira RM, Braga LW. Kinematic recordings while performing a modified version of the halstead finger tapping test: age, sex, and education effects. J Clin Exp Neuropsychol. (2020) 42:42–54. doi: 10.1080/13803395.2019.1665170
10. Prigatano GP, de Oliveira SB, Goncalves CWP, Denucci SM, Pereira RM, Braga LW. Inhibitory control of adjacent finger movements while performing a modified version of the halstead finger tapping test: Effects of age, education and sex. J Int Neuropsychol Soc. (2021) 27:813–24. doi: 10.1017/S1355617720001101
11. Ruff RM, Parker SB. Gender- and age-specific changes in motor speed and eye-hand coordination in adults: normative values for the finger tapping and grooved pegboard tests. Percept Mot Skills. (1993) 76:1219–30. doi: 10.2466/pms.1993.76.3c.1219
12. Christianson M, Leathem J. Development and standardisation of the computerised finger tapping test: comparison with other finger tapping instruments. NZ J Psychol. (2004) 33:4449.
13. Tanner BA, Bowles RL. Preliminary data on a computerized test of finger speed and endurance. Behav Res Meth Instrum Comput. (1995) 27:160161. doi: 10.3758/BF03204722
14. Gualtieri CT, Johnson LG. Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Arch Clin Neuropsychol. (2006) 21:623–43. doi: 10.1016/j.acn.2006.05.007
15. Austin D, McNames J, Klein K, Jimison H, Pavel M. A statistical characterization of the finger tapping test: Modeling, estimation, and applications. IEEE J Biomed Health Inform. (2015) 19:501–7. doi: 10.1109/JBHI.2014.2384911
16. Bermeo A, Bravo M, Huerta M, Bermeo J, Punin B, Barzallo B. 2017 IEEE second ecuador technical chapters meeting (ETCM). (2017). p. 1–6.
17. Turgeon M, Wing AM, Taylor LW. Timing and aging: Slowing of fastest regular tapping rate with preserved timing error detection and correction. Psychol Aging. (2011) 26:150. doi: 10.1037/a0020606
18. Schmidt SL, Oliveira RM, Krahe TE, Filgueiras CC. The effects of hand preference and gender on finger tapping performance asymmetry by the use of an infra-red light measurement device. Neuropsychologia. (2000) 38:529–34. doi: 10.1016/S0028-3932(99)00120-7
19. Cousins MS, Corrow C, Finn M, Salamone JD. Temporal measures of human finger tapping: effects of age. Pharmacol Biochem Behav. (1998) 59:445–9. doi: 10.1016/S0091-3057(97)00443-7
20. Todor JI, Smiley-Oyen AL. Force modulation as a source of hand differences in rapid finger tapping. Acta Psychol. (1987) 65:6573. doi: 10.1016/0001-6918(87)90047-3
21. Prigatano GP, Borgaro SR. Qualitative features of finger movement during the Halstead finger oscillation test following traumatic brain injury. J Int Neuropsychol Soc. (2003) 9:128–33. doi: 10.1017/S1355617703000134
22. Gordon AM, Quinn L, Reilmann R, Marder K. Coordination of prehensile forces during precision grip in Huntington's disease. Exp Neurol. (2000) 163:136–48. doi: 10.1006/exnr.2000.7348
23. Quinn L, Reilmann R, Marder K, Gordon AM. Altered movement trajectories and force control during object transport in Huntington's disease. Mov Disord. (2001) 16:469–80. doi: 10.1002/mds.1108
24. Reilmann R, Bohlen S, Kirsten F, Ringelstein EB, Lange HW. Assessment of involuntary choreatic movements in Huntington's disease–toward objective and quantitative measures. Mov Disord. (2011) 26:2267–73. doi: 10.1002/mds.23816
25. Schaeffer E, Maetzler W, Liepelt-Scarfone I, Sass C, Reilmann R, Berg D. Quantitative motor assessment of dyskinesias in Parkinson's disease. J Neural Transm. (2015) 122:1271–8. doi: 10.1007/s00702-015-1383-7
26. Tabrizi SJ, Scahill RI, Owen G, Durr A, Leavitt BR, Roos RA, et al. Predictors of phenotypic progression and disease onset in premanifest and early-stage Huntington's disease in the TRACK-HD study: analysis of 36-month observational data. Lancet Neurol. (2013) 12:637–49. doi: 10.1016/S1474-4422(13)70088-7
27. Klöppel S, Gregory S, Scheller E, Minkova L, Razi A, Durr A, et al. Compensation in preclinical Huntington's Disease: evidence from the track-On HD study. EBioMedicine. (2015) 2:1420–9. doi: 10.1016/j.ebiom.2015.08.002
28. Reilmann R, McGarry A, Grachev ID, Savola J-M, Borowsky B, Eyal E, et al. Safety and efficacy of pridopidine in patients with Huntington's disease (PRIDE-HD): a phase 2, randomised, placebo-controlled, multicentre, dose-ranging study. Lancet Neurol. (2019) 18:165–76. doi: 10.1016/S1474-4422(18)30391-0
29. Rosselli M, Ardila A, Bateman JR, Guzman M. Neuropsychological test scores, academic performance, and developmental disorders in spanish-speaking children. Dev Neuropsychol. (2001) 20:355–73. doi: 10.1207/S15326942DN2001_3
30. van der Plas E, Schubert R, Reilmann R, Nopoulos PC. A feasibility study of quantitative motor assessments in children using the Q-motor suite. J Huntingtons Dis. (2019) 8:333–8. doi: 10.3233/JHD-190353
31. Aoki T, Fukuoka Y. Finger tapping ability in healthy elderly and young adults. Med Sci Sports Exerc. (2010) 42:449–55. doi: 10.1249/MSS.0b013e3181b7f3e1
32. Ashendorf L, Vanderslice-Barr JL, McCaffrey RJ. Motor tests and cognition in healthy older adults. Appl Neuropsychol. (2009) 16:171–6. doi: 10.1080/09084280903098562
33. Elias MF, Robbins MA, Walter LJ, Schultz NR. The influence of gender and age on Halstead-Reitan neuropsychological test performance. J Gerontol. (1993) 48:P278–81. doi: 10.1093/geronj/48.6.P278
34. Godefroy O, Roussel M, Despretz P, Quaglino V, Boucart M. Age-related slowing: perceptuomotor, decision, or attention decline? Exp Aging Res. (2010) 36:169–89. doi: 10.1080/03610731003613615
35. Hubel KA, Reed B, Yund EW, Herron TJ, Woods DL. Computerized measures of finger tapping: effects of hand dominance, age, and sex. Percept Mot Skills. (2013) 116:929–52. doi: 10.2466/25.29.PMS.116.3.929-952
36. Leckliter IN, Matarazzo JD. The influence of age, education, IQ, gender, and alcohol abuse on Halstead-Reitan neuropsychological test battery performance. J Clin Psychol. (1989) 45:484–512. doi: 10.1002/1097-4679(198907)45:4<484::aid-jclp2270450402>3.0.co;2-l
37. McCurry SM, Gibbons LE, Uomoto JM, Thompson ML, Graves AB, Edland SD, et al. Neuropsychological test performance in a cognitively intact sample of older Japanese American adults. Arch Clin Neuropsychol. (2001) 16:447–59. doi: 10.1093/arclin/16.5.447
38. Shimoyama I, Ninchoji T, Uemura K. The finger-tapping test. a quantitative analysis. Arch Neurol. (1990) 47:681–4. doi: 10.1001/archneur.1990.00530060095025
39. Dorfberger S, Adi-Japha E, Karni A. Sex differences in motor performance and motor learning in children and adolescents: an increasing male advantage in motor learning and consolidation phase gains. Behav Brain Res. (2009) 198:165–71. doi: 10.1016/j.bbr.2008.10.033
40. Peters M, Campagnaro P. Do women really excel over men in manual dexterity? J Exp Psychol Hum Percept Perform. (1996) 22:1107. doi: 10.1037/0096-1523.22.5.1107
41. Roivainen E. Gender differences in processing speed: a review of recent research. Learn Individ Differ. (2011) 21:145–9. doi: 10.1016/j.lindif.2010.11.021
42. Yeudall LT, Reddon JR, Gill DM, Stefanyk WO. Normative data for the Halstead-Reitan neuropsychological tests stratified by age and sex. J Clin Psychol. (1987) 43:346–67. doi: 10.1002/1097-4679(198705)43:3<346::aid-jclp2270430308>3.0.co;2-q
43. Mostile G, Jankovic J. Alcohol in essential tremor and other movement disorders. Mov Disord. (2010) 25:2274–84. doi: 10.1002/mds.23240
44. Foulds J, Stapleton J, Swettenham J, Bell N, McSorley K, Russell MA. Cognitive performance effects of subcutaneous nicotine in smokers and never-smokers. Psychopharmacology. (1996) 127:31–8. doi: 10.1007/BF02805972
45. Perkins KA, Lerman C, Coddington SB, Jetton C, Karelitz JL, Scott JA, et al. Initial nicotine sensitivity in humans as a function of impulsivity. Psychopharmacology. (2008) 200:529–44. doi: 10.1007/s00213-008-1231-7
46. Perkins KA, Gerlach D, Broge M, Grobe JE, Sanders M, Fonte C, et al. Dissociation of nicotine tolerance from tobacco dependence in humans. J Pharmacol Exp Ther. (2001) 296:849–56.
47. Perkins KA, Grobe JE, Fonte C, Goettler J, Caggiula AR, Reynolds WA, et al. Chronic and acute tolerance to subjective, behavioral and cardiovascular effects of nicotine in humans. J Pharmacol Exp Ther. (1994) 270:628–38.
48. Sobin C, Sackeim HA. Psychomotor symptoms of depression. Am J Psychiatry. (1997) 154:4–17. doi: 10.1176/ajp.154.1.4
49. Teismann H, Wersching H, Nagel M, Arolt V, Heindel W, Baune BT, et al. Establishing the bidirectional relationship between depression and subclinical arteriosclerosis–rationale, design, and characteristics of the BiDirect Study. BMC Psychiatry. (2014) 14:174. doi: 10.1186/1471-244X-14-174
50. Wersching H, Berger K. [New cohorts. the BiDirect study]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. (2012) 55:822–3. doi: 10.1007/s00103-012-1491-6
51. Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. (1977) 1:385–401. doi: 10.1177/014662167700100306
52. R Core Team,. R: A Language Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2022). Available online at: https://www.R-project.org/
53. Teismann H, Kissler J, Berger K. Investigating the roles of age, sex, depression, and anxiety for valence and arousal ratings of words: a population-based study. BMC psychology. (2020) 8:118. doi: 10.1186/s40359-020-00485-3
54. Heaton RK, Grant I, Matthews CG. Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographic Corrections, Research Findings, and Clinical Applications. Psychological Assessment Resources (1991).
55. Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology. (2010) 210:453–69. doi: 10.1007/s00213-010-1848-1
Keywords: manual motor performance, quantitative motor (Q-Motor), finger tapping, observational, cross-sectional, Purdue Pegboard, community-dwelling adults
Citation: Teismann H, Schubert R, Reilmann R and Berger K (2022) Effects of age and sex on outcomes of the Q-Motor speeded finger tapping and grasping and lifting tests-findings from the population-based BiDirect Study. Front. Neurol. 13:965031. doi: 10.3389/fneur.2022.965031
Received: 09 June 2022; Accepted: 30 August 2022;
Published: 30 September 2022.
Edited by:
Santiago Perez-Lloret, Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), ArgentinaReviewed by:
Vrutangkumar V. Shah, Oregon Health and Science University, United StatesCopyright © 2022 Teismann, Schubert, Reilmann and Berger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Henning Teismann, aC50ZWlzbWFubkB1bmktbXVlbnN0ZXIuZGU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.