
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 05 August 2022
Sec. Stroke
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.955725
 Beom Joon Kim1*
Beom Joon Kim1* Bijoy K. Menon2
Bijoy K. Menon2 Joonsang Yoo3
Joonsang Yoo3 Jung Hoon Han4
Jung Hoon Han4 Bum Joon Kim5
Bum Joon Kim5 Chi Kyung Kim4
Chi Kyung Kim4 Jae Guk Kim6
Jae Guk Kim6 Joon-Tae Kim7
Joon-Tae Kim7 Hyungjong Park8
Hyungjong Park8 Sung Hyun Baik9
Sung Hyun Baik9 Moon-Ku Han1
Moon-Ku Han1 Jihoon Kang1
Jihoon Kang1 Jun Yup Kim1
Jun Yup Kim1 Keon-Joo Lee1
Keon-Joo Lee1 Jong-Moo Park10
Jong-Moo Park10 Kyusik Kang11
Kyusik Kang11 Soo Joo Lee6
Soo Joo Lee6 Jae-Kwan Cha12
Jae-Kwan Cha12 Dae-Hyun Kim12
Dae-Hyun Kim12 Jin-Heon Jeong12
Jin-Heon Jeong12 Tai Hwan Park13
Tai Hwan Park13 Sang-Soon Park13
Sang-Soon Park13 Kyung Bok Lee14
Kyung Bok Lee14 Jun Lee15
Jun Lee15 Keun-Sik Hong16
Keun-Sik Hong16 Yong-Jin Cho16
Yong-Jin Cho16 Hong-Kyun Park16
Hong-Kyun Park16 Byung-Chul Lee17
Byung-Chul Lee17 Kyung-Ho Yu17
Kyung-Ho Yu17 Mi-Sun Oh17
Mi-Sun Oh17 Dong-Eog Kim18
Dong-Eog Kim18 Wi-Sun Ryu18
Wi-Sun Ryu18 Kang-Ho Choi7
Kang-Ho Choi7 Jay Chol Choi19
Jay Chol Choi19 Joong-Goo Kim19
Joong-Goo Kim19 Jee-Hyun Kwon20
Jee-Hyun Kwon20 Wook-Joo Kim20
Wook-Joo Kim20 Dong-Ick Shin21
Dong-Ick Shin21 Kyu Sun Yum21
Kyu Sun Yum21 Sung-Il Sohn8
Sung-Il Sohn8 Jeong-Ho Hong8
Jeong-Ho Hong8 Chulho Kim22
Chulho Kim22 Sang-Hwa Lee22
Sang-Hwa Lee22 Juneyoung Lee23
Juneyoung Lee23 Mohammed A. Almekhlafi2
Mohammed A. Almekhlafi2 Andrew Demchuk2
Andrew Demchuk2 Hee-Joon Bae1 for the Clinical Research Collaboration for Stroke in Korea investigators†
Hee-Joon Bae1 for the Clinical Research Collaboration for Stroke in Korea investigators†Background and purpose: There is much uncertainty in endovascular treatment (EVT) decisions in patients with acute large vessel occlusion (LVO) and mild neurological deficits.
Methods: From a prospective, nationwide stroke registry, all patients with LVO and baseline NIHSS <6 presenting within 24 h from the time last known well (LKW) were included. Early neurological deterioration (END) developed before EVT was prospectively collected as an increasing total NIHSS score ≥2 or any worsening of the NIHSS consciousness or motor subscores during hospitalization not related to EVT. Significant hemorrhage was defined as PH2 hemorrhagic transformation or hemorrhage at a remote site. The modified Rankin Scale (mRS) was prospectively collected at 3 months.
Results: Among 1,083 patients, 149 (14%) patients received EVT after a median of 5.9 [3.6–12.3] h after LKW. In propensity score-matched analyses, EVT was not associated with mRS 0-1 (matched OR 0.99 [0.63–1.54]) but increased the risk of a significant hemorrhage (matched OR, 4.51 [1.59–12.80]). Extraneous END occurred in 207 (19%) patients after a median of 24.5 h [IQR, 13.5–41.9 h] after LKW (incidence rate, 1.41 [95% CI, 1.23–1.62] per 100 person-hours). END unrelated to EVT showed a tendency to modify the effectiveness of EVT (P-for-interaction, 0.08), which decreased the odds of having mRS 0–1 in mild LVO patients without END (adjusted OR, 0.63 [0.40–0.99]).
Conclusions: The use of EVT in patients with acute LVO and low NIHSS scores may require the assessment of individual risks of early deterioration, hemorrhagic complications and expected benefit.
Multiple recent randomized clinical trials (RCTs) have demonstrated the superiority of endovascular treatment (EVT) over medical management in ischemic stroke patients with anterior circulation large vessel occlusions (LVOs) presenting within 24 h from the time last known well (LKW) (1). The majority of these RCTs excluded patients with mild neurological deficits who comprise a substantial proportion of acute ischemic stroke patients due to LVO (2). Despite their non-disabling presentation, the clinical outcomes of mild LVO patients are not as favorable as expected (3). Several recent observational studies have reported that EVT may be associated with increased hemorrhagic complications and no overall clinical benefit in these patients (4–6). However, other observational studies have suggested potential benefits of EVT in these patients (7–11). More robust evidence from large high-quality registries is needed to help physicians make treatment decisions in these patients until ongoing trials present their results in a few years.
There is much uncertainty in the treatment decision for mild LVO patients. The decision to offer EVT is based on a consideration of the risks and benefits, and the latter of which is likely to be limited due to the mild neurological deficit at presentation. However, the early clinical course of mild LVO patients is dynamic, and early neurological deterioration (END) occurs in 10 to 20% of patients (12–15). Because END occurs some time after admission, these patients are often ineligible to receive recanalization treatments, and in-hospital logistics for emergent treatment tend to be delayed (16). These patients may also have received thrombolytics or antithrombotics that increase the risk of treatment-related hemorrhage. However, the ability to predict who among these patients is at higher risk of END is still limited.
Using prospectively collected data from the South Korean national registry of consecutive ischemic stroke patients [Clinical Research Collaboration for Stroke in Korea (CRCS-K)], we analyzed detailed clinical and imaging information in patients presenting with acute LVO but with mild stroke symptoms. We evaluated the effectiveness of EVT for mild LVO patients and then examined whether the effectiveness differs due to END that occurs before endovascular treatment.
Data were obtained from the CRCS-K registry, an ongoing, nationwide, multicenter acute stroke registry that prospectively sources records of patients with acute stroke or transient ischemic attack (TIA) admitted within seven days of onset (17). Between January 1, 2015 and March 31, 2019, a total of 36,339 admissions were recorded in the CRCS-K registry database. Among these admissions, patients who (1) reached hospital within 24 h after the time last known well (n = 24,596), (2) had a baseline NIHSS score <6 points (n = 15,436) and (3) had anterior circulation LVO (ICA or M1 or proximal M2 segment of MCA) confirmed by neuroimaging (n = 1083) were included (Supplementary Material I). Patients were treated per institutional protocols based on national guidelines at the time of practice (18, 19). The local institutional review boards (IRBs) of all participating centers of the CRCS-K registry approved the study with a waiver of consent. Secondary use of the registry data and additional review of medical records for this study were approved by IRBs [B-2007-622-105].
The baseline demographics of all included subjects were retrieved from the CRCS-K registry database. The data definitions have been published elsewhere (17). Stroke etiology was classified by the Trial of Org 10172 in Acute Stroke Treatment with an MRI-based algorithm (20, 21). END was defined as any new neurological symptoms or signs that satisfied one or more of the following conditions: an increase in the total NIHSS score ≥2; an increase in the NIHSS 1a, 1b, or 1c subscore (level of consciousness) ≥1; or an increase in the NIHSS 5a, 5b, 6a or 6b subscore (motor) ≥1. END was also defined as any new neurological symptoms or signs that occurred during hospitalization directly due to the index stroke (17, 22). Only ENDs that occurred before the initiation of EVT or in medically treated patients were analyzed for the present study. END was further categorized by the NIHSS score increase at the time of END as mild END (NIHSS increase <4) and severe END (NIHSS score increase ≥4). Data on END were prospectively collected. The modified Rankin Scale (mRS), recurrent stroke and death up to 3 months after stroke were prospectively collected during a regular clinic visit or through a structured telephone interview conducted by an appropriately trained nurse.
All neuroimaging data were retrospectively collected and independently evaluated by a central imaging core lab. Images were assessed for the Alberta Stroke Program Emergent CT Score (ASPECTS), collateral grade, location of cerebral arterial occlusion, tandem occlusion, white matter hyperintensities (Fazekas grade), old infarction, cerebral microbleeds, angiographic collateral grading (ASITN/SIR) and expanded treatment in cerebral ischemia after endovascular treatment. The collateral grade was categorized as good (grade 4 or 5), intermediate (2 or 3) or poor (0 or 1). Advanced white matter hyperintensity was defined as patients having Fazekas grade 2 or 3. Hemorrhages on follow-up CT or MR images were evaluated by the Heidelberg bleeding classification (HBC) (23); parenchymal hemorrhage 2 and/or HBC class III hemorrhages were deemed significant.
The central image lab consisted of vascular neurologists (JHH, BJK, BJK, CKK and J-TK), interventional neurologists (JGK, HP and JSY) and interventional radiologists (SHB). All images were independently evaluated by at least two raters (Supplementary Material II). Any discrepancy in reading between raters was adjudicated by a panel (BJK, JSY and SHB) to reach the final assessment. Details on image collection, anonymization, storage and back-up as well as image reading processes are provided in Supplementary Material III.
Statistical analyses were performed based on a prespecified plan (Supplementary Material IV). Baseline patient characteristics were summarized and compared using chi-squared tests for categorical data, independent t tests for parametric data and a test of medians for non-parametric data. To estimate the association between baseline variables and END occurrence, a multivariable logistic regression model was constructed using clinically relevant variables or with univariate P values < 0.20. To compare patients who received EVT vs. those who did not, a propensity score (PS) for receiving EVT was estimated from clinically relevant variables (Supplementary Material V). The primary clinical outcome for analysis was 90-day mRS 0-1. Patients who received EVT and controls who received medical management were matched 1:1 with a caliper of 0.2 and without replacement. The inverse probability of receiving EVT was estimated and used as an individual weight in a separate model with robust sandwich estimators for standard errors (24). Covariates with standardized mean differences of 0.20 or more after weighting were further incorporated into this model (25). Crude and multivariable logistic regression models without the PS techniques were built as sensitivity analyses. The effectiveness and safety of EVT were also tested against various outcome indices. Pre-specified subgroup analyses were performed using multivariable logistic regression models for mRS 0–1 without propensity score balancing. Significance levels were set at P < 0.05 for two-tailed tests, except when testing for multiplicative interactions when a two-tailed P < 0.10 was considered statistically significant (26). All tests were considered exploratory and hypothesis-generating; no adjustment was therefore made for multiple tests (27). All statistical analyses were performed using R, version 4.0.2 (R Foundation for Statistical Computing).
Among 36,339 admissions to 16 hospitals over 4 years, 1,083 patients met the eligibility criteria with an mean age of 67.2 years ± 13.1 years, 669 males (62%), a median NIHSS score of 2 points [interquartile range (IQR), 1–4] and a median LKW to hospital arrival time of 4.3 h [1.6–10.9]. Among these patients, 223 (21%) had extracranial internal carotid artery (ICA) occlusion, 69 (6%) had intracranial ICA occlusion, 377 (35%) had proximal middle cerebral artery (M1) occlusion and 337 (31%) had distal middle cerebral artery (M2 and beyond) occlusions. Tandem occlusions (combined extracranial ICA and M1 or M2 occlusions) were found in 77 (7%) patients. A good collateral grade was found in 154 (75%) patients with extracranial ICA occlusion, 34 (51%) patients with intracranial ICA occlusion, 134 (38%) patients with M1 segment MCA occlusion, 218 (67%) patients with proximal M2 segment MCA occlusion and 33 (46%) patients with tandem occlusions. Excellent functional status (90-day mRS 0–1) was observed in 576 (54%) patients, while mortality within 90 days was noted in 36 (3%) patients (Table 1 and Supplementary Figure I).
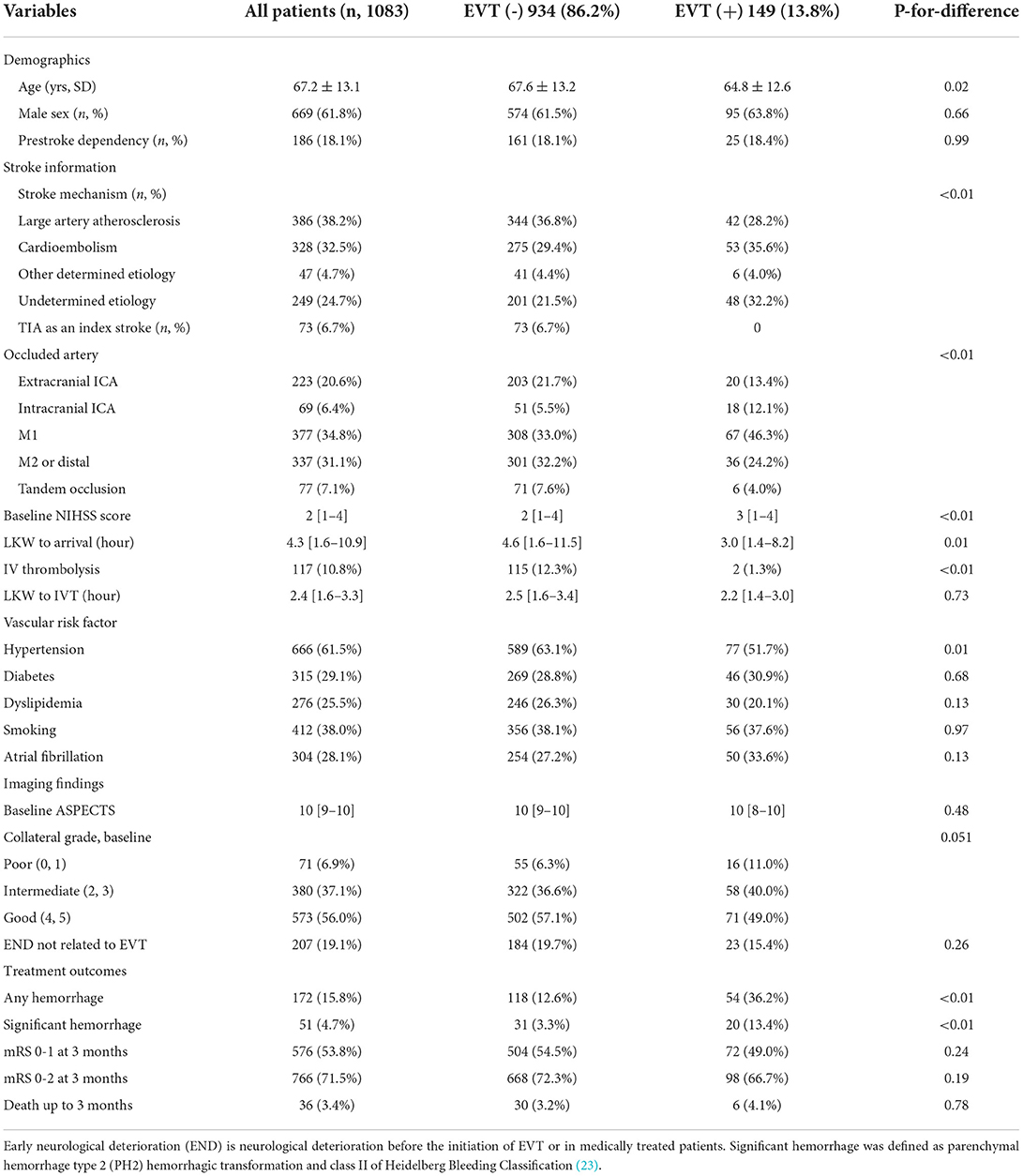
Table 1. Clinical profiles of patients stratified according to patients receiving or not receiving endovascular treatment.
EVT was performed in 149 (14%) patients with a median of 5.9 h [IQR 3.6–12.3 h] from LKW. Patients who received EVT were younger, arrived earlier, had intracranial ICA or M1 segment MCA occlusion rather than extracranial ICA or M2 occlusion at baseline and had a history of hypertension. Moreover, IV thrombolysis was given in only two patients (1%) in the EVT group, while 115 (12%) patients were in the medically managed group. Patients who received EVT were more likely to have a significant hemorrhage but not excellent functional recovery at 3 months (mRS 0–1; Table 1).
When using regression models incorporating propensity scores with 1:1 matching, EVT was not associated with better functional recovery 3 months after stroke. However, EVT increased the odds of having significant hemorrhage (PS-matched OR, 4.51 [1.59–12.80]) or any hemorrhage (PS-matched OR, 3.17 [95% CI, 1.76–5.69]; Table 2). The results from the unadjusted logistic regression model and inverse probability weighting model are provided in Supplementary Figure III.
Subgroup analyses showed that the effectiveness of EVT was potentially modified by occlusion location (P-for-interaction, 0.06), underlying stroke mechanism (P-for-interaction, 0.09) and the occurrence of END prior to EVT (P-for-interaction, 0.08; Figure 1). Multivariable logistic regression models adjusted for baseline demographics, vascular risk factors, location of the occlusion, baseline collaterals, ASPECTS and preceding END showed that the odds of having a 90-day mRS score 0–1 by EVT decreased in patients with baseline extracranial ICA occlusion (adjusted OR 0.30 [95% CI, 0.10–0.87]), patients with atherosclerotic stroke (adjusted OR 0.48 [95% CI, 0.22–1.06] and patients without END (adjusted OR 0.63 [95% CI, 0.40–0.99]). There was no difference in the proportion of patients who received EVT achieving 90-day mRS 0–1 among those who had END prior to EVT (45.5%) vs. those without preceding END (49.6%; Supplementary Data I).
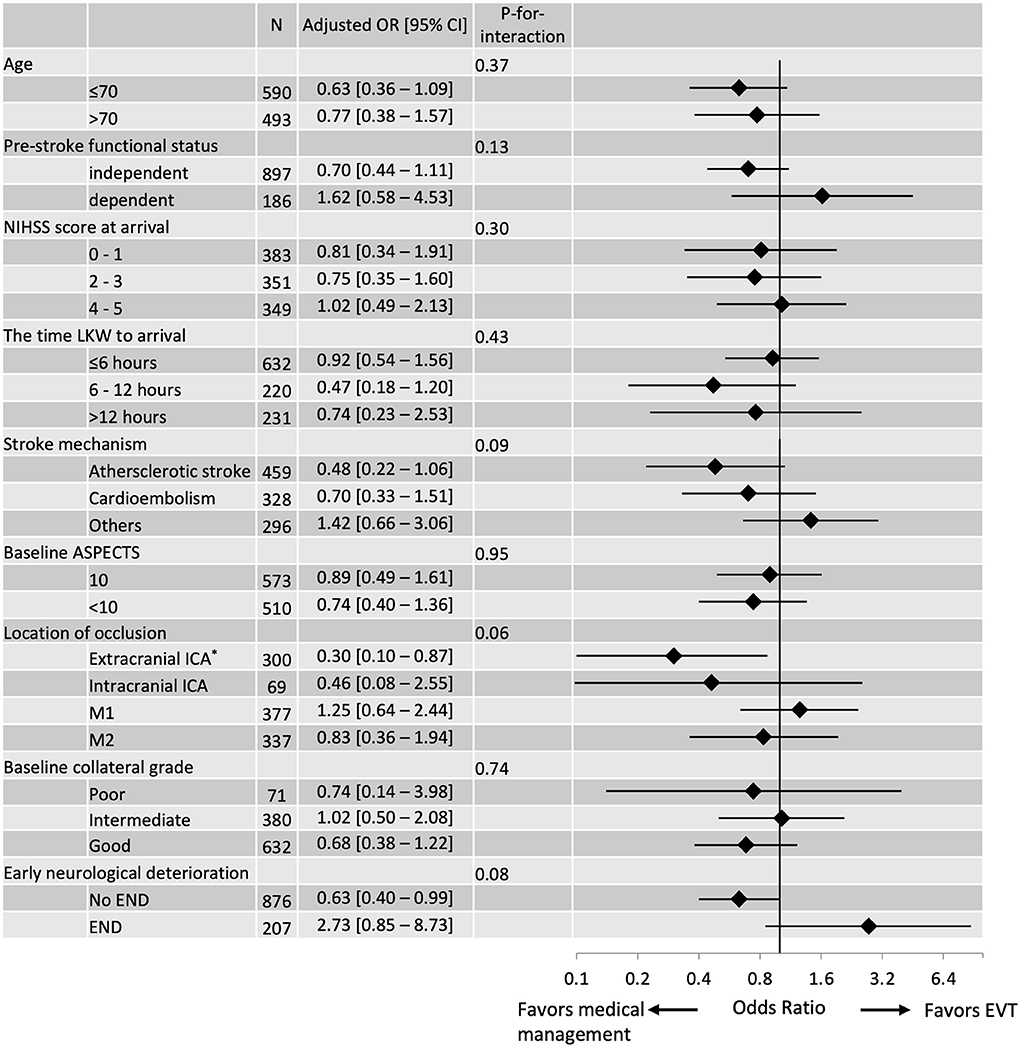
Figure 1. Subgroup analyses of EVT effectiveness were assessed using 90-day mRS 0–1 and stratified by selected baseline characteristics. The vertical line signifies the null point (OR 1.00). Adjusted ORs and 95% CIs were calculated using multivariable logistic regression models without applying the propensity score. *Extracranial ICA includes tandem lesions.
END before initiation of EVT or in medically treated patients occurred in 207 (19%) patients (Figure 2A), and the median [IQR] duration between LKW time and END reporting was 24.5 h [13.5–41.9]. The incidence rate [95% CI] of END was 1.41 [1.23–1.62] per 100 person-hours, and 85% of ENDs occurred within 48 h from the LKW (Figure 2B). The NIHSS score after the occurrence of END was a median of 7 (4–10), which increased by a median of 4 (2–8) from baseline. Patients with END were more likely to have a higher baseline NIHSS score, a history of hypertension and extracranial ICA occlusion on baseline imaging. Patients with END had a higher proportion of unfavorable imaging and clinical outcomes (Table 3 and Supplementary Figure II). Mild END (NIHSS score increase <4) occurred in 97 (9.0%) subjects, four of whom had EVT; severe END (NIHSS score increase ≥4) developed in 109 (10.1%) patients, 19 (17.4%) of whom received EVT (Supplementary Data I).
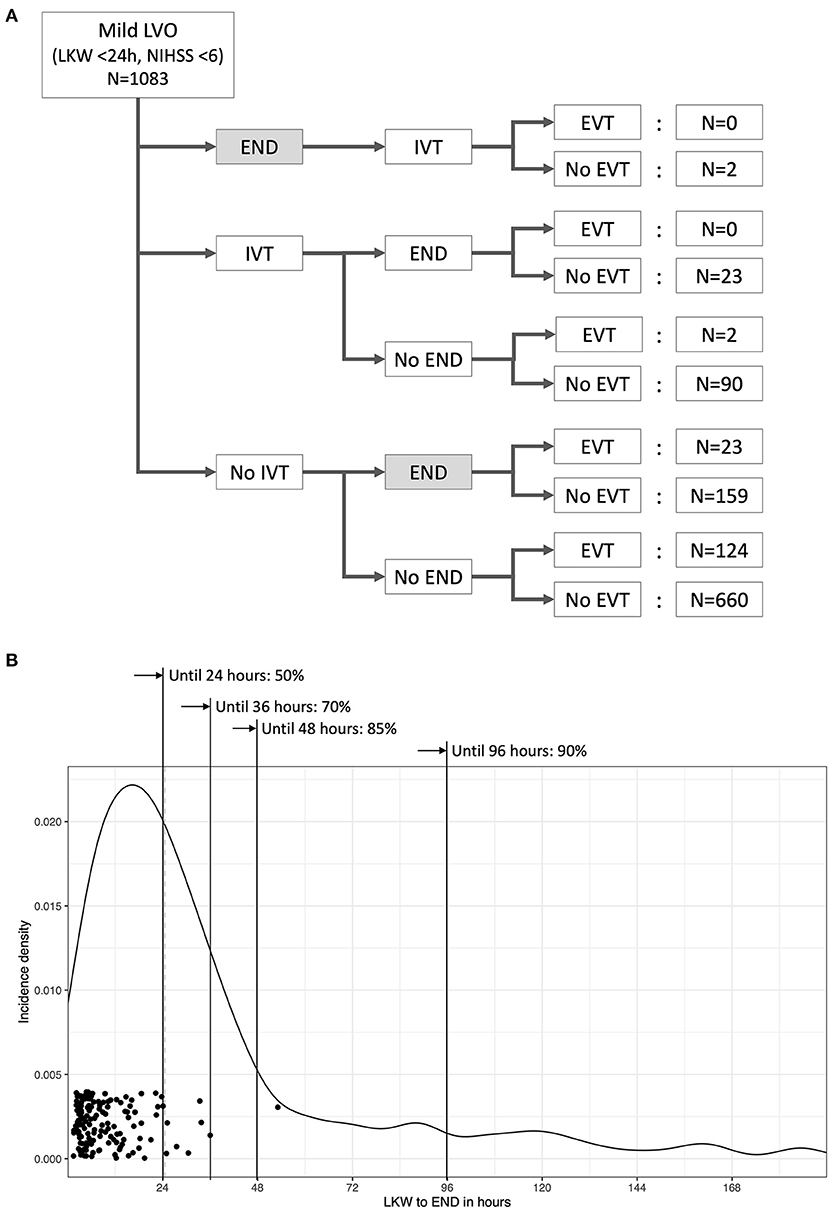
Figure 2. Temporal profile of early neurological deterioration (END) related to recanalization treatments for acute LVO patients with mild neurological deficits. Only ENDs captured before the initiation of EVT were counted for the present study. Of 207 ENDs (19% of all patients) that occurred before the initiation of EVT, 23 developed after intravenous thrombolysis. Five ENDs were symptomatic hemorrhages, one END occurred after intravenous thrombolysis and four ENDs occurred without recanalization treatment (A). The median time from the last known well (LKW) to END was 24.5 h, and 85% of ENDs developed within 48 h from the time LKW. Dots represent the time of arterial puncture for EVT (B).
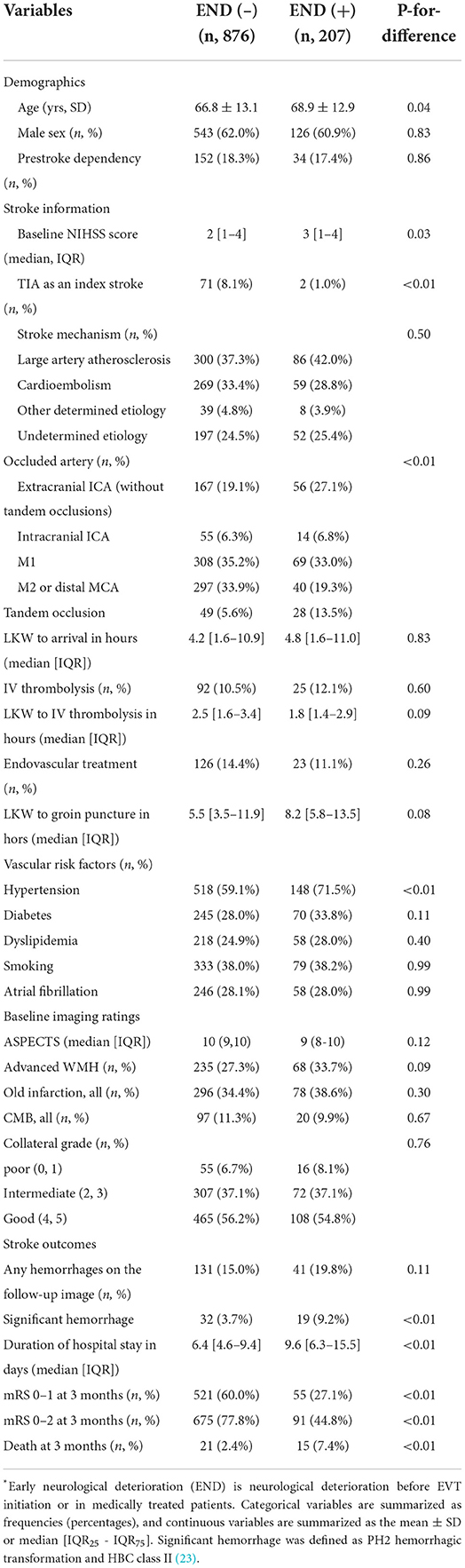
Table 3. Comparison of acute LVO patients with low NIHSS scores (<6) by early neurological deterioration (END).
The time from LKW to END occurrence was shorter in patients who had EVT (median 6.7 h [IQR, 4.0–12.2] when compared to a median of 26.2 h (16–39), [41–44] in those who were treated (P-for-difference, <0.01). The NIHSS at END occurrence was a median of 9 (7–13) in those who received EVT and a median of 7 (4–10) in medically managed patients (P-for-difference, 0.27). In the multivariable logistic regression model in which the location of occlusion was mutually exclusive, the presence of extracranial ICA occlusion (adjusted OR 1.72, 95% CI [1.09–2.73]), presence of tandem occlusion (adjusted OR, 2.79 [1.57–4.95]), history of hypertension (adjusted OR, 1.49 [1.03–2.14]) and a higher NIHSS (adjusted OR 1.12 per 1-point increase, [1.02–1.23]) were significantly associated with higher odds of END occurrence (for full results, see Supplementary Table III).
In this large nationwide registry-based study of 1,083 acute LVO patients with low NIHSS scores, early neurological deterioration (END) occurred in approximately 20% of patients, mostly within 48 h from the time LKW. EVT in these patients may potentially result in increased hemorrhagic complications without an overall clinical benefit. However, the potential benefits vs. risks associated with EVT are likely modified by occlusion location, underlying stroke etiology and proceeding END.
Clinical decision-making in patients with acute ischemic stroke due to LVOs presenting with mild stroke symptoms is challenging. The mild nature of symptoms indicates that the risks of invasive treatments, such as EVT, need to be balanced against the risks of neurological deterioration as part of natural history. Physicians have therefore relied on observational studies to provide necessary evidence on this topic. The present study, with its large sample size, prospectively collected data from a nationwide acute stroke registry and high-quality imaging characterization and outcome ascertainment, adds substantially to the current body of literature and strength of evidence (28). The patient demographics and prevalence of END in the present study were similar to those of previously published studies from North America and Europe (4, 12, 13, 29).
However, data on when patients with acute LVOs and mild stroke worsen neurologically, i.e., have END, are conflicting. In a previous report on 32 patients from a single center, END developed after a median of 5.2 h from arrival with a prehospital delay of a median of 10.5 h (7). In an analysis of 347 IV thrombolysis-treated patients, 48% of ENDs occurred within 2 h after thrombolysis (13). In comparison, Park et al. reported that 61% of ENDs developed within 48 h from the time LKW according to their analysis of all subjects from a nationwide registry (22). The present study demonstrated that END was likely to occur within 48 h from LKW in 85% of patients. Interestingly, >50% of ENDs occurs 24 h or more after LKW. Therefore, these patients are less likely to be offered EVT after END based on current EVT practice guidelines, thus attesting to the need to predict END occurrence early after stroke onset (30, 31). The efficacy of EVT in these delayed progressive ischemic stroke patients beyond the conventional “time window” should be investigated.
In the present study, the administration of EVT increased the odds of hemorrhagic complications without any comparable increase in the proportion of patients achieving good clinical outcomes at 90 days. Therefore, these results add to the body of literature that advocates caution in offering EVT to such patients outside of ongoing clinical trials (4, 28, 32). The possibility that the effect of EVT may be modified by the occurrence of END prior to EVT administration is intriguing. From our subgroup analysis, mild LVO patients without END did not benefit from EVT. Thus, waiting for END to occur and then offering EVT is one way of altering the analysis of risks and benefits with EVT in favor of that therapy. However, this option also indicates that patients may only be offered EVT late when the potential risks of therapy are higher and the potential benefits are lower (16). Although we have reported benefits with EVT in patients presenting even beyond 24 hours (33), more data will be needed before such therapy is offered to patients with worsening clinical symptoms beyond 24 h from LKW. An alternative approach may be an assessment of the risk of END. The present study showed that patients with higher baseline NIHSS scores, a history of hypertension and the presence of extracranial ICA occlusion or tandem occlusion on baseline imaging are at higher risk of END. A risk score that identifies patients at high risk for END may help in determining the best candidates for EVT from mild LVO patients (13, 30).
Detailed imaging evaluation at baseline was a strength of the present study. In contrast to expectations, we did not find any association among baseline collateral status, the occurrence of END and effect of EVT, which may be because these patients with LVOs and mild symptoms invariably tend to have good collaterals (56% in the present study). As long as the collateral circulation provides sufficient cerebral perfusion to the ischemic area, invasive treatment may be delayed based on the premise that new leptomeningeal collaterals may develop after ischemia (34–36). However, these leptomeningeal collaterals are tenuous vessels, indicating that even subtle changes in cerebral perfusion pressure may alter hemodynamics to the extent that may cause END (37, 38). A more detailed analysis of cerebral circulation over time will be needed to understand the complex pathophysiology of leptomeningeal collaterals and how they affect cerebral blood flow over time in these patients.
There are ongoing prospective studies evaluating the clinical benefit of endovascular recanalization for acute LVO patients with mild neurological severity. “Endovascular Therapy for low NIHSS Ischemic Strokes (ENDOLOW)” from North America (NCT04167527) and “Minor Stroke Therapy Evaluation (MOSTE)” from Europe (NCT03796468) are two randomized clinical trials expected to prove the efficacy of EVT. “Mild Acute Ischemic Stroke With Large Vessel Occlusion (MISTWAVE)” is a prospective registry recruiting EVT cases with ICA, M1, M2 or basilar artery occlusion who had NIHSS scores <6 at baseline (NCT03731351). Stronger evidence from large high-quality datasets is needed to help physicians make the best treatment decisions in these patients until those prospective results will be available in a few years.
The present study had limitations. Data were obtained from patients predominantly of East Asian ethnicity who have a higher prevalence of intracranial stenosis (39). Differences in stroke etiology and hemorrhagic tendency due to ethnicity of patients may affect the extrapolation of these results to other populations. Practice differences, such as higher utilization of MRI for follow-up, may affect the relative prevalence of outcomes, such as hemorrhage, in the present study. Diagnostic imaging acquisition parameters and treatment strategies for acute LVO patients with mild neurological deficits also varied by hospital (Supplementary Table II). The use of propensity score matching and regression analysis likely mitigated some of this variability.
Among acute LVO cases presenting with low NIHSS scores of ≤5 points, one-fifth of patients developed END, and 85% of patients developed END within 48 h of the LKW time. EVT was associated with increased hemorrhagic complications but no functional improvement even after balancing the baseline characteristics. The effectiveness of EVT was modified by stroke mechanisms, location of the occlusion and preceding END. The results of the present study did not permit general treatment recommendations for acute LVO patients with mild neurological deficits. Treatment decisions for the use of EVT for mild LVO patients require a prudent judgment based on the individual risk of stroke progression, expected benefit from the treatment and potential risk of complications, such as hemorrhagic transformation. Ongoing clinical trials will further inform this challenging clinical decision.
The STROBE statement is provided as Supplementary Data III.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Review Board of the Seoul National University Bundang Hospital [B-2007-622-105]. The patients/participants provided their written informed consent to participate in this study.
BeK: conception and design of the study. BuK, M-KH, JiK, JuK, K-JL, J-MP, KK, SL, J-KC, D-HK, J-HJ, TP, S-SP, KL, JunL, JuneL, K-SH, Y-JC, H-KP, B-CL, K-HY, M-SO, D-EK, W-SR, K-HC, JC, J-GK, J-HK, W-JK, D-IS, KY, S-IS, J-HH, CK, S-HL, and H-JB: acquisition of data. BeK, JY, JH, BuK, CKK, JGK, J-TK, HP, and SB: image analysis. BuK and BM: drafting and revision of the manuscript. All authors critical intellectual contribution for revision and approval of manuscript.
This research was supported by a fund (2020ER620200#) by the Research Fund of Korea Centers for Disease Control and Prevention.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.955725/full#supplementary-material
1. Goyal M, Menon BK, van Zwam WH, Dippel DWJ, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
2. Noorian AR, Sanossian N, Shkirkova K, Liebeskind DS, Eckstein M, Stratton SJ, et al. Los Angeles motor scale to identify large vessel occlusion: prehospital validation and comparison with other screens. Stroke. (2018) 49:565–72. doi: 10.1161/STROKEAHA.117.019228
3. Romano JG, Smith EE, Liang L, Gardener H, Camp S, Shuey L, et al. Outcomes in mild acute ischemic stroke treated with intravenous thrombolysis: a retrospective analysis of the get with the guidelines-stroke registry. JAMA Neurol. (2015) 72:423–31. doi: 10.1001/jamaneurol.2014.4354
4. Goyal N, Tsivgoulis G, Malhotra K, Ishfaq MF, Pandhi A, Frohler MT, et al. Medical management vs mechanical thrombectomy for mild strokes: an international multicenter study and systematic review and meta-analysis. JAMA Neurol. (2020) 77:16–24. doi: 10.1001/jamaneurol.2019.3112
5. Nagel S, Bouslama M, Krause LU, Kupper C, Messer M, Petersen M, et al. Mechanical thrombectomy in patients with milder strokes and large vessel occlusions. Stroke. (2018) 49:2391–7. doi: 10.1161/STROKEAHA.118.021106
6. Saito T, Itabashi R, Yazawa Y, Uchida K, Yamagami H, Sakai N, et al. Clinical outcome of patients with large vessel occlusion and low national institutes of health stroke scale scores: subanalysis of the rescue-Japan registry 2. Stroke. (2020) 51:1458–63. doi: 10.1161/strokeaha.119.028562
7. Haussen DC, Bouslama M, Grossberg JA, Anderson A, Belagage S, Frankel M, et al. Too good to intervene? Thrombectomy for large vessel occlusion strokes with minimal symptoms: an intention-to-treat analysis. J Neurointerv Surg. (2017) 9:917–21. doi: 10.1136/neurintsurg-2016-012633
8. Haussen DC, Lima FO, Bouslama M, Grossberg JA, Silva GS, Lev MH, et al. Thrombectomy versus medical management for large vessel occlusion strokes with minimal symptoms: an analysis from stopstroke and gestor cohorts. J Neurointerv Surg. (2018) 10:325–9. doi: 10.1136/neurintsurg-2017-013243
9. Alexandre AM, Valente I, Pedicelli A, Pezzullo AM, Colo F, Scarcia L, et al. Mechanical thrombectomy in acute ischemic stroke due to large vessel occlusion in the anterior circulation and low baseline national institute of health stroke scale score: a multicenter retrospective matched analysis. Neurol Sci. (2022) 43:3105–12. doi: 10.1007/s10072-021-05771-5
10. Heldner MR, Chaloulos-Iakovidis P, Panos L, Volbers B, Kaesmacher J, Dobrocky T, et al. Outcome of patients with large vessel occlusion in the anterior circulation and low nihss score. J Neurol. (2020) 267:1651–62. doi: 10.1007/s00415-020-09744-0
11. Shang XJ, Shi ZH, He CF, Zhang S, Bai YJ, Guo YT, et al. Efficacy and safety of endovascular thrombectomy in mild ischemic stroke: results from a retrospective study and meta-analysis of previous trials. BMC Neurol. (2019) 19:150. doi: 10.1186/s12883-019-1372-9
12. Saleem Y, Nogueira RG, Rodrigues GM, Kim S, Sharashidze V, Frankel M, et al. Acute neurological deterioration in large vessel occlusions and mild symptoms managed medically. Stroke. (2020) 51:1428–34. doi: 10.1161/STROKEAHA.119.027011
13. Seners P, Ben Hassen W, Lapergue B, Arquizan C, Heldner MR, Henon H, et al. Prediction of early neurological deterioration in individuals with minor stroke and large vessel occlusion intended for intravenous thrombolysis alone. JAMA Neurol. (2021) 78:321–8. doi: 10.1001/jamaneurol.2021.2025
14. Mazya MV, Cooray C, Lees KR, Toni D, Ford GA, Bar M, et al. Minor stroke due to large artery occlusion when is intravenous thrombolysis not enough? Results from the sits international stroke thrombolysis register. Eur Stroke J. (2018) 3:29–38. doi: 10.1177/2396987317746003
15. Volny O, Zerna C, Tomek A, Bar M, Rocek M, Padr R, et al. Thrombectomy vs medical management in low nihss acute anterior circulation stroke. Neurology. (2020) 95:e3364–e72. doi: 10.1212/WNL.0000000000010955
16. Akbik F, Xu H, Xian Y, Shah S, Smith EE, Bhatt DL, et al. Trends in reperfusion therapy for in-hospital ischemic stroke in the endovascular therapy era. JAMA Neurol. (2020) 77:1486–95. doi: 10.1001/jamaneurol.2020.3362
17. Kim BJ, Park J-M, Kang K, Lee SJ, Ko Y, Kim JG, et al. Case characteristics, hyperacute treatment, and outcome information from the clinical research center for stroke-fifth division registry in South Korea. J Stroke. (2015) 17:38–53. doi: 10.5853/jos.2015.17.1.38
18. Hong KS, Ko SB Yu KH, Jung C, Park SQ, Kim BM, et al. Update of the Korean clinical practice guidelines for endovascular recanalization therapy in patients with acute ischemic stroke. J Stroke. (2016) 18:102–13. doi: 10.5853/jos.2015.01655
19. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the american heart association/American stroke association. Stroke. (2018) 49:e46–e110. doi: 10.1161/STR.0000000000000158
20. Adams HP Jr., Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. definitions for use in a multicenter clinical trial. toast. trial of org 10172 in acute stroke treatment. Stroke. (1993) 24:35–41. doi: 10.1161/01.STR.24.1.35
21. Ko Y, Lee S, Chung JW, Han MK, Park JM, Kang K, et al. Mri-based algorithm for acute ischemic stroke subtype classification. J Stroke. (2014) 16:161–72. doi: 10.5853/jos.2014.16.3.161
22. Park TH, Lee JK, Park MS, Park SS, Hong KS Ryu WS, et al. Neurologic deterioration in patients with acute ischemic stroke or transient ischemic attack. Neurology. (2020) 95:e2178–e91. doi: 10.1212/WNL.0000000000010603
23. Kummer Rv B., Campbell BCV, Demchuk A, Goyal M, Hill MD, et al. The heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. (2015) 46:2981–6. doi: 10.1161/STROKEAHA.115.010049
24. Zeileis A. Econometric computing with hc and hac covariance matrix estimators. J Stat Software. (2004) 11:1–17. doi: 10.18637/jss.v011.i10
25. Nguyen TL, Collins GS, Spence J, Daures JP, Devereaux PJ, Landais P, et al. Double-adjustment in propensity score matching analysis: choosing a threshold for considering residual imbalance. Bmc Med Res Methodol. (2017) 17:78. doi: 10.1186/s12874-017-0338-0
26. Pan G, Wolfe DA. Test for qualitative interaction of clinical significance. Stat Med. (1997) 16:1645–52.
27. Rubin M. Do p values lose their meaning in exploratory analyses? It depends how you define the familywise error rate. Rev Gen Psychol. (2017) 21:269–75. doi: 10.1037/gpr0000123
28. McCarthy DJ, Tonetti DA, Stone J, Starke RM, Narayanan S, Lang MJ, et al. More expansive horizons: a review of endovascular therapy for patients with low nihss scores. J Neurointerv Surg. (2021) 13:146–51. doi: 10.1136/neurintsurg-2020-016583
29. Kim J-T, Park M-S, Chang J, Lee JS, Choi K-H, Cho K-H. Proximal arterial occlusion in acute ischemic stroke with low nihss scores should not be considered as mild stroke. PLoS ONE. (2013) 8:e70996. doi: 10.1371/journal.pone.0070996
30. Gwak DS, Kwon JA, Shim DH, Kim YW, Hwang YH. Perfusion and diffusion variables predict early neurological deterioration in minor stroke and large vessel occlusion. J Stroke. (2021) 23:61–8. doi: 10.5853/jos.2020.01466
31. Turc G, Bhogal P, Fischer U, Khatri P, Lobotesis K, Mazighi M, et al. European stroke organisation (Eso) - European society for minimally invasive neurological therapy (esmint) guidelines on mechanical thrombectomy in acute ischemic stroke. J Neurointerv Surg. (2019) 4:6–12. doi: 10.1177/2396987319832140
32. Sarraj A, Hassan A, Savitz SI, Grotta JC, Cai C, Parsha KN, et al. Endovascular thrombectomy for mild strokes: how low should we go? Stroke. (2018) 49:2398–405. doi: 10.1161/STROKEAHA.118.022114
33. Kim BJ, Menon BK, Kim JY, Shin DW, Baik SH, Jung C, et al. Endovascular treatment after stroke due to large vessel occlusion for patients presenting very late from time last known well. JAMA Neurol. (2020) 78:21–9. doi: 10.1001/jamaneurol.2020.2804
34. Sarraj A, Hassan AE, Grotta J, Blackburn S, Day A, Abraham M, et al. Early infarct growth rate correlation with endovascular thrombectomy clinical outcomes: analysis from the select study. Stroke. (2021) 52:57–69. doi: 10.1161/STROKEAHA.120.030912
35. Ospel JM, Hill MD, Kappelhof M, Demchuk AM, Menon BK, Mayank A., et al. Which acute ischemic stroke patients are fast progressors? Results from the escape trial control arm. Stroke. (2021) 52:1847–50. doi: 10.1161/STROKEAHA.120.032950
36. Kim BJ, Singh N, Menon BK. Hemodynamics of leptomeningeal collaterals after large vessel occlusion and blood pressure management with endovascular treatment. J Stroke. (2021) 23:343–57. doi: 10.5853/jos.2021.02446
37. Campbell BC, Christensen S, Tress BM, Churilov L, Desmond PM, Parsons MW, et al. Failure of collateral blood flow is associated with infarct growth in ischemic stroke. J Cereb Blood Flow Metab. (2013) 33:1168–72. doi: 10.1038/jcbfm.2013.77
38. Jeong HG, Kim BJ, Kim H, Jung C, Han MK, Liebeskind DS, et al. Blood pressure drop and penumbral tissue loss in nonrecanalized emergent large vessel occlusion. Stroke. (2019) 50:2677–84. doi: 10.1161/STROKEAHA.119.025426
Keywords: endovascular recanalization, mild stroke, low NIHSS score, early neurological deterioration, CRCS-K, multicenter registry
Citation: Kim BJ, Menon BK, Yoo J, Han JH, Kim BJ, Kim CK, Kim JG, Kim J-T, Park H, Baik SH, Han M-K, Kang J, Kim JY, Lee K-J, Park J-M, Kang K, Lee SJ, Cha J-K, Kim D-H, Jeong J-H, Park TH, Park S-S, Lee KB, Lee J, Hong K-S, Cho Y-J, Park H-K, Lee B-C, Yu K-H, Oh M-S, Kim D-E, Ryu W-S, Choi K-H, Choi JC, Kim J-G, Kwon J-H, Kim W-J, Shin D-I, Yum KS, Sohn S-I, Hong J-H, Kim C, Lee S-H, Lee J, Almekhlafi MA, Demchuk A and Bae H-J (2022) Effectiveness and safety of EVT in patients with acute LVO and low NIHSS. Front. Neurol. 13:955725. doi: 10.3389/fneur.2022.955725
Received: 29 May 2022; Accepted: 12 July 2022;
Published: 05 August 2022.
Edited by:
Kristian Barlinn, University Hospital Carl Gustav Carus, GermanyReviewed by:
Apostolos Safouris, Metropolitan Hospital, GreeceCopyright © 2022 Kim, Menon, Yoo, Han, Kim, Kim, Kim, Kim, Park, Baik, Han, Kang, Kim, Lee, Park, Kang, Lee, Cha, Kim, Jeong, Park, Park, Lee, Lee, Hong, Cho, Park, Lee, Yu, Oh, Kim, Ryu, Choi, Choi, Kim, Kwon, Kim, Shin, Yum, Sohn, Hong, Kim, Lee, Lee, Almekhlafi, Demchuk and Bae. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Beom Joon Kim, S2ltLkJKLlN0cm9rZUBnbWFpbC5jb20=
†A list of collaborators is provided in the Supplementary Data II
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.