
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 20 September 2022
Sec. Epilepsy
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.964761
 Fitri Octaviana1,2*
Fitri Octaviana1,2* Kanadi Sumapraja3,4†
Kanadi Sumapraja3,4† Winnugroho Wiratman1,2†
Winnugroho Wiratman1,2† Luh Ari Indrawati1,2†
Luh Ari Indrawati1,2† Astri Budikayanti1,2†
Astri Budikayanti1,2†Objective: Menstrual disorders are more common in women with epilepsy than in those without epilepsy. This study aimed to examine the characteristics of reproductive function in women with epilepsy at an Indonesian national referral hospital.
Methods: A case-control study was conducted from March 2020 to March 2021. Women with and without epilepsy aged ≥18 years were enrolled. All women were premenopausal before epilepsy diagnosis. Data on demographic characteristics, menstrual profiles, epileptic syndrome, seizure type, seizure frequency, etiology, localization, and anticonvulsant medication were collected. Hormone levels (follicle stimulating hormone, luteinizing hormone, prolactin, and estradiol) were measured.
Results: A total of 72 women with and 50 without epilepsy (controls) were included. Dysmenorrhea was more common in women with epilepsy than in those without (59.7 vs. 20%, p < 0.001; odds ratio: 5.931 [95% confidence interval: 2.566–13.709]). Marriage rates were higher in women without epilepsy (82 vs. 45.8%, p < 0.001). No difference was found in hormone levels between the groups. The frequency of seizures was associated with prolactin and estradiol levels (p < 0.001). Polytherapy with clobazam was associated with menstrual cycle regularity. In women with epilepsy with menstrual disorders, valproic acid was associated with higher estradiol levels (p = 0.001) and lamotrigine with lower follicle stimulating hormone levels (p = 0.008).
Significance: Women with epilepsy experienced more dysmenorrhea. A higher frequency of seizures associated with lower prolactin and estradiol levels. Polytherapy with clobazam was associated with irregular menstrual cycles, while valproic acid and lamotrigine was associated with estradiol and follicle stimulating hormone levels.
Epilepsy is a neurological disorder that commonly affects women, with an estimated prevalence of 6.85 cases per 1,000 population (1, 2) and range of ~0.3–0.7% in women of childbearing age (3, 4). Approximately 1.5 million women with epilepsy (WWE) in the United States and 1.3 million WWE in India belong to the reproductive age group (5, 6). Epilepsy is considered an inherited disease by certain inhabitants, thus influencing certain people to hesitate marrying WWE. A study in India, which is similar to Indonesia in social and economic aspects, revealed that marriage rates among WWE in India were far lower than those among women without epilepsy (WWoE) (7). However, confirmed data in Indonesia are still lacking. Stigma surrounding epilepsy in Indonesia remains profound (8).
Epilepsy and reproductive hormones have a complex bidirectional relationship, and the reproductive health of WWE requires special consideration, especially in patients who have been using anti-seizure medications (ASMs) for a long period of time (9). Reproductive hormones, such as follicle stimulating hormone (FSH), luteinizing hormone (LH), progesterone, and estradiol, are important determinants of female reproductive function. These hormones affect oocyte maturation, ovulation, and menstruation. A previous study demonstrated that hormonal changes in WWE affect neuronal activity and increase the risk of worsening seizures; for example, estrogen has an excitatory effect on neurons that can generate seizures (10). Epilepsy can also affect reproductive endocrine function through the hypothalamic-pituitary-adrenal (HPA) axis (11). Several studies have shown an increase in the frequency of menstrual disorders, fertility problems, and hormonal dysfunction in WWE. However, fluctuating hormonal levels can also affect the frequency of seizures and metabolism of ASMs and vice versa (2). In one study, menstrual disorders were reported in one in three WWE compared to one in seven women in the general population (12).
The increasing prevalence of menstrual disorders in WWE can also be attributed to the side effects of certain ASMs. Cytochrome P450 inducers, such as carbamazepine and phenytoin, can affect circulating hormone levels, while continued use of clonazepam can affect menstrual cycle regularity (9). Other ASMs such as valproic acid (VPA) can also cause side effects, such as polycystic ovaries and hyperandrogenism (13). The relationship between epilepsy and menstrual disorders remains unclear. The purpose of this study was to determine the characteristics of reproductive function in WWE, with a focus on dysmenorrhea and menstrual disorders, and to evaluate differences in reproductive hormone levels between WWE and WWoE.
This study was approved by the Faculty of Medicine Universitas Indonesia Ethics Committee and Cipto Mangunkusumo Hospital research section (ethical review number: 1393/UN2.F1/ETIK/PPM.00.02/2019). We conducted a case-control study from March 2020 to March 2021 at Dr. Cipto Mangunkusumo National Referral Hospital, Jakarta. All patients provided written informed consent. The subjects were recruited using convenient consecutive sampling method. The sample size was determined using the calculation for 2 groups with a 95% confidence interval (95% CI) and power of 80%, using the prevalence of menstrual disorders in WWE and WWoE based on previous study (7). The inclusion criteria for the case group (WWE) and control group (WWoE) were those aged ≥18 years and already had their menstrual cycle. The exclusion criteria for the case group were WWE who had already had menopause before being diagnosed epilepsy, while the exclusion criteria for control group were WWoE who already had menopause and had comorbidities such as autoimmune disease and other chronic diseases. Subjects in the control group who had menstrual disorders were recruited from patients who visited the gynecology outpatient clinic at Dr Cipto Mangunkusumo National Referral Hospital, while subjects in the control group without menstrual disorders were recruited from patients who visited general outpatient clinics at the same hospital and did not have any symptoms of menstrual disorders. Thereafter, WWE and WWoE with menstrual disorders were compared, and a similar comparison was made between WWE and WWoE without menstrual disorders.
Demographic and clinical data were obtained primarily through interviews and secondarily through medical records. Blood samples were subsequently taken from both groups after the interview to measure levels of reproductive hormones which were FSH, LH, prolactin, and estradiol. The confounder factor was the blood samples were taken randomly at unspecified times without regard to phases of the menstrual cycle in both groups.
Independent variables comprised the subjects' age, onset of epilepsy, duration of epilepsy, seizure type and frequency, type and number of ASMs consumed, history of status epilepticus, etiology of epilepsy, localization of epilepsy, age of menarche, and marital history. The classification of epilepsy types and etiology were defined based on the International League Against Epilepsy 2017 classification guidelines (14). To determine structural etiology and localization of epilepsy, EEG and brain MRI were performed on all WWE. All patients on ASM polytherapy were further analyzed to determine the association between ASM combinations and menstrual disorders or dysmenorrhea. ASMs were further categorized further into the following three categories: (a) inducers, which included carbamazepine, phenytoin, and phenobarbital; (b) inhibitors, which included VPA; and (c) miscellaneous for the remaining types of ASMs. Subjects with controlled seizures were defined as those who had been seizure free throughout the preceding year.
Dependent variables included menstrual disorders and dysmenorrhea. Menstrual disorders were defined as any deviation from the normal menstrual cycle based on the 2011 International Federation of Gynecology and Obstetrics guidelines (15). In this study, we classified menstrual disorders based on frequency and regularity. Menstrual cycles were considered of normal frequency if they were within the range of 21–35 days and abnormal if they were outside that range. Regularity was classified as normal if the variation between cycles was limited to 2–20 days over a 12-months period. Dysmenorrhea was defined as discomfort and pain during the menstrual period (16).
Data were analyzed using SPSS version 20.0. Data on subject characteristics measured in categorical form are presented in proportions. Data in numerical form were analyzed using the Kolmogorov–Smirnov test to determine the distribution of the data. Normally distributed data were interpreted using a p-value > 0.05 to indicate statistical significance and are presented as the mean and standard deviation (SD), while abnormally distributed data were interpreted using a p-value < 0.05 to indicate statistical significance and are presented as the median and range. To determine the potential factors associated with menstrual disorders and dysmenorrhea, an initial bivariate analysis was conducted to determine the factors that had a statistically significant effect. The chi-square test or Fisher's exact test was used for categorical variables, and the student's t-test or Mann–Whitney test was used for numerical variables.
Seventy-two WWE and 50 WWoE participated in this study. There were no missing data in this study. The demographic characteristics of WWE compared to those of WWoE are displayed in Table 1. The mean age of WWE was younger than that of WWoE (p < 0.05). The age of menarche was comparable between the groups (~12 years). The numbers of WWE (case) and WWoE (control) who had menstrual disorders were similar (30 and 32, respectively). However, the number of WWE was higher than that of WWoE in the normal menses group (42 and 18, respectively). Dysmenorrhea was more common in WWE than in WWoE (59.7 vs. 20%; odds ratio [OR]: 5.931, 95% CI: 2.566–13.709). WWE were less likely to be married than WWoE (45.8 vs. 82%; p < 0.001). Most WWE were aged ≤ 40 years (83.3%), and the majority (73.6%) were aged >10 years at the time of epilepsy onset. The most common type of seizure encountered was the focal type (95.8%), with most subjects claiming to have uncontrolled seizures (63.9%). Only eight patients (11.1%) had a history of status epilepticus in their lifetime. Most WWE reported one or two seizures per month (76.1%).
The analysis was performed separately between groups with or without menstrual disorders, as shown in Table 2. The demographic characteristics of the subjects based on the presence of menstrual disorders are displayed in Table 2. In the group with menstrual disorders, there were 30 WWE and 32 WWoE. Most WWE and WWoE with menstrual disorders were aged ≤ 40 years (80 and 84.4%, respectively), and the age at onset of menarche was ≥12 years in most patients (83.3 and 90.6%, respectively). Dysmenorrhea was significantly more common in WWE than in WWoE in the menstrual disorders group (56.7 vs. 9.4%; OR: 12.64, CI: 3.146–50.794). In the group without menstrual disorders, there were 42 WWE and 18 WWoE. The proportion of WWE who had dysmenorrhea was higher than that of WWoE (61.9 and 38.9%, respectively); however, the difference was not statistically significant.
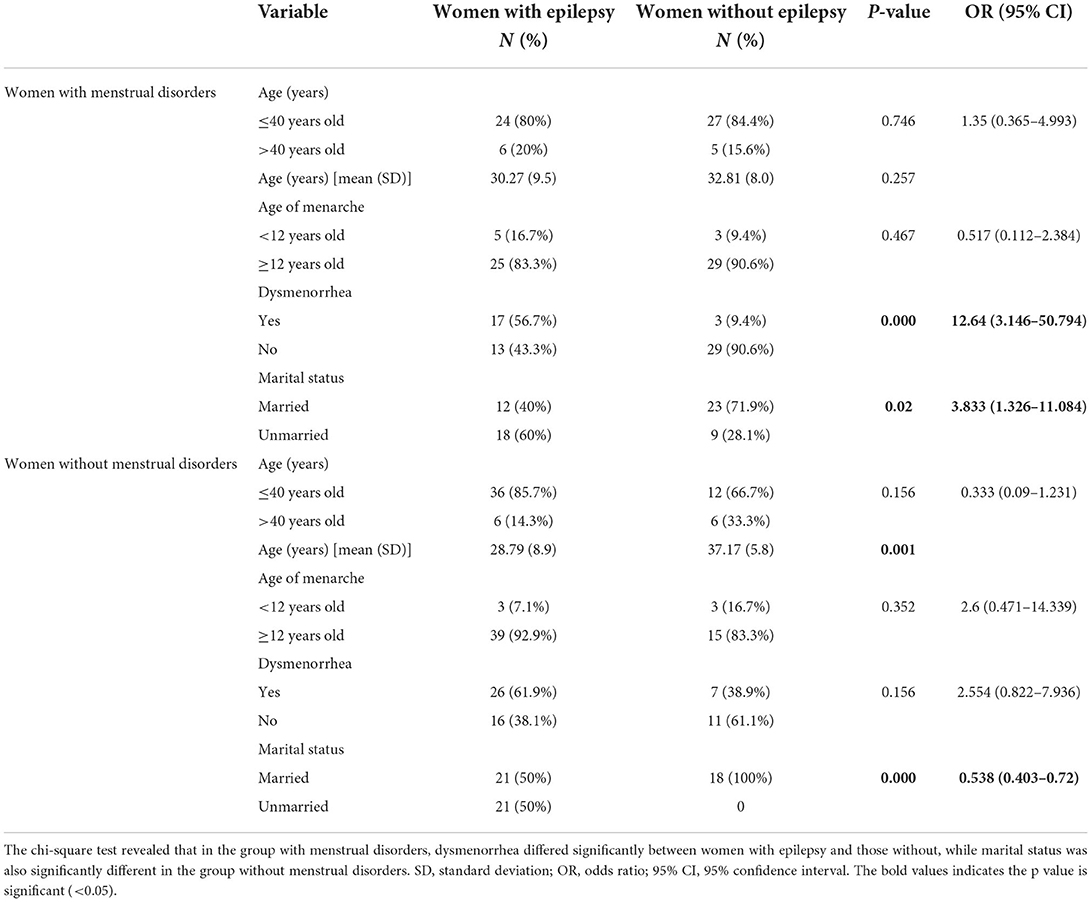
Table 2. Comparison of demographic and hormone characteristics between women with menstrual disorders and women without menstrual disorders.
Table 3 details the association of dysmenorrhea and menstrual disorders in WWE. No factors were related to the incidence of dysmenorrhea or menstrual disorders in WWE. However, clobazam was significantly associated with menstrual disorders (p = 0.033). All patients who used clobazam were on polytherapy.
The characteristics of reproductive hormones among WWE and WWoE with and without menstrual disorders are shown in (Supplementary S1). Although the levels of reproductive hormones (FSH, LH, prolactin, and estradiol) were higher in WWE than in WWoE, the difference was not significant (p > 0.05). In the group without menstrual disorders, there were 42 WWE and 18 WWoE. Compared with the group with menstrual disorders, the average level of both WWE and WWoE reproductive hormones (FSH, LH, and prolactin) in the group without menstrual disorders was lower, with estradiol being the only exception. Among WWE, the average estradiol level was higher in those without menstrual disorders than in those with menstrual disorders (209.5 [509.9] and 101.72 pg/mL [123.1], respectively).
Supplementary S2 shows the association of reproductive hormones with demographic and clinical factors in WWoE. Hormone levels FSH, LH, and prolactin were associated with menstrual disorders in WWoE (p < 0.05). While estradiol level was observed to be higher in WWoE who undergoes menarche at the age <12 years old.
In contrast to WWoE, menstrual disorders didn't show to be associated with hormone levels in WWE (Table 4). However, there was a significant association between seizure frequency and prolactin and estradiol levels in WWE (p < 0.001). Prolactin and estradiol levels tended to be lower with higher number of seizure frequency. FSH level was lower in WWE aged ≤ 40 years than in those aged >40 years (p < 0.001).
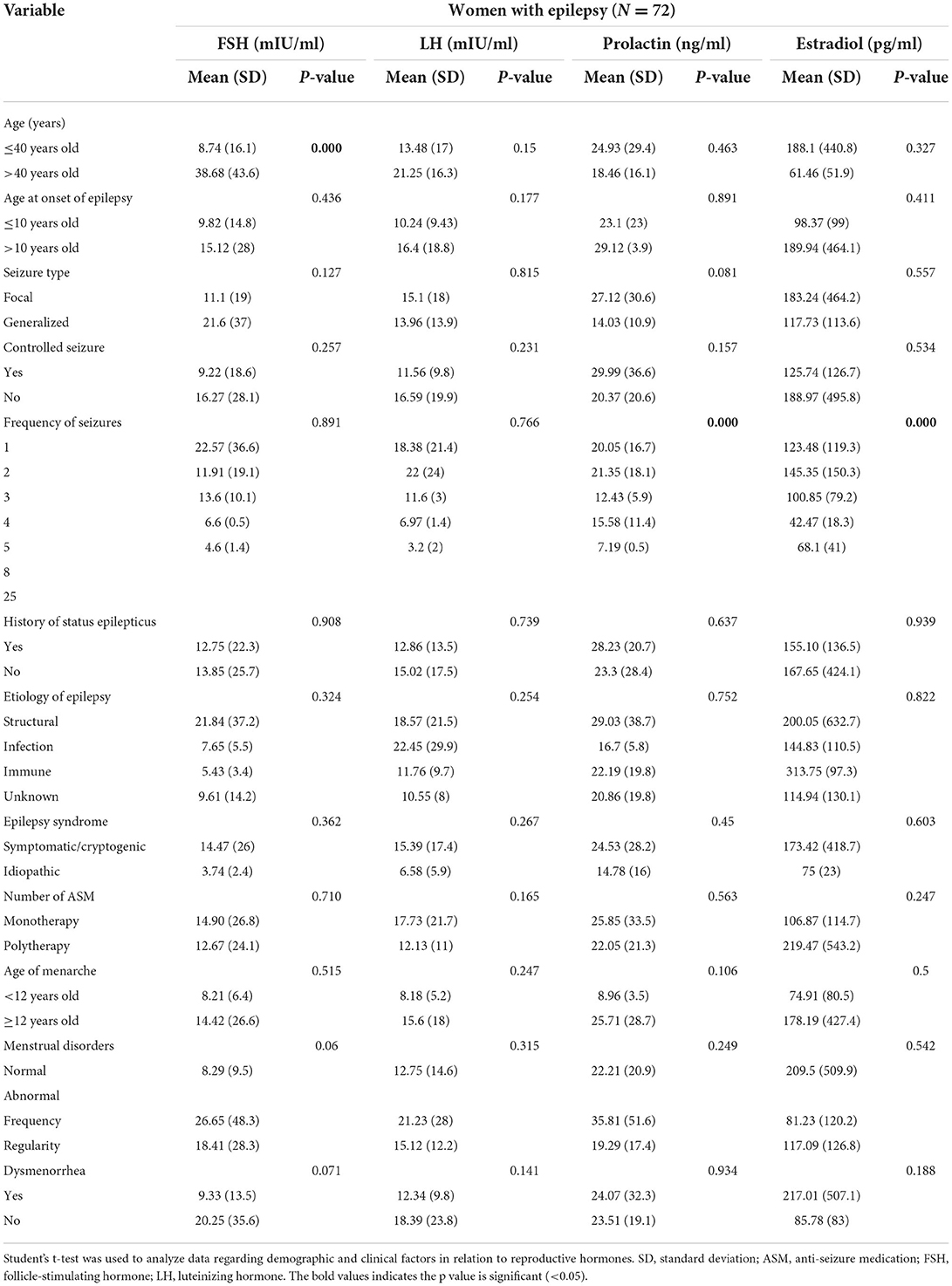
Table 4. The association of reproductive hormone with demographic and clinical factors in women with epilepsy.
Inducer ASMs were the most widely used ASMs (32.1%), followed by inhibitor ASMs (13%). Supplementary S3 presents the association of certain types of ASMs with reproductive hormones. There was no association between the use of ASM and reproductive hormone levels in WWE in the total cohort. However, in subgroup analysis based on the presence of menstrual disorders, some ASMs exhibited a direct relationship with certain hormones. In WWE with menstrual disorders, VPA was associated with higher estradiol levels, while lamotrigine was associated with lower FSH levels. In WWE without menstrual disorders, topiramate was associated with higher prolactin levels.
This is the first study conducted on WWE in Indonesia. We found no significant difference in age at menarche between WWoE and WWE, which are consistent with the findings of the previous (16). In contrast, there was a difference in marital status between the groups: marriage rates were lower in WWE than in WWoE. General population in Indonesia tend to be unwilling to marry people with epilepsy (PWE). Despite of good knowledge of epilepsy among general population, they were still reluctant to have deep bond with PWE. This could lead to low self-esteem and high unmarried rate among WWE (8). Similarly, a study in India also found that WWE had a lower marriage rate than the general population (9).
The clinical characteristics of the subjects included in this study were identical to those included in previous studies on epilepsy that displayed focal onset epilepsy as a common seizure type (17). The mainstream etiologies in our subjects were either structural or unknown; this is in accordance with several other studies that revealed structural (head trauma, cerebrovascular accidents) or unknown problems as the leading causes of epilepsy (18, 19). In terms of epilepsy syndromes, symptomatic/cryptogenic were more common than idiopathic types in all groups, which was also demonstrated in a Canadian study, where symptomatic, cryptogenic, and idiopathic epilepsy occurred in 44.4, 47.6, and 8% of subjects, respectively (20).
The prevalence of menstrual disorders among WWE in this study was higher than that reported in other studies. Menstrual disorders were reported in 28.8% of WWE in Poland7 and 39.7% of those in Nigeria (10). The irregularity of menstrual cycle in WWE could be associated with the decrease of luteal phase. This phenomenon was also seen in depression and anxiety (21). The correlation of epilepsy with menstrual disorders is thought to be due to the effect of epilepsy itself or an adverse effect of ASMs (clonazepam) on the neuroendocrine regulation of the menstrual cycle (7, 22). The prevalence of menstrual disorders in WWE who had an onset of epilepsy before menarche was higher than that in WWE with an age of epilepsy onset after menarche (7). However, there were no significant associations of age of epilepsy onset and age at menarche with menstrual disorders in this study, which is consistent with the findings of previous studies (7).
Our study showed there was no significant association between menstrual disorders in WWE and ASM. Bosak et al. (7) demonstrated no significant difference in the incidence of irregular menstruation in patients taking valproic acid, lamotrigine, levetiracetam, lacosamide, or topiramate but noted a significant association with clonazepam. Similarly, our study revealed that polytherapy with clobazam was the only ASM to have a significant relationship with menstrual disorders. Benzodiazepines, such as clonazepam and clobazam, are commonly used to treat exacerbations of hormonally affected seizures (5). The mechanism underlying the relationship between the use of benzodiazepines and menstrual disorders has not been elucidated. The number of ASMs and type of polytherapy combination were not significantly associated with dysmenorrhea and menstrual disorders; hence, duration of drug usage and drug dosage were not analyzed further.
The incidence of dysmenorrhea in this study was higher in WWE (59.7%) than in WWoE (20%). Psychological factors, such as depression, stress, and anxiety, are thought to have a reciprocal relationship with the severity of dysmenorrhea and menstrual disorders. The occurrence of monthly dysmenorrhea can increase the risk of depression and vice versa. Women with dysmenorrhea are more prone to depression and anxiety (23). However this study did not include depression as a variable influencing dysmenorrhea in WWE. In addition, disturbances in the HPA axis in WWE can affect the production of reproductive hormones, which then play a role in the incidence of dysmenorrhea (24). We did not found any association between demographic and clinical factors to dysmenorrhea in WWE.
The results of this study revealed that prolactin, estradiol, FSH, and LH levels in WWE were higher than those in WWoE. FSH levels were found to be higher in the sample population aged >40 years old in both WWE and WWoE. This finding is consistent with known physiological changes, as FSH production indeed increases with age. However, after closer scrutiny, FSH levels appear significantly higher in WWE (Table 4) than in WWoE (Supplementary S2). An earlier study also indicated that WWE generally have higher levels of FSH, LH, prolactin, and estradiol than WWoE (25). Herzog et al. (26) proposed that an element of lateralization was involved in reproductive hormone disorders among WWE. Unfortunately, we did not distinguish between symptomatic epilepsy with left and right-sided foci.
In this study, a higher frequency of seizures was associated with lower prolactin and estradiol levels in the blood. The mechanisms involved remain vague, and further research is required to elucidate them. The proconvulsant nature of cortisol leads to further susceptibility and frequency of future seizures (27). The association between estrogen and seizure frequency has not been fully understood. Estradiol levels were reduced in WWE compared with those in WWoE in this study. In contrast, estrogen has both effects as pro- and anticonvulsant properties as well (28). Therefore, the lower level of estrogen could become the associated factors toward high frequency seizure in WWE and vice versa. The high number of seizure frequency could alter the pulsatile secretion of estrogen.
The interaction between epilepsy and ASMs can cause sexual hormone imbalance (4). One study concluded that monotherapy with VPA causes a decrease in FSH and LH levels in WWE (29). An earlier study demonstrated that oxcarbazepine reduces estrogen and progesterone levels, while lamotrigine reduces estradiol levels (30). Our findings differ slightly in the sense that in WWE with menstrual disorders, VPA was associated with higher estradiol levels, while lamotrigine was associated with lower FSH levels. An in-vitro study demonstrated that VPA directly inhibits ovarian steroid production, namely FSH and estradiol synthesis (31); however, other studies show that VPA tends to increase estradiol activity, either through inhibition of liver enzymes or enhancement of estrogen receptors (32). Lower FSH levels have also been discovered in patients who underwent monotherapy with lamotrigine, regardless of their menstrual phase (33). In WWE without menstrual disorders, topiramate was connected to higher prolactin levels. However, the mechanism underlying the association of topiramate and prolactin is unclear due to limited data. No further association has been identified between epilepsy syndromes and etiology on reproductive hormone levels. A possible reason for the discrepancy between our study and previous studies could be the insufficient sample size and timing of retrieving blood samples.
The limitations of our study are that we did not consider the various phases of the menstrual cycle, therefore analysis of hormone levels in women without menstrual disorders could potentially be exposed to bias. Unfortunately, we did not record seizure data within 24 h of blood collection to determine the association between seizures and hormone levels, especially of prolactin. Other limitations includes absent information on lateralization of structural etiology and lack of body mass index and other clinical data (comorbidities and routine consumption of drugs) on WWoE in comparison with WWE. Due to limited time during sample collection in this pandemic era, the number of WWoE without menstrual disorders did not achieve the same level as that of the case group. Unfortunately, we could not conduct multicenter research, since we could not visit other hospital at the beginning of the pandemic era.
In conclusion, WWE were more prone to suffer from dysmenorrhea than WWoE. Unsurprisingly there was a significant difference in WWoE hormone levels, in those with and without menstrual disorders. However, this phenomenon was not observed in WWE; hence, menstrual disorders in WWE could be caused by external, non-hormonal factors. Further research opportunities are suggested with a larger sample size and multicenter to comprehend the association between dysmenorrhea and depression, as well as the focal epileptic discharge location association toward the occurrence of menstrual disorders in WWE. Moreover, additional research on menstrual disorders in WWE needs to be warranted, involving serial reproductive hormone levels examination in relation to certain phases of the menstrual cycle.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Faculty of Medicine Universitas Indonesia Ethics Committee and Cipto Mangunkusumo Hospital research section (Ethical review number: 1393/UN2.F1/ETIK/PPM.00.02/2019). The patients/participants provided their written informed consent to participate in this study.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The authors would like to thank Universitas Indonesia for funding this research through the PUTI Grant (Contract number: NKB-1304/UN2.RST/HKP.05.00/2020).
We would like to thank Sugiharto, MD, Christine Sari, MD, and Theodore Nathan, MD, for assisting in data collection and providing valuable contributions to this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.964761/full#supplementary-material
1. Fiest KM, Sauro KM, Wiebe S, et al. Prevalence and incidence of epilepsy. Neurology. (2017) 88:296–303. doi: 10.1212/WNL.0000000000003509
2. Stephen LJ, Harden C, Tomson T, Brodie MJ. Management of epilepsy in women. Lancet Neurol. (2019) 18:481–91. doi: 10.1016/S1474-4422(18)30495-2
3. Sayehmiri K, Tavan H, Sayehmiri F, Mohammadi I, Carson KV. Prevalence of epilepsy in Iran: a meta-analysis and systematic review. Iran J Child Neurol. (2014) 8:9–17.
4. Atarodi-Kashani Z, Kariman N, Ebadi A, Alavi Majd H, Beladi-Moghadam N. Sexual function and related factors in Iranian woman with epilepsy. Seizure. (2017) 52:147–53. doi: 10.1016/j.seizure.2017.10.001
5. Sazgar M. Treatment of women with epilepsy. Continuum. (2019) 25:408–30. doi: 10.1212/CON.0000000000000713
6. Santhosh NS, Sinha S, Satishchandra P. Epilepsy: Indian perspective. Ann Indian Acad Neurol. (2014) 17:S3–11. doi: 10.4103/0972-2327.128643
7. Bosak M, Słowik A, Turaj W. Menstrual disorders and their determinants among women with epilepsy. Neuropsychiatr Dis Treat. (2018) 14:2657–64. doi: 10.2147/NDT.S179438
8. Budikayanti A, Primardi A, Indrawati LA, Hamid D, Indriyani J, Rahmi I, et al. The stigma paradox: perception of quality-of-life in people with epilepsy among themselves, the family, and the general population in Indonesian urban areas. Epilepsy Res. (2022) 183:106938. doi: 10.1016/j.eplepsyres.2022.106938
9. Agarwal P, Mehndiratta MM, Antony AR, Kumar N, Dwivedi RN, Sharma P, et al. Epilepsy in India: nuptiality behaviour and fertility. Seizure. (2006) 15:409–15. doi: 10.1016/j.seizure.2006.04.005
10. Osalusi BS, Ogunjimi L, Yaria J, Ale A, Ogunsemi O, Akinyinka A, et al. The hormonal changes among Nigerian women with epilepsy. Afr J Sci Nat. (2020) 10:32–8. doi: 10.46881/ajsn.v10i0.173
11. Wulsin A, Solomon MB, Privitera M, Danzer SC, Herman JP. Hypothalamic-pituitary-adrenocortical axis dysfunction in epilepsy. Physiol Behav. (2016) 166:22–31. doi: 10.1016/j.physbeh.2016.05.015
12. Noe K. Chapter 12: epilepsy in women. In: Cascino GD, Sirven JI, Tatum WO, editors. Epilepsy. Hoboken, NY: Wiley (2021). p. 217–27. doi: 10.1002/9781119431893.ch12
13. Sidhu HS, Srinivasa R, Sadhotra A. Evaluate the effects of antiepileptic drugs on reproductive endocrine system in newly diagnosed female epileptic patients receiving either valproate or lamotrigine monotherapy: a prospective study. Epilepsy Res. (2018) 139:20–7. doi: 10.1016/j.eplepsyres.2017.10.016
14. Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia. (2017) 58:512–21. doi: 10.1111/epi.13709
15. Fraser IS, Critchley HOD, Broder M, Munro MG. The FIGO recommendations on terminologies and definitions for normal and abnormal uterine bleeding. Semin Reprod Med. (2011) 29:383–90. doi: 10.1055/s-0031-1287662
16. Stefanidou M, Montouris G. Reproductive and sexual health concerns in transition-age adolescents and young adults with epilepsy. Semin Pediatr Neurol. (2020) 36:100855. doi: 10.1016/j.spen.2020.100855
17. Guekht A, Hauser WA, Milchakova L, Churillin Y, Shpak A, Gusev E. The epidemiology of epilepsy in the Russian Federation. Epilepsy Res. (2010) 92:209–18. doi: 10.1016/j.eplepsyres.2010.09.011
18. Wirrell EC, Grossardt BR, Wong-Kisiel LCL, Nickels KC. Incidence and classification of new-onset epilepsy and epilepsy syndromes in children in Olmsted County, Minnesota from 1980 to 2004: a population-based study. Epilepsy Res. (2011) 95:110–8. doi: 10.1016/j.eplepsyres.2011.03.009
19. Beghi E. The epidemiology of epilepsy. Neuroepidemiology. (2020) 54:185–91. doi: 10.1159/000503831
20. Téllez-Zenteno JF, Hernández-Ronquillo L, Buckley S, Zahagun R, Rizvi S. A validation of the new definition of drug-resistant epilepsy by the international league against epilepsy. Epilepsia. (2014) 55:829–34. doi: 10.1111/epi.12633
21. Handy AB, Greenfield SF, Yonkers KA, Payne LA. Psychiatric symptoms across the menstrual cycle in adult women: a comprehensive review. Harv Rev Psychiatry. (2022) 30:100–17. doi: 10.1097/HRP.0000000000000329
22. Pennell PB. Hormonal aspects of epilepsy. Neurol Clin. (2009) 27:941–65. doi: 10.1016/j.ncl.2009.08.005
23. Bahrami A, Sadeghnia H, Avan A, Mirmousavi SJ, Moslem A, Eslami S, et al. Neuropsychological function in relation to dysmenorrhea in adolescents. Eur J Obstet Gynecol Reprod Biol. (2017) 215:224–9. doi: 10.1016/j.ejogrb.2017.06.030
24. Atif M, Rehan Sarwar M, Scahill S. The relationship between epilepsy and sexual dysfunction: a review of the literature. Springerplus. (2016) 5:2070. doi: 10.1186/s40064-016-3753-5
25. Hamed SA. The effect of epilepsy and antiepileptic drugs on sexual, reproductive and gonadal health of adults with epilepsy. Expert Rev Clin Pharmacol. (2016) 9:807–19. doi: 10.1586/17512433.2016.1160777
26. Herzog AG. Disorders of reproduction in patients with epilepsy: primary neurological mechanisms. Seizure. (2008) 17:101–10. doi: 10.1016/j.seizure.2007.11.025
27. Hooper A, Paracha R, Maguire J. Seizure-induced activation of the HPA axis increases seizure frequency and comorbid depression-like behaviors. Epilepsy Behav. (2018) 78:124–33. doi: 10.1016/j.yebeh.2017.10.025
28. Miziak B, Chroscinska-Krawczayk M, Czuczwar SJ. Neurosteroids and seizure activity. Front Endocrinol. (2020) 11:541802. doi: 10.3389/fendo.2020.541802
29. Rathore C, Henning OJ, Luef G, Radhakrishnan K. Sexual dysfunction in people with epilepsy. Epilepsy Behav. (2019) 100:106495. doi: 10.1016/j.yebeh.2019.106495
30. Reimers A. New antiepileptic drugs and women. Seizure. (2014) 23:585–91. doi: 10.1016/j.seizure.2014.05.004
31. Glister C, Satchell L, Michael AE, Bicknell AB, Knight PG. The anti-epileptic drug valproic acid (VPA) inhibits steroidogenesis in bovine theca and granulosa cells in vitro. PLoS ONE. (2012) 7:e49553. doi: 10.1371/journal.pone.0049553
32. Stempin S, Andres S, Bumke Scheer M, Rode A, Nau H, Seidel A, et al. Valproic acid and its derivatives enhanced estrogenic activity but not androgenic activity in a structure dependent manner. Reprod Toxicol. (2013) 42:49–57. doi: 10.1016/j.reprotox.2013.07.019
Keywords: menstrual disorders, epilepsy, women, reproductive hormones, dysmenorrhea
Citation: Octaviana F, Sumapraja K, Wiratman W, Indrawati LA and Budikayanti A (2022) Characteristics of menstrual disorders and reproductive hormones in women with epilepsy at an Indonesian national referral hospital. Front. Neurol. 13:964761. doi: 10.3389/fneur.2022.964761
Received: 09 June 2022; Accepted: 29 August 2022;
Published: 20 September 2022.
Edited by:
Mohammad Taheri, University Hospital Jena, GermanyReviewed by:
Omid Mirmosayyeb, University at Buffalo, United StatesCopyright © 2022 Octaviana, Sumapraja, Wiratman, Indrawati and Budikayanti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fitri Octaviana, Zml0cmkub2N0YXZpYW5hQHVpLmFjLmlk
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.