
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Neurol., 14 December 2022
Sec. Endovascular and Interventional Neurology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.962987
This article is part of the Research TopicMechanical Thrombectomy and Development of Thrombectomy DevicesView all 10 articles
 Yongxin Zhang1†
Yongxin Zhang1† Pei Liu1†
Pei Liu1† Zifu Li1
Zifu Li1 Ya Peng2
Ya Peng2 Wenhuo Chen3
Wenhuo Chen3 Liyong Zhang4
Liyong Zhang4 Jianfeng Chu5
Jianfeng Chu5 Dong Kuai6
Dong Kuai6 Zhen Chen7
Zhen Chen7 Wei Wu8
Wei Wu8 Yun Xu9
Yun Xu9 Yong Zhang10
Yong Zhang10 Bin Zhou11
Bin Zhou11 Yu Geng12
Yu Geng12 Congguo Yin13
Congguo Yin13 Jiang Li14
Jiang Li14 Ming Wang15
Ming Wang15 Naichi Zhai16
Naichi Zhai16 Xiaoxiang Peng17
Xiaoxiang Peng17 Zhong Ji18
Zhong Ji18 Yaping Xiao19
Yaping Xiao19 Xingen Zhu20
Xingen Zhu20 Xueli Cai21
Xueli Cai21 Lei Zhang1
Lei Zhang1 Bo Hong22
Bo Hong22 Pengfei Xing1
Pengfei Xing1 Hongjian Shen1
Hongjian Shen1 Yongwei Zhang1
Yongwei Zhang1 Minghua Li23
Minghua Li23 Meixia Shang24
Meixia Shang24 Jianmin Liu1*
Jianmin Liu1* Pengfei Yang1*
Pengfei Yang1*Objective: The Neurohawk retriever is a new fully radiopaque retriever. A randomized controlled non-inferiority trial was conducted to compare the Neurohawk and the Solitaire FR in terms of safety and efficacy. In order to evaluate the efficacy and safety of endovascular treatment in acute ischemic stroke (AIS) caused by intracranial atherosclerotic disease (ICAD) larger vessel occlusion (LVO), a sub-analysis was performed.
Methods: Acute ischemic stroke patients aged 18–80 years with LVO in the anterior circulation were randomly assigned to undergo thrombectomy with either the Neurohawk or the Solitaire FR. The primary efficacy endpoint was successful reperfusion (mTICI 2b-3) rate by the allocated retriever. A relevant non-inferiority margin was 12.5%. Safety outcomes were symptomatic intracranial hemorrhage (sICH) and all-cause mortality within 90 days. Secondary endpoints included first-pass effect (FPE), modified FPE, and favorable outcomes at 90 days. In subgroup analysis, the patients were divided into the ICAD group and non-ICAD group according to etiology, and baseline characteristics, angiographic, and clinical outcomes were compared.
Results: A total of 232 patients were involved in this analysis (115 patients in the Neurohawk group and 117 in the Solitaire group). The rates of successful reperfusion with the allocated retriever were 88.70% in the Neurohawk group and 90.60% in the Solitaire group (95%CI of the difference, −9.74% to 5.94%; p = 0.867). There were similar results in FPE and mFPE in both groups. The rate of sICH seemed higher in the Solitaire group (13.16% vs. 7.02%, p = 0.124). All-cause mortality and favorable outcome rates were comparable as well. In subgroup analysis, 58 patients were assigned to the ICAD group and the remaining 174 to the non-ICAD group. The final successful reperfusion and favorable outcome rates showed no statistically significant differences in two groups. Mortality within 90 days was relatively lower in the ICAD group (6.90% vs. 17.24%; p = 0.054).
Conclusion: The Neurohawk retriever is non-inferior to the Solitaire FR in the mechanical thrombectomy of large vessel occlusion-acute ischemic stroke (LVO-AIS). The sub-analysis suggested that endovascular treatment including thrombectomy with the retriever and essential rescue angioplasty is effective and safe in AIS patients with intracranial atherosclerotic disease-larger vessel occlusion (ICAD-LVO).
Clinical trial registration: https://clinicaltrials.gov/ct2/show/NCT04995757, number: NCT04995757.
Mechanical thrombectomy has become the standard of care for large vessel occlusion-acute ischemic stroke (LVO-AIS) since the five landmark trials were published (1–6). The high reperfusion rates and short procedure shown in these trials were driven by the use of stent-like retrievers. The Solitaire FR (Medtronic Inc., California, USA) has been one of the most frequently used retrievers (7, 8). However, various novel thrombectomy devices with improved visibility have been developed and tested. Although the physical properties of different novel thrombectomy devices have been investigated in vitro, the efficacy and safety of any device should also be demonstrated in clinical trials.
The Neurohawk (MicroPort NeuroTech Company, Shanghai, China) is a stent-like retriever with the closed-cell design. There are three radiopaque marks at the distal end of the retriever. In addition, three radiopaque wires twined around the entire struts of the retriever make it a fully radiopaque device, which allow the physician to visualize the overall placement of the retriever and the combination with thrombus. The useable section of the Neurohawk retriever is composed of large cells and small cells. The large cells is nearly twice as large as the small cells, which is dedicated to catching hard and large-sized thrombus, while the small cells is more conducive to embedding soft and small thrombus (Figure 1). This multi-center randomized controlled trial was designed and carried out to assess the efficacy and safety of this new device comparatively with the Solitaire FR.
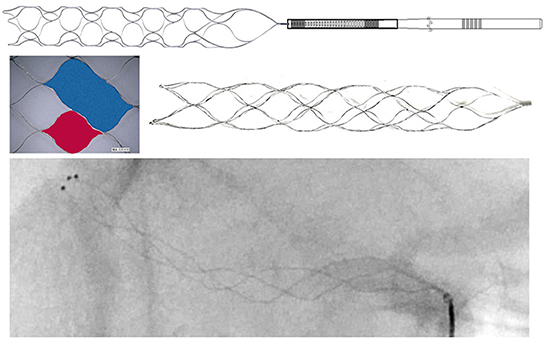
Figure 1. There are three radiopaque marks at the distal end of the Neurohawk retriever. And three radiopaque wires twined around the entire struts. The useable section of the Neurohawk retriever is composed of large cells (blue area) and small cells (red area).
As the etiologies of large vessel occlusion were different among ethnic groups, intracranial atherosclerotic disease-larger vessel occlusion (ICAD-LVO) account for a large proportion of patients with acute ischemic stroke (AIS) among Asians. It remains uncertain whether patients with acute ICAD-related occlusion can benefit from mechanical thrombectomy as those with embolism do. Prospective comparative data focusing on efficacy and safety of mechanical thrombectomy between patients with ICAD and those with non-ICAD are scarce. Therefore, a subgroup comparative analysis was conducted.
This study was a prospective, multicenter, single-blind, randomized, controlled, non-inferiority clinical trial comparing the safety and effectiveness of patients with LVO-AIS treated with either the Neurohawk or the Solitaire FR. The clinical trial followed the principles of law and science, and was approved by the ethics committee at each participating site. This trial planned to enroll 238 patients in 21 tertiary care centers, which were each required to have performed at least 30 endovascular thrombectomy procedures during the previous year.
All patients or their legally authorized representatives provided written informed consent before enrollment. Inclusion criteria were: (1) 18–80 years of age; (2) AIS secondary to internal carotid artery (ICA) or middle cerebral artery (MCA) (M1 or M2) occlusion; (3) ability to undergo puncture within 6 h of symptom onset. Key exclusion criteria were: (1) a pre-stroke modified Rankin Scale (mRS) score (9) ≥2; (2) a baseline National Institutes of Health Stroke Scale (NIHSS) score <2 or >25; (3) massive cerebral infarction defined as an Alberta Stroke Program Early CT Score (ASPECTS) (10) <6 or >1/3 of blood supplying areas on CT/diffusion weighted imaging; (4) concomitant use of oral anticoagulation drugs and INR >3.0, or a platelet count <30 * 109 /L (11).
According to guidelines, intravenous thrombolysis was administered before mechanical thrombectomy in all eligible patients (12). Depending on the patient's condition, procedures were performed with the patient under local anesthesia, conscious sedation or general anesthesia. The choice of the thrombectomy device was made according to random allocation, which was performed utilizing a 1:1 ratio. Randomization was accomplished by employing a Web-based system with stratification according to each participating site, occlusion segment of artery and the NIHSS. Treatment-group assignment was known to the operating physicians but blinded to the patients. The instructions of the Neurohawk were very similar to those of the Solitaire FR. Due to the presence of the fully radiopaque, the push-and-fluff technique (13), which may lead to better device opening with optimized wall apposition, could be used in the Neurohawk group. Stent retriever combined with aspiration catheter was allowed in both arms. Other retrievers, such as the Trevo (Stryker, Kalamazoo, MI, USA) or other techniques were allowed as salvage measures after unsuccessful recanalization with the Neurohawk or the Solitaire FR. The etiology of occlusion was assessed based on the medical history, risk factors, and angiographic characteristics. For patients with an underlying intracranial stenosis, repeated angiography was performed to exclude potential vasospasm or dissection after the first recanalization. Once atherosclerosis related occlusion was identified, salvage measures, including administration of tirofiban (glycoprotein IIb/IIIa inhibitor) or balloon (Gateway, Boston Scientific, Natick, MA, USA) angioplasty and/or placement of a permanent stent (Enterprise, Johnson and Johnson, Raynham, MA, USA; Wingspan, Stryker, Kalamazoo, MI, United States) or an Apollo balloon mounted stent (MicroPort, Shanghai, China), were allowed (14). Daily oral dual antiplatelet therapy with 100 mg of aspirin and 75 mg of clopidogrel was started post-procedure and continued for 3 months, followed by life-long 100 mg aspirin.
The primary efficacy endpoint was successful reperfusion rate by the allocated retriever, defined as the percentage of patients achieving modified thrombolysis in cerebral infarction (mTICI) 2b or 3 (15). Secondary endpoints included first-pass effect (FPE, defined as achieving mTICI 3 with a single pass), modified FPE (defined as achieving mTICI 3/2b with a single pass) (16–18), the time from groin puncture to reperfusion, NIHSS at 30 ± 6 h, and favorable clinical outcome (defined as a mRS of 0–2 at 90 ± 14 days). The mRS at 90 days was determined by outpatient follow-up or telephone interview conducted by independent physicians unaware of treatment-group assignment in each center. Safety endpoint measurements were the rate of symptomatic intracranial hemorrhage (sICH) within 30 ± 6 h after intervention, all-cause mortality within 90 days, and all-cause adverse events within 90 days. The sICH was defined as any ICH identified by CT scan combined with a four-point increase in NIHSS or death. Images of the procedure were read by two independent neuroradiologists from the core lab, with consensus required in case of discrepancy. In subgroup analysis, the patients were divided into the ICAD and non-ICAD groups according to the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification (19). Baseline characteristics, treatments, and angiographic and clinical outcomes were also compared between the two groups.
The primary study hypothesis was that the successful reperfusion rate of the Neurohawk would be non-inferior to that of the Solitaire FR, with a relevant non-inferiority margin of 12.5%. The non-inferiority margin was calculated by using a two-step method based on the clinical practical significance according to the “Guidelines for the Design of Clinical Trials of Medical Devices” by National Medical Products Administration. All statistical analyses followed the intention-to-treat principle. Baseline data are presented as descriptive statistics according to treatment assignment. The non-inferiority test was based on the asymptotic Z-test. Two-sided 95% confidence intervals (CIs) of the differences in the rate of successful reperfusion between the groups were estimated by the Cochran–Mantel–Haenszel chi-squared test with adjusting centers. Statistical tests for continuous variables used Student's t-test or the Wilcoxon Mann-Whitney rank-sum test, and categorical variables were tested using the chi-squared test or Fisher's exact test. All statistical tests were two-tailed with a significance level of 0.05, using the SAS software, version 9.4 (SAS Institute, Cary, North Carolina).
A total of 239 patients were enrolled in this trial. A study flow diagram and protocol deviation details are shown in Figure 2. One patient had thrombus dissolution before the retriever reached the target vessel; two patients had chronic occlusion, and one showed aggravating heart failure after general anesthesia. One patient had ICA dissection and two cases had multiple cerebral artery occlusions, which did not align with the inclusion criteria. A total of 115 patients were treated with the Neurohawk, while 117 underwent thrombectomy with the Solitaire FR. Digital subtraction angiographic (DSA) imaging data were missing for one patient in each group. Five patients (two in the Neurohawk group and three in the Solitaire group) were lost to follow-up for mRS assessment at 90 days.
The patient baseline characteristics were similar in both treatment groups, and are detailed in Table 1. The median patient age was 66.6 years. A total of 95 patients had a history of atrial fibrillation and 149 patients had hypertension. Median NIHSS scores were 16 in the Neurohawk group and 17 in the Solitaire group. The median ASPECTS was 8. The most common target vessels were the MCA M1 (117 cases), the ICA (87 cases), and the MCA M2 (26 cases).
Procedural results and outcomes are shown in Table 2. In 92 patients, bridging intravenous fibrinolysis was administrated. General anesthesia was performed in 40.87% of the Neurohawk group and 33.33% of the Solitaire group. There were no differences in bridging therapy and anesthesia method between the two groups. In all 115 patients of the Neurohawk group, the Neurohawk was used as the single retriever with no rescuing needed using other retrievers. The use of a retriever combined with aspiration was similar between the two groups. However, more balloon guide catheters were used in the Solitaire group (p = 0.038).
The rates of successful reperfusion with the assigned retriever were 88.70% in the Neurohawk group and 90.60% in the Solitaire group (95%CI of difference, −9.74% to 5.94%; p = 0.867). And the median times of pass were two in both group. First-pass complete reperfusion (mTICI 3) was achieved in 61/232 (26.96% vs. 25.64%) and first-pass successful reperfusion (mTICI 3/2b) was achieved in 93/232 (42.61% vs. 37.61%). There were similar FPE and mFPE rates in both groups. Angioplasty with balloon and/or stent use was performed as a remedial measure to maintain a stable flow in 27 patients of the Neurohawk group and 24 of the Solitaire group (p = 0.586). Median time from groin puncture to successful reperfusion was similar in both treatment groups (p = 0.772). Finally, similar proportions of patients in the Neurohawk and Solitaire groups achieved successful reperfusion (90.43% vs. 91.45%, respectively).
Regarding the major safety outcomes, the rate of sICH within 36 h seemed higher in the Solitaire group (13.16% vs. 7.02%, respectively), but the difference was not statistically significant (p = 0.124). All-cause mortality rates within 90 days were 12.17% in the Neurohawk group and 17.09% in the Solitaire group, with no significant difference (p = 0.289). There were no significant differences between the two groups in terms of NIHSS at 30 h and favorable outcome (mRS 0–2) at 90 days.
According to the etiology of occlusion, 58 patients were assigned to the ICAD group and the remaining 174 patients to the non-ICAD group. In subgroup analysis, baseline, clinical, and angiographic data between these two groups were compared, and are summarized in Table 3. Compared with the non-ICAD group, individuals with ICAD-LVO had younger age, lower initial NIHSS, and longer time from onset to puncture. Besides, MCA M1 occlusion was more frequent among the patients with ICAD. In terms of treatment method, the proportions of bridging intravenous fibrinolysis were similar in both groups. However, the use of a balloon guide catheter was more preferred in the non-ICAD group (1.72% vs. 17.24%, p = 0.003). To maintain a stable flow, 79.31% of patients with ICAD-LVO needed rescue measures. Time from groin puncture to successful reperfusion was longer in the ICAD-LVO group (p = 0.004). However, there was no statistically significant difference in successful reperfusion rate between the two groups. In the ICAD group, the successful reperfusion rates were 96.97% in Neurohawk group and 92.0% in Solitaire group (p = 0.804). The ICAD group seemed to have a lower frequency of sICH, but without statistical significance. Although mortality within 90 days was relatively lower in the ICAD group (6.90% vs. 17.24%; p = 0.054), the rates of favorable outcome at 90 days were comparable between the two groups.
This randomized controlled trial demonstrated that the Neurohawk achieved comparable rates of successful reperfusion and PFE vs. the Solitaire FR for the treatment of patients with LVO-AIS. From a safety perspective, the rates of sICH were similar. These angiographic results translated to comparable proportions of patients with good clinical outcomes, with close to 60% of patients regaining functional independence at 90 days in each group. In subgroup analysis, almost 80% of individuals with ICAD-LVO needed rescue measures to maintain a favorable reperfusion, including balloon and/or stent angioplasty, which would prolong the time of the procedure. Even so, patients with ICAD-LVO AIS had similar successful reperfusion rate and favorable outcome rate at 90 days vs. those with non-ICAD-LVO AIS. In addition, patients with ICAD-LVO AIS had a relatively lower mortality rate within 90 days.
The Neurohawk retriever is a closed-cell designed retriever with full radiopaque visibility, which can be delivered through a 0.021-inch microcatheter. The available working lengths of the Neurohawk are 25 mm with diameter of 4 mm, and 30 mm with diameter of 6 mm. For a retriever with only several radiopaque marks, physicians could see the ends of the device but not the retrieval area, indicating that they often had to make assumptions regarding the positional relationship between the clot and opened cells. However, the radiopaque Neurohawk retriever allows the physician to visualize the placement of struts at the location of the clot. In addition, it allows a particular deployment maneuver, the push and fluff technique, which may lead to better device opening and optimized wall apposition (20, 21). After partially unsheathing the retriever by retracting the delivery microcatheter, the forward force is applied to the device delivery wire and the forward tension continues to push the device into maximal expansion. Therefore, the larger stent cell area may allow for incorporation of higher volumes of clot.
This study is one of the few randomized controlled trials that focus on the efficacy and safety of a new device for thrombectomy. Although new devices for thrombectomy are constantly emerging, most of them are validated for clinical effects in single-arm studies. Usually, the data from a single group are compared with previous studies of other devices. However, direct comparison of prognoses between single-arm studies and previous registries could produce bias from inhomogeneous baselines and inconsistent operator experiences (22–27). In this study, the prospective randomization design generated a well-balanced baseline between the two groups. From the perspective of angiographic results and clinical outcomes, the Neurohawk was demonstrated to be non-inferior to the Solitaire FR in the treatment of LVO-AIS in the anterior circulation. Although the visibility of Neurohawk may improve the usage experience, the angiographic endpoint showed no difference. One reason might be the high rate of mTICI 2b-3 seen with the modern endovascular technology results in a ceiling effect, making the measurement insensitive. The subtle difference caused by minor improvements may require larger sample sizes and more sensitive measurements to confirm.
While embolism and extracranial atherosclerotic disease is the leading cause of AIS in Caucasian patients, ICAD-LVO cases are more prevalent among Asians (28, 29). In this study, one-fourth of cases resulted from ICAD-LVO. The characteristics of occlusions arising from ICAD and non-ICAD differed in terms of therapeutic responses. For instance, subsequent plaque irritation and platelet aggregation are persistent even after mechanical thrombectomy, which often leads to re-occlusion. Another concern for these patients is whether the use of antiplatelet drugs post-angioplasty would increase the risk of hemorrhagic complications. However, limited studies have so far assessed the efficacy and safety of mechanical thrombectomy in AIS due to ICD-LVO, and the only information available is based on single center, retrospective studies conducted on few samples (14, 30–32).
In this study, patients with ICAD-LVO had lower initial NIHSS, which might be attributed to ischemic preconditioning and better collateral compensation. This finding was in line with the EAST study in which admission NIHSS in the ICAD group was lower than that of the embolic group (33). Endovascular treatment of AIS underlying ICAD-LVO is technically more complex. About four-fifth of patients with ICAD-LVO received angioplasty, while only five patients in the non-ICAD group needed single stent implantation. The EAST study in China showed 63.8% (30/47) of patients were considered to be eligible for rescue treatment. In our previous meta-analysis of endovascular treatment of ICAD-LVO, the most common rescue therapy was stenting with or without balloon angioplasty (32.7%), followed by single balloon angioplasty (12.3%) (30). Besides, our data showed additional rescue therapy was indeed reflected by significantly longer procedure time, in concordance with the previous study. Finally, our findings corroborate studies that also demonstrated similar angiographic and clinical outcomes were obtained in the treatment of acute ICAD-LVO.
This study had several underlying limitations because of the restrictive nature of the randomized controlled non-inferiority trial design, including the limited sample size, and strict inclusion and exclusion criteria. In addition, the mRS was accessed by physicians in each center, which can lead to heterogenous judgments. The sub-analysis also had several limitations. Data for this sub-analysis were derived from the original trial, and therefore, selection bias was inevitable. There was heterogeneity in the intraoperative antiplatelet regimen among different centers.
This randomized clinical trial demonstrated that the Neurohawk retriever is non-inferior to the Solitaire FR in the mechanical thrombectomy of LVO-AIS. Meanwhile, the sub-analysis suggested that endovascular treatment including thrombectomy with the retriever and essential rescue angioplasty is effective and safe in patients with AIS underlying ICAD-LVO.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Ethics Committee at each participating site (the full list is available as a Supplementary file). The patients/participants provided their written informed consent to participate in this study.
PY and JLiu: conceptualization, responsible for theoretical guidance and conceptual specification, methodology, funding acquisition, project research fund, data collection and collation, and supervision. PY, JLiu, YongxZ, PL, MS, and ML: responsible for the guidance of research methods, formal analysis, and investigation. YongxZ and PL: analyze the current research status and put forward the research direction, writing—original draft preparation, and writing—review and editing. YongxZ, PL, ZL, YP, WC, LiZ, JC, DK, ZC, WW, YXu, YongZ, BZ, YG, CY, JLi, MW, NZ, XP, ZJ, YXi, XZ, XC, LeZ, BH, PX, HS, and YongwZ: resources. All authors read and approved the final manuscript.
This study was funded by MicroPort NeuroTech (Shanghai, China). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
We thank the patients and their families for participating in this trial, we thank MicroPort NeuroTech employees who contributes to the clinical operations support and the success of the trial. We also thank the investigators, their study coordinators and especially the subjects who participated in the clinical trial for their diligent support throughout.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.962987/full#supplementary-material
1. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. (2016) 387:1723–31. doi: 10.1016/s0140-6736(16)00163-x
2. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. (2015) 372:11–20. doi: 10.1056/NEJMoa1411587
3. Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. (2015) 372:2296–306. doi: 10.1056/NEJMoa1503780
4. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. (2015) 372:1019–30. doi: 10.1056/NEJMoa1414905
5. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. (2015) 372:1009–18. doi: 10.1056/NEJMoa1414792
6. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med. (2015) 372:2285–95. doi: 10.1056/NEJMoa1415061
7. Malisch TW, Zaidat OO, Castonguay AC, Marden FA, Gupta R, Sun CJ, et al. Clinical and angiographic outcomes with the combined local aspiration and retriever in the North American Solitaire Stent-Retriever Acute Stroke (NASA) Registry. Interv Neurol. (2018) 7:26–35. doi: 10.1159/000480353
8. Zaidat OO, Castonguay AC, Gupta R, Sun CH, Martin C, Holloway WE, et al. North American Solitaire Stent Retriever Acute Stroke registry: post-marketing revascularization and clinical outcome results. J Neurointerv Surg. (2014) 6:584–8. doi: 10.1136/neurintsurg-2013-010895
9. van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. (1988) 19:604–7. doi: 10.1161/01.str.19.5.604
10. Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. (2000) 355:1670–4. doi: 10.1016/s0140-6736(00)02237-6
11. Yang P, Zhang Y, Liu J. LAST(2) CH(2)ANCE: a summary of selection criteria for thrombectomy in acute ischemic stroke. AJNR Am J Neuroradiol. (2017) 38:E58–9. doi: 10.3174/ajnr.A5249
12. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 Guidelines for the Early Management of Patients with Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2018) 49:e46–110. doi: 10.1161/str.0000000000000158
13. Haussen DC, Rebello LC, Nogueira RG. Optimizating clot retrieval in acute stroke: the push and fluff technique for closed-cell stentrievers. Stroke. (2015) 46:2838–42. doi: 10.1161/strokeaha.115.010044
14. Yang W, Zhang Y, Li Z, Zhang L, Li H, Hua W, et al. Differences in safety and efficacy of endovascular treatment for acute ischemic stroke: a propensity score analysis of intracranial atherosclerosis-related occlusion versus embolism. Clin Neuroradiol. (2021) 31:457–64. doi: 10.1007/s00062-020-00899-x
15. Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke. (2013) 44:2650–63. doi: 10.1161/strokeaha.113.001972
16. Yoo AJ, Soomro J, Andersson T, Saver JL, Ribo M, Bozorgchami H, et al. Benchmarking the extent and speed of reperfusion: first pass TICI 2c-3 is a preferred endovascular reperfusion endpoint. Front Neurol. (2021) 12:669934. doi: 10.3389/fneur.2021.669934
17. Abbasi M, Liu Y, Fitzgerald S, Mereuta OM, Arturo Larco JL, Rizvi A, et al. Systematic review and meta-analysis of current rates of first pass effect by thrombectomy technique and associations with clinical outcomes. J Neurointerv Surg. (2021) 13:212–16. doi: 10.1136/neurintsurg-2020-016869
18. Zaidat OO, Castonguay AC, Linfante I, Gupta R, Martin CO, Holloway WE, et al. First pass effect: a new measure for stroke thrombectomy devices. Stroke. (2018) 49:660–6. doi: 10.1161/strokeaha.117.020315
19. Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke. (2001) 32:2735–40. doi: 10.1161/hs1201.100209
20. Ohshima T, Kawaguchi R, Nagano Y, Miyachi S, Matsuo N, Takayasu M. Experimental direct measurement of clot-capturing ability of stent retrievers. World Neurosurg. (2019) 121:e358–63. doi: 10.1016/j.wneu.2018.09.106
21. Tomasello A, Ribò M, Gramegna LL, Melendez F, Rosati S, Moreu M, et al. Procedural approaches and angiographic signs predicting first-pass recanalization in patients treated with mechanical thrombectomy for acute ischaemic stroke. Interv Neuroradiol. (2019) 25:491–96. doi: 10.1177/1591019919847623
22. Mattle HP, Scarrott C, Claffey M, Thornton J, Macho J, Riedel C, et al. Analysis of revascularisation in ischaemic stroke with EmboTrap (ARISE I study) and meta-analysis of thrombectomy. Interv Neuroradiol. (2019) 25:261–70. doi: 10.1177/1591019918817406
23. Brouwer PA, Yeo LLL, Holmberg A, Andersson T, Kolloch J, KuntzeSöderqvist Å, et al. Thrombectomy using the EmboTrap device: core laboratory-assessed results in 201 consecutive patients in a real-world setting. J Neurointerv Surg. (2018) 10:964–68. doi: 10.1136/neurintsurg-2018-013765
24. Sakai N, Ota S, Matsumoto Y, Kondo R, Satow T, Kubo M, et al. Efficacy and safety of REVIVE SE thrombectomy device for acute ischemic stroke: river JAPAN (reperfuse ischemic vessels with endovascular recanalization device in Japan). Neurol Med Chir (Tokyo). (2018) 58:164–72. doi: 10.2176/nmc.oa.2017-0145
25. Siemonsen S, Forkert ND, Bernhardt M, Thomalla G, Bendszus M, Fiehler J. ERIC acute stroke recanalization: a study using predictive analytics to assess a new device for mechanical thrombectomy. Int J Stroke. (2017) 12:659–66. doi: 10.1177/1747493017700661
26. Kallenberg K, Solymosi L, Taschner CA, Berkefeld J, Schlamann M, Jansen O, et al. Endovascular stroke therapy with the aperio thrombectomy device. J Neurointerv Surg. (2016) 8:834–9. doi: 10.1136/neurintsurg-2015-011678
27. Kahles T, Garcia-Esperon C, Zeller S, Hlavica M, Añon J, Diepers M, et al. Mechanical thrombectomy using the new ERIC retrieval device is feasible, efficient, and safe in acute ischemic stroke: a swiss stroke center experience. AJNR Am J Neuroradiol. (2016) 37:114–9. doi: 10.3174/ajnr.A4463
28. Kim BJ, Kim JS. Ischemic stroke subtype classification: an asian viewpoint. J Stroke. (2014) 16:8–17. doi: 10.5853/jos.2014.16.1.8
29. Qureshi AI, Caplan LR. Intracranial atherosclerosis. Lancet. (2014) 383:984–98. doi: 10.1016/s0140-6736(13)61088-0
30. Li H, Zhang Y, Zhang L, Li Z, Xing P, Zhang Y, et al. Endovascular treatment of acute ischemic stroke due to intracranial atherosclerotic large vessel occlusion: a systematic review. Clin Neuroradiol. (2020) 30:777–87. doi: 10.1007/s00062-019-00839-4
31. Yang D, Hao Y, Zi W, Wang H, Zheng D, Li H, et al. Effect of retrievable stent size on endovascular treatment of acute ischemic stroke: a multicenter study. AJNR Am J Neuroradiol. (2017) 38:1586–93. doi: 10.3174/ajnr.A5232
32. Sun B, Shi Z, Pu J, Yang S, Wang H, Yang D, et al. Effects of mechanical thrombectomy for acute stroke patients with etiology of large artery atherosclerosis. J Neurol Sci. (2019) 396:178–83. doi: 10.1016/j.jns.2018.10.017
Keywords: acute ischemic stroke, endovascular treatment, new fully radiopaque retriever, intracranial atherosclerotic disease, a randomized controlled trial
Citation: Zhang Y, Liu P, Li Z, Peng Y, Chen W, Zhang L, Chu J, Kuai D, Chen Z, Wu W, Xu Y, Zhang Y, Zhou B, Geng Y, Yin C, Li J, Wang M, Zhai N, Peng X, Ji Z, Xiao Y, Zhu X, Cai X, Zhang L, Hong B, Xing P, Shen H, Zhang Y, Li M, Shang M, Liu J and Yang P (2022) Endovascular treatment of acute ischemic stroke with a fully radiopaque retriever: A randomized controlled trial. Front. Neurol. 13:962987. doi: 10.3389/fneur.2022.962987
Received: 29 September 2022; Accepted: 26 October 2022;
Published: 14 December 2022.
Edited by:
Juyu Chueh, Tufts University, United StatesReviewed by:
Mostafa Meshref, Al-Azhar University, EgyptCopyright © 2022 Zhang, Liu, Li, Peng, Chen, Zhang, Chu, Kuai, Chen, Wu, Xu, Zhang, Zhou, Geng, Yin, Li, Wang, Zhai, Peng, Ji, Xiao, Zhu, Cai, Zhang, Hong, Xing, Shen, Zhang, Li, Shang, Liu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianmin Liu, Y2hzdHJva2VAMTYzLmNvbQ==; Pengfei Yang, Y2h5YW5ncGZAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.