- 1Center for Stroke Research Berlin, Charité – Universitätsmedizin Berlin, Berlin, Germany
- 2German Centre for Cardiovascular Research DZHK, Charité –Universitätsmedizin Berlin, Berlin, Germany
- 3Klinik und Hochschulambulanz für Neurologie, Charité- Universitätsmedizin Berlin, Berlin, Germany
- 4German Center for Neurodegenerative Disease DZNE, Berlin Charité-Universitätsmedizin Berlin, Berlin, Germany
- 5Institute of Biometry and Clinical Epidemiology, University of Würzburg, Würzburg, Germany
- 6German Center for Neurodegenerative Disease DZNE, partner site Berlin Charité-Universitätsmedizin Berlin, Berlin, Germany
- 7NeuroCure Clinical Research Center, Charité – Universitätsmedizin Berlin, Berlin, Germany
- 8Department of Clinical Epidemiology, Leiden University Medical Center, Leiden, Netherlands
- 9Department of Neurology, Carl von Ossietzky University of Oldenburg, Oldenburg, Germany
Introduction: Low ankle-brachial index (ABI) ≤0. 9 is a marker for generalized atherosclerosis and a risk factor for cognitive decline in the general population.
Objective: To evaluate the impact of ABI ≤0.9 on cognitive function up to 3 years after first-ever ischemic stroke.
Methods: Data was used from the “PROspective Cohort with Incident Stroke-Berlin” (PROSCIS-B; NCT01363856). ABI was measured at baseline and categorized into normal (1.4–0.9) vs. low (≤0.9). Cognitive function was assessed with the Montreal Cognitive Assessment (MoCA) and the Mini-Mental-State-Examination (MMSE) at baseline and with the Telephone Interview for Cognitive Status-modified (TICS-m) at 1–3 years of follow-up. We performed confounder adjusted generalized linear models (GLM) to calculate relative risks (RR) for cognitive impairment at baseline (MMSE≤26; MoCA≤25) and linear mixed models (LMM) to estimate the impact of low ABI on TICS-m over time.
Results: We included 325 patients [mean age: 66 (SD = 13); 38% female, median NIHSS = 2 (IQR = 1–4), ABI≤0.9: 59 (18%)]. Patients with low ABI were at increased risk of cognitive impairment at baseline (adjusted RR for MoCA≤25 = 1.98; 95%-CI:1.24 to 3.16). TICS-m scores were consistently lower over time in patients with low ABI (adjusted ß = −1.96; 95%-CI:−3.55 to −0.37). Independent of ABI, cognitive function did not decline over time (adjusted ß:0.29; 95%-CI:−0.06 to 0.64).
Conclusion: In patients with mild to moderate first-ever ischemic stroke, low ABI is associated with reduced cognitive function over a 3-year follow-up.
Study Registration: https://clinicaltrials.gov; Unique identifier: NCT01363856.
Introduction
The ankle-brachial index (ABI) is a reliable, non-invasive, and inexpensive screening tool for peripheral artery disease (PAD) (1, 2). Low ABI ≤ 0.9 is a sensitive marker for generalized atherosclerosis and independently associated with cognitive impairment and decline in the general population (3, 4).
However, observational studies investigating the impact of low ABI on cognition after stroke are limited, particularly regarding long-term cognitive outcome (5). Stroke occurrence in itself is associated with ensuing cognitive impairment and decline (6). Between 20 and 80% of stroke survivors suffer from cognitive deficits, whereby the severity and recurrence of stroke are related to subsequent cognitive decline (6, 7). Low ABI could be a useful marker to detect cognitive impairment as a stroke-related disability and monitor secondary prevention therapies. A cross-sectional study of 103 hospitalized patients with acute lacunar stroke found that low ABI is associated with cognitive impairment in the acute stroke phase using the Montreal Cognitive Assessment (MoCA; cutoff score at ≤23) (5). Nonetheless, data on low ABI and cognitive function over time after ischemic stroke are lacking.
Aim
We aimed to investigate whether low ABI ≤ 0.9 is associated with reduced cognitive function at baseline and subsequent decline over a three-year follow-up after first-ever ischemic stroke.
Methods
Study design and population
This study is part of the Prospective Cohort with Incident Stroke Berlin (PROSICS-B), an observational, hospital-based prospective cohort study, described in detail elsewhere (8). Between January 2010 and May 2013 stroke patients were recruited at three stroke units of the Charité – Universitätsmedizin Berlin within 7 days after stroke onset (see flow chart in Supplementary Figure 1). Inclusion criteria were patients with (1) first-ever ischemic stroke, primary intracranial hemorrhage, or cerebral venous sinus thrombosis, (2) aged 18 and older, (3) written informed consent by patient or legal guardian prior to study participation. Exclusion criteria were (1) prior stroke (definition according to WHO criteria), (2) patients with brain tumor or brain metastasis, (3) participation in an interventional trial.
Patients were followed up annually with telephone-based interviews for up to 3 years. Patients who had suffered a prior stroke, presented brain tumors, brain metastases or participated in an intervention study were excluded. Only patients with mild to moderate ischemic stroke events, defined by a score of < 16 in the National Institutes of Health Stroke Scale (NIHSS), were included in the analysis. The assessment of ABI and MoCA were introduced to the study as an amendment in January 2011, ~1 year after patient enrollment had started according to the requirements of the ethical committee.
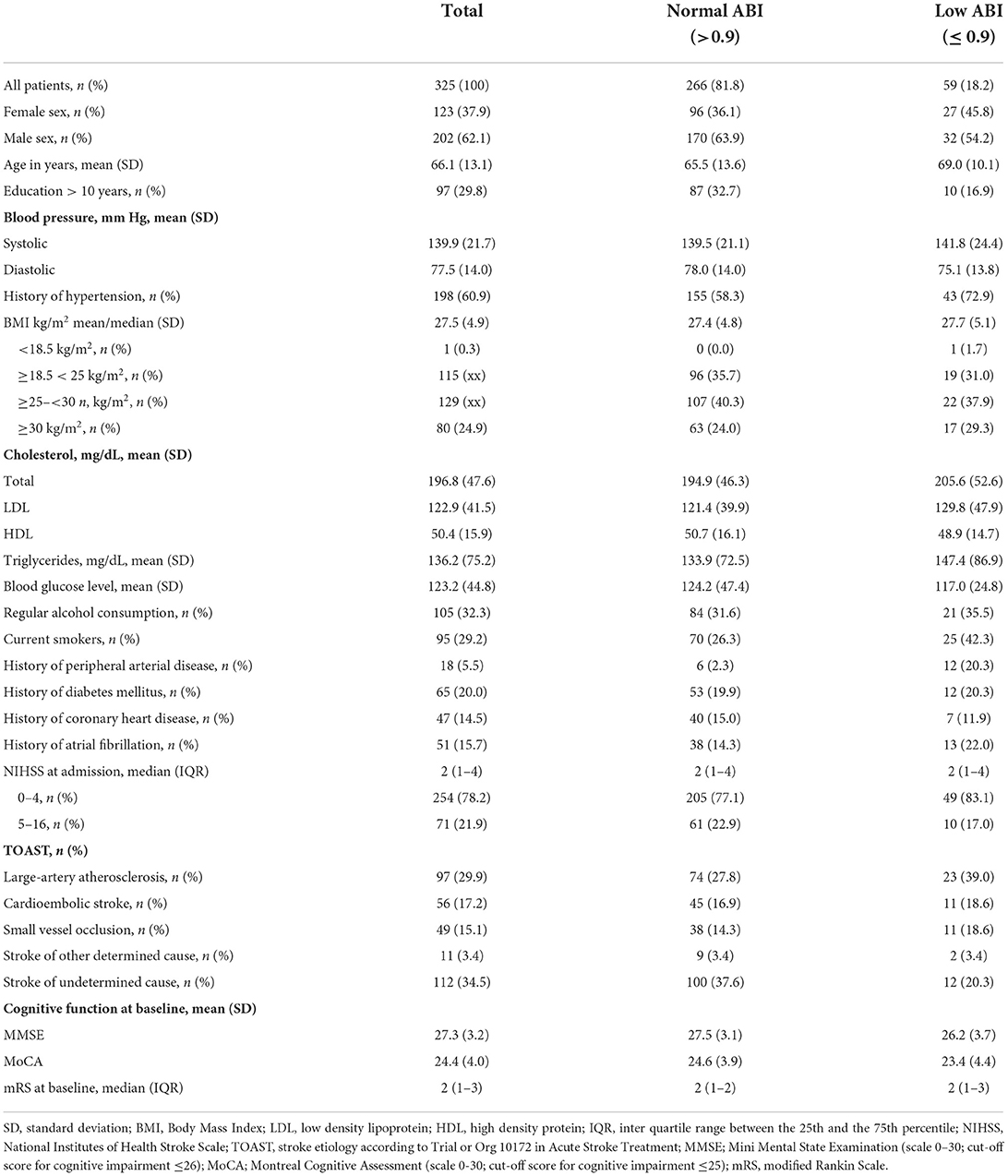
Patient characteristics
Baseline characteristics were assessed upon admission, including sociodemographic characteristics (e.g., age, sex and education, living situation and lifestyle habits), laboratory blood measures, stroke severity, and cardiovascular risk factors (BMI, current smoking and alcohol consumption, history of hypertension, diabetes mellitus and coronary heart disease).
ABI measurement
ABI was measured using a manual cuff and a portable Doppler ultrasound device. Participants were assessed in supine position following a 5-min resting period. The systolic blood pressure was determined for the brachial arteries, posterior tibial and dorsal pedis arteries bilaterally. Measurements were assessed at all three stroke centers following a pre-established standard operating procedure.
In accordance with the guidelines issued by the American Heart Association, ABI was calculated on each side by dividing the higher value of the posterior tibial or dorsal pedis artery systolic blood pressure by the higher systolic blood pressure of both brachial arteries (9). We used the lower ABI value. Low ABI was defined as ≤ 0.9. Participants with abnormally high ABI ≥ 1.4, indicative of non-compressible blood vessel calcification, were excluded (9).
Outcome definitions
Our outcome of interest was cognitive function after stroke at baseline and over a three-year follow-up period. Cognitive function at baseline was assessed using the Montreal Cognitive Assessment (MoCA) and the Mini Mental State Examination (MMSE). Cognitive impairment was defined as a MoCA score ≤ 25 or a MMSE score ≤ 26 (10–12). Cognitive function over time was measured with the Modified Telephone Interview for Cognitive Status (TICS-m), a screening instrument for cognitive dysfunction with 20 items and a maximum score of 50, which was administered annually via telephone interview over a three-year follow-up (13).
Statistical methods
Generalized linear models (GLM) were used to calculate crude and confounder adjusted relative risks (RR) for cognitive impairment at baseline (MoCA ≤ 25; MMSE ≤ 26) in patients with low versus normal ABI and corresponding 95% confidence intervals (CI). We performed linear mixed models (LMM) to calculate effect sizes (ß) and corresponding 95% CI for the crude and confounder adjusted association between low vs. normal ABI and cognitive function over a three-year follow-up, using the annual TICS-m score.
To explore whether the severity of low ABI had an effect on cognitive function in the sense of a dose-response relationship, we further categorized low ABI into mildly low (0.75–0.9) and moderately to severely low ABI (< 0.75) and recalculated our main analyses with these subgroups compared to normal ABI.
Multiple models were used to control for possible sources of confounding. Potential confounding factors were selected a priori according to their presumable impact on both the exposure (ABI) and the outcome (cognitive function). Model 1 adjusted for the sociodemographic variables age (continuous), sex and years of education received (≤ 10 years of schooling, > 10 years of schooling). Model 2 additionally adjusted for the cardiovascular risk factors BMI (kg/m2), current smoking (yes/no), history of diabetes mellitus (yes/no) and hypercholesterolemia (yes/no), and history of coronary heart disease (yes/no) and atrial fibrillation (yes/no). Model 3 moreover adjusted for stroke severity as defined by the NIHSS (in 2 categories: 0–4 and 5–15).
We conducted different sensitivity analyses, described in Methods I of the Supplementary material.
Data were prepared using IBM Statistics for Windows, version 25 (IBM Corp, Armonk, NY). All descriptive and statistical analyses were calculated using Stata version 14.1 (Stata Corp, College Station, TX). Data visualizations were performed in the R project (R 4.0.0) using the ggplot2 package.
Ethics approval
Patients or their legal guardians gave written informed consent prior to study participation. The study was approved by the Charité – Universitätsmedizin Berlin ethics committee (EA1/218/09) and was conducted according to the declaration of Helsinki.
Results
Study population
This analysis included 325 patients with mild to moderate ischemic stroke (NIHSS score < 16) and data on ABI (for detailed information on patient inclusion and exclusion see the flow chart in Supplemental Figure 1).
Mean age at study enrollment was 66 years (SD, 13), 38% (n = 123) were female. Median NIHSS was 2 [inter quartile range (IQR), 1–4], 30% (n = 95) were smokers upon study admission and 19% (n = 58) had a history of hypercholesterolemia. 31% (n = 97) had received a school education for more than 10 years. 18% (n = 59) had ABI ≤ 0.9. Of those, 59% (n = 35) had mildly low ABI (0.75 – 0.9) and 41% (n = 24) had moderately to severely low ABI (< 0.75). Table 1 gives a detailed overview of patient baseline characteristics.
Cognitive function at baseline
Patients with low ABI had a higher risk of baseline cognitive impairment than patients with normal ABI (MoCA adjusted RR in Model 3: 1.98; 95%-CI: 1.24 to 3.16). The risk for cognitive impairment was most pronounced in patients with moderately to severely low ABI (< 0.75) (adjusted RR for Model 3 for MoCA: 2.60; 95%-CI: 1.46 to 4.64). However, it must be noted that the MoCA was only assessed in 75% (n = 243) of the patients with ABI measurement. Estimates for cognitive function at baseline assessed with MoCA and MMSE are shown in the Supplementary Table 1.
Cognitive function over time
Over all follow-up time-points, patients with low ABI had lower TICS-m scores than patients with normal ABI (adjusted ß for Model 3: −1.96; 95%-CI: −3.55 to −0.37). Cognitive function was most impaired in patients with moderately to severely low ABI (adjusted ß for Model 3: −2.26; 95%-CI: −5.24 to 0.72). Cognitive function over time is illustrated in Figure 1. Cognitive function did not decline over time (adjusted ß for Model 3: 0.29; 95%-CI: −0.06 to 0.64). Results of crude and adjusted linear mixed models are shown in Table 2. Results of a sensitivity analysis stratifying low ABI can be found in Supplementary Table 2. Sensitivity analyses excluding patients who experienced a recurrent stroke or died during the three-year follow up (n = 44) yielded similar results (adjusted ß for Model 3: −1.90; 95%-CI: −3.57 to −0.24), as did using multiple imputation by chained events (MICE) (adjusted ß for Model 3: −1.28; 95%-CI: −2.55 to −0.01). These results are depicted in Supplementary Tables 3, 4.
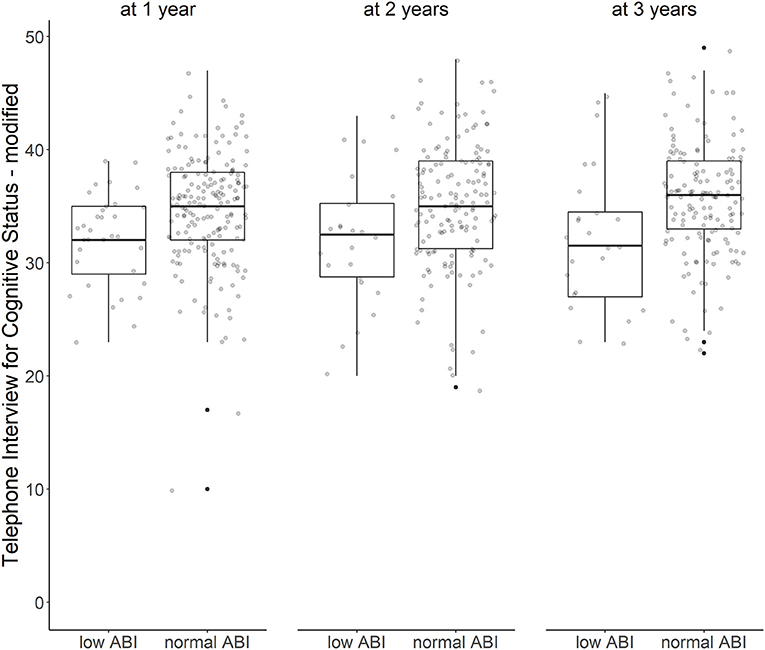
Figure 1. ABI and cognitive function over time. Cognitive function after first ischemic stroke with low vs. normal ankle-brachial index at 1, 2, and 3 years after stroke (Tukey's boxplots with jitter/scatterplot).
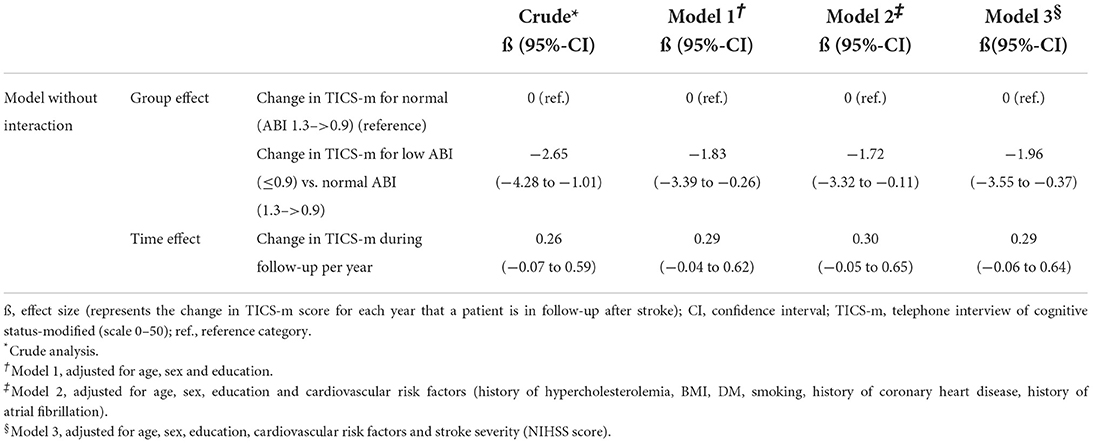
Table 2. Association of normal (>0.9) vs. low (≤ 0.9) ABI with cognitive function based on TICS-m over a 3-year follow-up.
Discussion
The main finding of this study is that low ABI (≤ 0.9), measured after an index stroke, is associated with reduced cognitive function, both at baseline and over a 3-year follow-up compared to normal ABI. This effect is independent of age, sex, educational level and several cardiovascular risk factors and most pronounced when comparing participants with moderately to severely low ABI (< 0.75) to those with normal ABI.
Our findings expand on recent observations that low ABI is linked to cognitive impairment immediately post-stroke by demonstrating detrimental effects on cognitive function also in the long-term (5). Our results support atherosclerosis as an underlying pathomechanism for cognitive disfunction and as a contributing factor in the development of dementia (e.g., Alzheimer's disease) (14). These findings expand the use of ABI as a sensitive screening mechanism, controlling for the progression of atherosclerotic blood vessel calcification, to a biomarker for cognitive dysfunction after stroke. Research has shown that statin use helps reduce the risk of post-stroke cognitive impairment, underlining the impact of atherosclerosis as a modifiable risk factor on unfavorable stroke outcomes (15). While low ABI as a surrogate marker can be improved through protective interventions, further research is needed to examine whether modifying ABI also modifies the risk for future cognitive impairment (16).
Strengths and limitations
Strengths of the study include its prospective design with a homogenous cohort, long follow-up period of 3 years with annual screenings and the assessment of cognitive function both at baseline and during follow-up. However, several limitations need to be addressed. First, we only included patients with mild to moderate ischemic stroke. This impedes the generalizability of our results to severe stroke patients and patients with other than ischemic stroke subtypes. However, patients who suffered severe stroke events are more likely to have low ABI at baseline and suffer from more severe cognitive impairment over time (7, 17). Secondly, predominantly male patients (62.3%) and a relatively high proportion of “strokes of undetermined cause” (35%) were included into the study. This should be taken into account when interpreting the data. Furthermore, we only assessed ABI at baseline, not during annual follow-ups. Further studies are needed to examine whether ABI is a modifiable risk factor for cognitive impairment. Thirdly, while our results showed that cognitive function was reduced both at baseline and over time in participants with low vs. normal ABI, cognitive performance did not significantly decline over time in either group. This may indicate that post-stroke cognition over time is predicted by baseline cognitive function following stroke occurrence (18). Then again, participants had only suffered mild-to-moderate ischemic strokes and may thus have remained mostly cognitively unimpaired. Moreover, the serial application of the TICS-m might have led to practice effects, masking cognitive decline (19). In addition, the cognitive tests we administered assessed global cognition, so no conclusions can be drawn as to whether the observed associations are more prominent in certain cognitive domains.
Conclusions
In conclusion, our 3-year prospective cohort study including mild-to-moderate first-ever ischemic stroke patients showed that low ABI at stroke occurrence was associated with reduced cognitive function both at baseline and over a 3-year follow-up period. The associations were strongest in participants with moderately and severely low ABI.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee Charité. The patients/participants provided their written informed consent to participate in this study.
Author contributions
MS, BS, and TL: study conception and design. MS, PS, LB, SH, and TL: data collection. MS, SP, BS, PS, and TL: analysis and interpretation of results. MS, PS, LB, SH, SP, PH, ME, and TL: draft manuscript preparation. All authors reviewed the results and approved the final version of the manuscript.
Funding
The PROSCIS-B study received funding from the Federal Ministry of Education and Research via the Grant Center for Stroke Research Berlin (01 EO 0801) up until May 2018.
Acknowledgments
The authors would like to thank J. Thümmler for her part in the collection and cleaning of data for PROSCIS-B.
Conflict of interest
PH received research grants from the German Ministry of Research and Education, German Research Foundation, European Union, Charité, Berlin Chamber of Physicians, German Parkinson Society, University Hospital Würzburg, Robert-Koch-Institute, German Heart Foundation, Federal Joint Committee (G-BA) within the Innovationfond, Charité–Universitätsmedizin Berlin (within MonDAFIS; supported by an unrestricted research grant to the Charité from Bayer), University Göttingen (within FIND-AF-randomized; supported by an unrestricted research grant to the University Göttingen from Boehringer-Ingelheim), and University Hospital Heidelberg (within RASUNOA-prime; supported by an unrestricted research grant to the University Hospital Heidelberg from Bayer, BMS, Boehringer-Ingelheim, Daiichi Sankyo), outside submitted work. ME received grant support from Bayer, the German Research Foundation (DFG), the German Federal Ministry of Education and Research (BMBF), the German Center for Neurodegenerative Diseases (DZNE), the German Centre for Cardiovascular Research (DZHK), the European Union, Corona Foundation, and Fondation Leducq; fees paid to the Charité from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Pfizer, Daiichi Sankyo, Amgen, GlaxoSmithKline, Sanofi, Covidien, Novartis, all outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.963262/full#supplementary-material
References
1. Xu D, Li J, Zou L, Xu Y, Hu D, Pagoto SL, et al. Sensitivity and specificity of the ankle-brachial index to diagnose peripheral artery disease: a structured review. Vasc Med. (2010) 15:361–9. doi: 10.1177/1358863X10378376
2. Fowkes FGR. The measurement of atherosclerotic peripheral arterial disease in epidemiological surveys. Int J Epidemiol. (1988) 17:248–54. doi: 10.1093/ije/17.2.248
3. Newman AB, Siscovick DS, Manolio TA, Polak J, Fried LP, Borhani NO, et al. Ankle-arm index as a marker of atherosclerosis in the cardiovascular health study. Circulation. (1993) 88:837–45. doi: 10.1161/01.CIR.88.3.837
4. Guerchet M, Aboyans V, Nubukpo P, Lacroix P, Clément JP, Preux PM. Ankle-brachial index as a marker of cognitive impairment and dementia in general population. A systematic review. Atherosclerosis. (2011) 216:251–7. doi: 10.1016/j.atherosclerosis.2011.03.024
5. Huang J, Tang J, Zhang Y, Zhang J, Tan Z, Shi S. Association between ankle brachial index, brachial-ankle pulse wave velocity, and mild cognitive impairment in patients with acute lacunar infarction. Eur Neurol. (2020) 83:147–53. doi: 10.1159/000504844
6. Sun JH, Tan L, Yu JT. Post-stroke cognitive impairment: epidemiology, mechanisms and management. Ann Transl Med. (2014) 2:80. doi: 10.3978/j.issn.2305-5839.2014.08.05
7. Schneider JA, Wilson RS, Bienias JL, Evans DA, Bennett DA. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology. (2004) 62:1148–55. doi: 10.1212/01.WNL.0000118211.78503.F5
8. Liman TG, Zietemann V, Wiedmann S, Jungehuelsing GJ, Endres M, Wollenweber FA, et al. Prediction of vascular risk after stroke - protocol and pilot data of the prospective Cohort with incident stroke (PROSCIS). Int J Stroke. (2013) 8:484–90. doi: 10.1111/j.1747-4949.2012.00871.x
9. Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, et al. Measurement and interpretation of the Ankle-Brachial Index: a scientific statement from the American Heart Association. Circulation. (2012) 126:2890–909. doi: 10.1161/CIR.0b013e318276fbcb
10. Tombaugh TN, Mclntyre NJ. The mini-mental state examination : a comprehensive review. J Am Geriatr Soc. (1992) 40:922–35. doi: 10.1111/j.1532-5415.1992.tb01992.x
11. Godefroy O, Fickl A, Roussel M, Auribault C, Bugnicourt JM, Lamy C, et al. Is the montreal cognitive assessment superior to the mini-mental state examination to detect poststroke cognitive impairment? A study with neuropsychological evaluation. Stroke. (2011) 42:1712–6. doi: 10.1161/STROKEAHA.110.606277
12. Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
13. Knopman DS, Roberts RO, Geda YE, Pankratz VS, Christianson TJH, Petersen RC, et al. Validation of the telephone interview for cognitive status-modified in subjects with normal cognition, mild cognitive impairment, or dementia. Neuroepidemiology. (2010) 34:34–42. doi: 10.1159/000255464
14. Hofman A, Ott A, Breteler MMB, Bots ML, Slooter AJC, Van Harskamp F, et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer's disease in the Rotterdam Study. Lancet. (1997) 349:151–4. doi: 10.1016/S0140-6736(96)09328-2
15. Douiri A, Mckevitt C, Emmett ES, Rudd AG, Wolfe CDA. Long-term effects of secondary prevention on cognitive function in stroke patients. Circulation. (2013) 128:1341–8. doi: 10.1161/CIRCULATIONAHA.113.002236
16. Gibbs BB, Dobrosielski DA, Althouse AD, Stewart KJ. The effect of exercise training on ankle-brachial index in type 2 diabetes. Atherosclerosis. (2013) 230:125–30. doi: 10.1016/j.atherosclerosis.2013.07.002
17. Ratanakorn D, Keandoungchun J, Tegeler CH. Prevalence and association between risk factors, stroke subtypes, and abnormal ankle brachial index in acute ischemic stroke. J Stroke Cerebrovasc Dis. (2012) 21:498–503. doi: 10.1016/j.jstrokecerebrovasdis.2010.11.011
18. Zietemann V, Georgakis MK, Dondaine T, Muller C, Mendyk AM, Kopczak A, et al. Early moca predicts long-term cognitive and functional outcome and mortality after stroke. Neurology. (2018) 91:E1838–50. doi: 10.1212/WNL.0000000000006506
Keywords: ankle-brachial index, ischemic stroke, outcome, post stroke cognitive function, atherosclerosis
Citation: Stillfried MRV, Sperber PS, Broersen LHA, Huo S, Piper SK, Heuschmann PU, Endres M, Siegerink B and Liman TG (2022) Low ankle-brachial index and cognitive function after stroke—the PROSpective with Incident Stroke Berlin (PROSCIS-B). Front. Neurol. 13:963262. doi: 10.3389/fneur.2022.963262
Received: 07 June 2022; Accepted: 31 August 2022;
Published: 28 September 2022.
Edited by:
Ana Catarina Fonseca, University of Lisbon, PortugalReviewed by:
Firoz Akhter, Stony Brook University, United StatesMarialuisa Zedde, IRCCS Local Health Authority of Reggio Emilia, Italy
Copyright © 2022 Stillfried, Sperber, Broersen, Huo, Piper, Heuschmann, Endres, Siegerink and Liman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thomas G. Liman, dGhvbWFzLmxpbWFuQGNoYXJpdGUuZGU=
 Maria R. V. Stillfried
Maria R. V. Stillfried Pia S. Sperber1,2
Pia S. Sperber1,2 Shufan Huo
Shufan Huo Sophie K. Piper
Sophie K. Piper Matthias Endres
Matthias Endres Bob Siegerink
Bob Siegerink Thomas G. Liman
Thomas G. Liman