
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 05 August 2022
Sec. Stroke
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.950045
Background: Patients with ischemic stroke are at high risk for post-stroke depression (PSD). There are limited data regarding the clinical impact of early PSD, assessed in hospitalized patients with acute ischemic stroke.
Methods: This hospital-based observational cohort study included consecutive patients with acute ischemic stroke or transient ischemic attack between July 2019 and June 2021. In the study hospital, all admitted patients were systematically screened for depression. The depression was screened using the Patient Health Questionnaire-9 (PHQ-9), and PHQ-9 positivity indicated early PSD, which was defined as a score of >4. Logistic regression analyses were used to compare the rates of poor functional outcomes at 3 months in patients with and without PHQ-9 positivity.
Results: Among 1339 patients admitted during the study period, 775 were included, with a median age of 68.0 years, and 316 (40.8%) were women. A total of 111 (14.3%) patients were PHQ-9 positive. History of cancer and early neurological deterioration were independently associated with PHQ-9 positivity. Poor functional outcomes at 3 months were observed in 147 patients (18.8%). PHQ-9 positivity independently showed a 2.2-fold increased risk of poor functional outcome at 3 months (Odds ratio 2.23; 95% confidence interval 1.05–4.73, P = 0.037).
Conclusions: Patients with history of cancer and early neurological deterioration were at risk for early PSD. Early PSD was independently associated with poor functional outcomes at 3 months. The identification of early depression could offer opportunities for further questioning and exploration of symptoms, as well as interventions.
Post-stroke depression (PSD) is one of the most common complications of stroke affecting approximately one-third of stroke survivors (1). PSD is independently associated with poor functional outcomes and higher mortality after stroke (2, 3). The current guideline recommends routine screening for PSD among stroke survivors by administration of a structured depression inventory (4). However, optimal timing, setting, methods, and follow-up for PSD screening are currently unclear (4).
The importance of early recognition and treatment of PSD has led to recommendations for depression screening in the early stages of patients hospitalized for acute ischemic stroke (5). Although numerous validated tools for the diagnosis of depression have been used to screen PSD in the subacute or chronic phase, data on the usefulness of these screening measures during the acute in-hospital phase of stroke are still limited (2, 6).
The Patient Health Questionnaire 9 (PHQ-9) is a 9-item self-administered depression screening and diagnostic tool for PSD, which is well validated in the chronic phase of ischemic stroke survivors (2, 7). A previous meta-analysis assessing PSD screening tools (24 studies, N = 2907) found that the 20-item Center of Epidemiological Studies-Depression Scale (CES-D), 21-item Hamilton Depression Rating Scale (HAMD) and PHQ-9 had high sensitivity for detecting PSD (8). Hence, a recent scientific statement from American Heart Association/American Stroke Association suggested that PHQ-9 may be more pragmatic, while CES-D and HAMD may not be feasible in a busy clinical practice despite high sensitivity (2, 4). Recent randomized trials including Effects of fluoxetine on functional outcomes after acute stroke trial and Assessment of Fluoxetine in Stroke Recovery trial, which evaluated the effect of fluoxetine on functional outcome after acute stroke, also defined secondary outcomes of depressive mood based on PHQ results (9, 10). Several small studies have further attempted to define early PSD using the PHQ-9 in patients with stroke within the acute period (11–13).
In the study hospital, all hospitalized patients with acute ischemic stroke were systematically screened using the PHQ-9 during admission to detect early PSD (5). In this study, we attempted to define early PSD using the PHQ-9 in hospitalized patients with acute ischemic stroke or transient ischemic attack (TIA). We sought to explore factors associated with early PSD, as well as the relationship between early PSD and short-term outcomes.
This study was approved by the institutional review board of Severance Hospital, Yonsei University Health System (no. 4–2021–1602), which waived the need for informed consent due to its retrospective nature.
This was a retrospective single-center based observational cohort study of patients with acute ischemic stroke or TIA who were prospectively registered in the Yonsei Stroke cohort (14). The registry enrolled consecutive patients with acute ischemic stroke or TIA within 7 days of stroke onset of those admitted to the stroke center.
All patients were thoroughly evaluated and managed based on current stroke guidelines (4). We collected patients' data on vascular risk factors such as hypertension, diabetes, dyslipidemia, body mass index (kg/m2), abdominal circumference, current smoking status, atrial fibrillation, cancer, and prior history of ischemic heart disease, stroke, or medication use. All patients underwent brain computed tomography (CT) and/or magnetic resonance imaging, cerebral angiographic studies (digital subtraction angiography, CT angiography, and/or magnetic resonance angiography), and chest radiography. Cardiac evaluations included 12-lead electrocardiography (ECG), continuous ECG monitoring during staying in a stroke unit and/or 24-h Holter monitoring; transesophageal echocardiography; and/or transthoracic echocardiography. During admission, the Korean version of the Mini-Mental State Examination (K-MMSE) was also evaluated along with education level (nil, primary school, or secondary school and above) and handedness (15). Stroke severity was assessed using the National Institute of Health Stroke Scale (NIHSS). Early neurological deterioration (END) was defined as any increase (≥1) in NIHSS score within 7 days after admission compared with baseline NIHSS score (16). After discharge, all patients were regularly followed up at 3 months, 1 year, and annually thereafter. Follow-up information was obtained by neurologists or clinical research assistants at the outpatient clinic through face-to-face interviews or through telephone interview using an in-house questionnaire (5). Poor functional outcome was defined as a modified Rankin scale (mRS) score of >2 at 3 months after stroke.
This study included patients with acute ischemic stroke or TIA who underwent PHQ-9 between July 2019 and June 2021. Patients with unknown mRS scores at 3 months were excluded from the study. Patients with depression before the onset of index stroke and/or prior antidepressant use, chronic alcoholism, or dementia were excluded. Further, patients without MMSE results were excluded from the study.
All patients admitted to the Severance Stroke Center were systematically screened for depression by trained nurses using the validated Korean version of PHQ-9 (17). PHQ-9 was usually performed within 3 days after admission especially during weekday daytime hours. For patients with END, PHQ was usually performed after the occurrence of END. If a patient could not undergo PHQ-9, the reason was consecutively clarified in the medical record as follows: poor systemic conditions, difficulties in communication due to aphasia and/or impaired mentality, poor cooperation, or other causes.
The PHQ-9 is a self-administered questionnaire completed by the patient (17, 18). It consists of nine questions [1. Little interest or pleasure in doing things? 2. Feeling down, depressed, or hopeless? 3. Trouble falling or staying asleep, or sleeping too much? 4. Feeling tired or having little energy? 5. Poor appetite or overeating? 6. Feeling bad about yourself - or that you are a failure or have let yourself or your family down? 7. Trouble concentrating on things, such as reading the newspaper or watching television? 8. Moving or speaking so slowly that other people could have noticed? or the opposite—being so fidgety or restless that you have been moving around a lot more than usual? 9. Thoughts that you would be better off dead, or of hurting yourself in some way?]. Each question is scored from 0 to 3 points based on the frequency of the specified symptoms in the preceding 2 weeks, reflecting the index stroke concomitantly. Scores range from 0 (no depression) to 27 (severe depression). PHQ-9 positivity, indicating early PSD, was defined as a score of >4 (more than mild depression) (7).
The corresponding action, including outpatient/inpatient psychiatric referral and initiation of antidepressant therapy, was decided by the treating physician. Escitalopram was usually prescribed to treat PSD in the study hospital (19). Besides treatment for depression, bupropion was used for smoking cessation (20).
Statistical analysis was performed using R version 4.0.4 (http://www.R-project.org). Univariable analysis was performed using the independent t-test, analysis of variance, or Kruskal–Wallis test for continuous variables, and the chi-square test for categorical variables, as appropriate. Multivariable logistic regression analysis was performed to identify which variable was independently associated with PHQ-9 positive and whether PHQ-9 was independently associated with poor functional outcome at 3 months. Analyses were adjusted for age, sex, and variables (P < 0.05) in univariable analyses. Subgroup analyses were performed to investigate whether the association between PHQ-9 positivity and poor functional outcome at 3 months differed according to the following factors: median age, sex, and significant variables associated with PHQ-9 positivity. Considering the decreased number of patients in each subgroup, multivariable models were adjusted for age and sex in the subgroup analyses. Statistical significance was set at P < 0.05.
Furthermore, to reduce potential confounding effects of baseline characteristics, propensity score matching (PSM) analysis was employed. The propensity score for the predicted probability of PHQ-9 positive in each patient was estimated with a logistic regression model, using age, sex, education, Medicaid, right handedness, K-MMSE, hypertension, diabetes, dyslipidemia, previous stroke, ischemic heart disease, current smoker, atrial fibrillation, cancer, body mass index, prestroke mRS > 2, right hemispheric stroke, NIHSS at admission, intravenous tissue plasminogen activator, intra-arterial thrombectomy, and END. We created a propensity score-matched cohort by matching each patient with PHQ-9 positive to a patient with PHQ-negative (a 1:2 match). A nearest-neighbor-matching algorithm was used to match the participants based on a difference of 0.1 multiplied by the standard deviation for linearly transformed propensity scores (logit-transformation). Covariate balance was evaluated by using standardized mean differences, with a standardized difference of <0.2 for a given covariate being considered to have achieved covariate balance. In the matched cohort, patient characteristics between both groups were compared using linear mixed model or conditional logistic regression. Multivariable conditional logistic regression analyses were performed to evaluate whether PHQ-9 was associated with poor functional outcome at 3 months after propensity score matching. Analyses were adjusted for length of hospital stay, NIHSS at discharge, mRS at discharge >2, home discharge, and antidepressant except bupropion.
During the study period, a total of 1339 consecutive patients with acute ischemic stroke or TIA were enrolled in the prospective registry, and 309 patients without PHQ-9 scores were excluded (poor systemic conditions [N = 33], communication difficulties [N = 205], poor cooperation [N = 31], or other causes [N = 40]). Seventeen patients had missing mRS scores at 3 months. Among 1,013 patients with PHQ-9 scores and proper follow-up data, we further excluded 21 patients with known depression and/or prior antidepressant use, 114 patients with chronic alcoholism, 87 patients with dementia, and 6 patients without K-MMSE results. Finally, 775 patients were included in this study (Figure 1). The median time interval between admission to PHQ-9 screening and stroke onset to PHQ-9 screening was 1 day (Inter-quartile range [IQR] 0–2.0 days) and 45 h (IQR 18–76 h), respectively.
Of the 775 patients enrolled (median age [IQR], 68.0 [59.0–77.0] years; and 316 [40.8%] women), 111 (14.3%) patients were PHQ-9 positive. The baseline characteristics according to the PHQ-9 are shown in Table 1. Both antidepressant and antidepressant except bupropion were more commonly prescribed in the PHQ-positive group. There was no difference in the bupropion description at discharge. Consultation to a psychiatrist during hospitalization was performed in 8 (1.0%) patients (2 [1.8%] in PHQ-9 positive vs. 6 [0.9%] in PHQ-9 negative group, P = 0.719).
After 1:2 PSM, a total of 300 patients were included in the matched cohort (100 for PHQ-9 positive and 200 for PHQ-9 negative). The matched cohort was well balanced in terms of standardized differences (Supplementary Table 1), and observed covariates according to PHQ-positive (Table 1).
Patients with PHQ-9 positivity were younger, had a history of cancer more commonly, a longer length of hospital stay, and a higher NIHSS score at admission (Table 1). The median time interval between END and PHQ-9 screening was 1 day (IQR 0–2.0 days).
In univariable logistic regression analyses (Table 2), a history of cancer or END was associated with PHQ-positivity. In the multivariable logistic regression analysis (Table 2), younger age, history of cancer, and END were independently associated with PHQ-9 positivity.
Patients with PHQ-9 positivity more frequently had poor functional outcomes (mRS score of >2) at 3 months than those who were PHQ-9 negative (33.3 vs. 16.4%; P < 0.001; Figure 2). When we additionally trichotomized PHQ-9 results (<5, negative; 5–9, mild; >9, moderate to severe), patients with more severe PHQ-9 results had worse functional outcomes at 3 months (P = 0.001; Supplementary Figure 1).
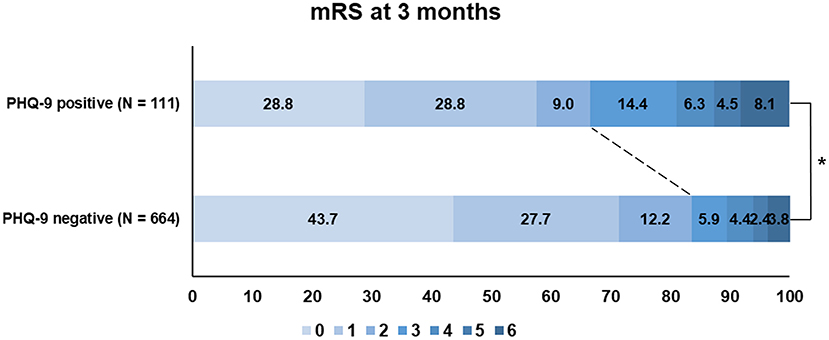
Figure 2. Functional outcome (modified Rankin Scale) at 3 months according to the PHQ-9 status. PHQ-9, Patient Health Questionnaire-9; mRS, modified Rankin Scale.
In the univariable logistic regression analysis (Supplementary Table 2), PHQ-9 positivity was associated with poor functional outcomes at 3 months (Odds ratio [OR] 2.55, 95% confidence interval [CI] 1.63–3.97, P < 0.001). In the multivariable logistic regression analysis after adjustment for age, sex, and variables that showed P < 0.05 in univariable analyses (Table 3), PHQ-9 positivity showed a 2.6-fold higher risk of poor functional outcome at 3 months (OR 2.58; 95% CI 1.26–5.28; P = 0.009; Table 3). When the PHQ-9 score was computed as a continuous variable in the same multivariable model, every increase by one point led to a 1.2-fold higher risk of poor functional outcome at 3 months in the multivariable analysis (OR 1.15; 95% CI 1.06–1.26; P = 0.002). The PSM analysis also showed PHQ-9 positivity showed 3.6-fold higher risk of poor functional outcome at 3 months (OR 6.72; 95% CI 1.82–24.78, P = 0.004; Table 3). When PHQ-9 was used as a continuous variable, every 1-point increase led to 1.3-fold higher risk of poor functional outcome at 3 months.
We divided our study population based on the median age of 68 years, sex, and variables, which were significantly associated with the occurrence of PHQ-9 positivity, the presence of cancer, and END. Significant associations between PHQ-9 positivity and poor functional outcome at 3 months were consistently observed in each subgroup (Figure 3).
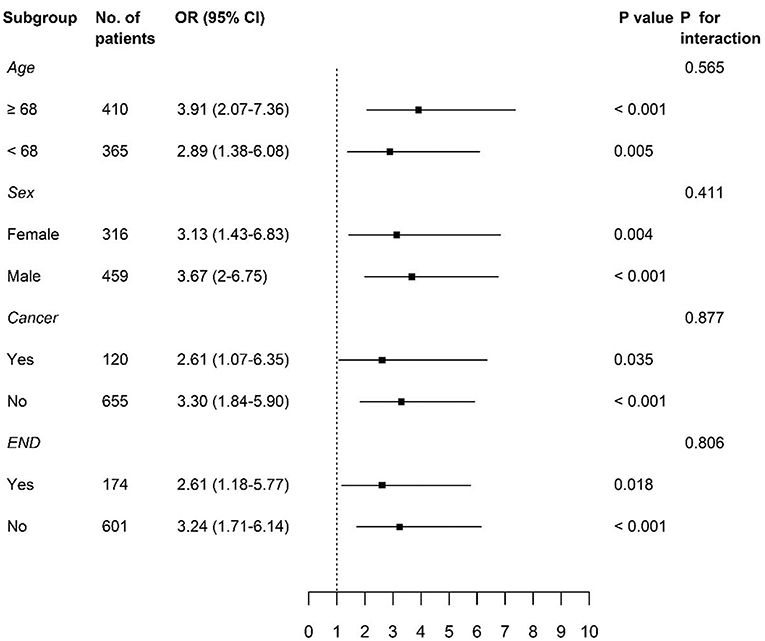
Figure 3. Subgroup analyses of adjusted odds ratios of PHQ-9 positive group for poor functional outcome at 3 months. PHQ-9, Patient Health Questionnaire-9; OR, odds ratio; CI, confidence interval; END, early neurological deterioration.
In this study, we showed that approximately 14% of patients with acute ischemic stroke or TIA had early PSD based on PHQ-9 assessment. History of cancer and occurrence of END were independent factors for early PSD. Furthermore, early PSD, evaluated using the PHQ-9 during hospitalization for acute ischemic stroke, was independently associated with poor functional outcomes.
PSD was independently associated with short-term functional outcomes at 3 months in this study. This is compatible with previous studies, which showed the association of PSD with worse functional outcomes after stroke (2, 3, 21). Although the pathophysiology of PSD is poorly understood, PSD might limit participation in rehabilitation, directly decrease physical, social, and cognitive function, and perhaps affect the biological process of neuroplasticity (2, 22). Some may argue that early PSD simply reflects a psychological response to a new disability, rather than true PSD (23). A previous study even showed that the timing of assessments for PSD could affect the prevalence of PSD (2). However, in this study, early PSD was independently associated with functional outcome at 3 months, even after adjustments for a diverse range of variables regarding neurologic deficit: NIHSS at admission and at discharge, END, and mRS at discharge. END, which was independently associated with early PSD, was not associated with functional outcome at 3 months when entered into the multivariable model with PHQ-9 positivity. PHQ-9 was consistently associated with poor functional outcomes at 3 months in the subgroup analyses. The importance of early PSD is also highlighted as a requirement for Joint Commission-sponsored Comprehensive Stroke Center certification in the United States (5). Furthermore, a previous study showed that both early PSD (in 2 weeks) and chronic PSD (at 1 year) were associated with worse long-term cardio-cerebrovascular outcomes during 12-year follow-up (21).
In our study, history of cancer and END were independently associated with early PSD. Younger age was not associated with early PSD in univariable analysis, but associated with early PSD in multivariable analysis. Although there are conflicts in the association between age and PSD (2, 3), younger age was suggested to be associated with early PSD (11, 24). Interestingly, our study showed that a history of cancer was associated with PSD. Depression is a common complication in cancer survivors, affecting up to one-fourth of cancer patients (25). Furthermore, depression is even suggested to be associated with increased incidence of cancer and mortality (26). With the rise in the survival rate of patients with cancer, the number of patients experiencing stroke is also escalating and warrants better care for these patients (27). Interestingly, early PSD was associated with END, not NIHSS score at admission. In previous studies, the association between NIHSS score at admission and early PSD was usually investigated; hence, the role of END in early PSD was not known (11, 12, 24). This might suggest that patients with worsened neurologic deficit during admission have a higher chance of having depression and a need for screening of the development or management for PSD. We also investigated a diverse range of factors, including education level, MMSE socioeconomic status (Medicaid), right handedness, and right hemispheric lesion, which have been suggested to be associated with PSD (2, 3). However, they were not associated with early PSD in this study.
This study had several strengths. It was conducted based on registry data, which included extensive and thorough data. Pre-morbid depression status, antidepressant use on admission, and antidepressant use at discharge, including the type of drug, were also evaluated. All admitted patients were systematically screened for PHQ-9 (5). If PHQ-9 could not be performed, the classified reason was recorded in a consecutive manner.
This study has several limitations. First, PHQ-9 positivity indicates not only early PSD, but a depressive tendency as well, which was maintained before stroke, as detected based on the frequency in the preceding 2 weeks of each question in the PHQ-9. Hence, in a previous study, the frequency since hospitalization of each question in the PHQ-9 was evaluated, although it had not been previously validated in the stroke population (11). Second, there may still be the argument regarding the clinical meaning of early PSD defined using the PHQ-9 in the acute period (23). Early screening of depression in hospitalized patients for stroke was investigated in several small studies, especially using the PHQ-9 (11–13), which, being a self-administered questionnaire was easily applicable; PHQ-9 was also used in this study. Future studies based on depression screening or confirmative examination in both acute and chronic periods of stroke might further increase our understanding of early PSD. Third, although it was suggested that there was association between medical adherence and depression, the association between medical adherence and early PSD could not be investigated in this study (28). Fourth, the results of this study should be interpreted carefully, because the study population had relatively mild stroke symptom and were frequently treated with bupropion for anti-smoking purpose.
Early PSD was associated with worse short-term functional outcomes. Considering the high prevalence of PSD, its early identification could offer opportunities for further questioning and exploration of symptoms, as well as interventions.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Institutional Review Board of Severance Hospital, Yonsei University Health System. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
MB: conception and design, collection, analysis, interpretation of data, and drafting of the manuscript. HyuL, IL, JH, HN, and HyeL: analysis and interpretation of the data and manuscript preparation. YK: conception and design, analysis and interpretation of data, drafting of the manuscript, and revising it critically for important intellectual content. All authors contributed to the article and approved the submitted version.
This study was supported by a faculty research grant of Yonsei University College of Medicine (6-2020-0202 and 6-2019-0191) and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HC19C0028).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.950045/full#supplementary-material
1. Hackett ML, Pickles K. Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int J Stroke. (2014) 9:1017–25. doi: 10.1111/ijs.12357
2. Towfighi A, Ovbiagele B, El Husseini N, Hackett ML, Jorge RE, Kissela BM, et al. Poststroke depression: a scientific statement for healthcare professionals from the american heart association/American stroke association. Stroke. (2017) 48:e30–43. doi: 10.1161/STR.0000000000000113
3. Kutlubaev MA, Hackett ML. Part II predictors of depression after stroke and impact of depression on stroke outcome: an updated systematic review of observational studies. Int J Stroke. (2014) 9:1026–36. doi: 10.1111/ijs.12356
4. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the american heart association/American stroke association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
5. Joint Commission. Comprehensive certification manual for disease-specific care including advanced programs for disease-specific care certification. (2015).
6. Kang HJ, Stewart R, Kim JM, Jang JE, Kim SY, Bae KY, et al. Comparative validity of depression assessment scales for screening poststroke depression. J Affect Disord. (2013) 147:186–91. doi: 10.1016/j.jad.2012.10.035
7. Williams LS, Brizendine EJ, Plue L, Bakas T, Tu W, Hendrie H, et al. Performance of the PHQ-9 as a screening tool for depression after stroke. Stroke. (2005) 36:635–8. doi: 10.1161/01.STR.0000155688.18207.33
8. Meader N, Moe-Byrne T, Llewellyn A, Mitchell AJ. Screening for poststroke major depression: a meta-analysis of diagnostic validity studies. J Neurol Neurosurg Psychiatry. (2014) 85:198. doi: 10.1136/jnnp-2012-304194
9. Hankey GJ, Hackett ML, Almeida OP, Flicker L, Mead GE, Dennis MS, et al. Safety and efficacy of fluoxetine on functional outcome after acute stroke (AFFINITY): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. (2020) 19:651–60. doi: 10.1016/S1474-4422(20)30207-6
10. Effects Effects of fluoxetine on functional outcomes after acute stroke (FOCUS): a pragmatic double-blind randomised controlled trial. Lancet. (2019) 393:265–74. doi: 10.1016/s0140-6736(18)32823-x
11. Karamchandani RR, Vahidy F, Bajgur S, Vu KYT, Choi HA, Hamilton RK, et al. Early depression screening is feasible in hospitalized stroke patients. PloS ONE. (2015) 10:e0128246. doi: 10.1371/journal.pone.0128246
12. Shankar L, Smith N, Uchino K, Thompson NR, Pozuelo L, Katzan IL. Evaluation of the patient health questionnaire-2 as a screening tool for depression during the acute Stroke admission. J Stroke Cerebrovasc Dis. (2017) 26:2519–26. doi: 10.1016/j.jstrokecerebrovasdis.2017.05.044
13. Wilkins SS, Akhtar N, Salam A, Bourke P, Joseph S, Santos M, et al. Acute post stroke depression at a primary stroke center in the middle east. PloS ONE. (2018) 13:e0208708. doi: 10.1371/journal.pone.0208708
14. Baik M, Nam HS, Heo JH, Park HJ, Kim BK, Park JY, et al. Advanced liver fibrosis predicts unfavorable long-term prognosis in first-ever ischemic stroke or transient ischemic attack. Cerebrovasc Dis. (2020) 49:474–80. doi: 10.1159/000510436
15. Kang, Y. W., Na, D.L., Hahn S.H. A validity study on the korean mini-mental state examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. (1997) 15:300–8
16. Kim YD, Song D, Kim EH, Lee KJ, Lee HS, Nam CM, et al. Long-term mortality according to the characteristics of early neurological deterioration in ischemic stroke patients. Yonsei Med J. (2014) 55:669–75. doi: 10.3349/ymj.2014.55.3.669
17. Han C, Jo SA, Kwak JH, Pae CU, Steffens D, Jo I, et al. Validation of the patient health questionnaire-9 Korean version in the elderly population: the Ansan Geriatric study. Compr Psychiatry. (2008) 49:218–23. doi: 10.1016/j.comppsych.2007.08.006
18. Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluat. Mental Disorders Patient Health Questionnaire JAMA. (1999) 282:1737–44. doi: 10.1001/jama.282.18.1737
19. Kim JS, Lee EJ, Chang DI, Park JH, Ahn SH, Cha JK, et al. Efficacy of early administration of escitalopram on depressive and emotional symptoms and neurological dysfunction after stroke: a multicentre, double-blind, randomised, placebo-controlled study. Lancet Psychiatry. (2017) 4:33–41. doi: 10.1016/S2215-0366(16)30417-5
20. Richmond R, Zwar N. Review of bupropion for smoking cessation. Drug Alcohol Rev. (2003) 22:203–20. doi: 10.1080/09595230100100642
21. Lee E-H, Kim J-W, Kang H-J, Kim S-W, Shin I-S, Kim J-T, et al. Effects of acute and chronic depression on 12-year long-term outcomes after stroke. Int J Geriatr Psychiatry. (2021) 36:1759–66. doi: 10.1002/gps.5597
22. Parikh RM, Robinson RG, Lipsey JR, Starkstein SE, Fedoroff JP, Price TR. The impact of poststroke depression on recovery in activities of daily living over a 2-year follow-up. Arch Neurol. (1990) 47:785–9. doi: 10.1001/archneur.1990.00530070083014
23. Sibon I, Lassalle-Lagadec S, Renou P, Swendsen J. Evolution of depression symptoms following stroke: a prospective study using computerized ambulatory monitoring. Cerebrovasc Dis. (2012) 33:280–5. doi: 10.1159/000334663
24. Shi Y, Yang D, Zeng Y, Wu W. Risk factors for post-stroke depression: a meta-analysis. Front Aging Neurosci. (2017) 9:218. doi: 10.3389/fnagi.2017.00218
25. Krebber AMH, Buffart LM, Kleijn G, Riepma IC, de Bree R, Leemans CR, et al. Prevalence of depression in cancer patients: a meta-analysis of diagnostic interviews and self-report instruments. Psychooncology. (2014) 23:121–30. doi: 10.1002/pon.3409
26. Wang Y-H, Li J-Q, Shi J-F, Que J-Y, Liu J-J, Lappin JM, et al. Depression and anxiety in relation to cancer incidence and mortality: a systematic review and meta-analysis of cohort studies. Mol Psychiatry. (2020) 25:1487–99. doi: 10.1038/s41380-019-0595-x
27. Yoo J, Kim YD, Park H, Kim BM, Bang OY, Kim HC, et al. Immediate and long-term outcomes of reperfusion therapy in patients with cancer. Stroke. (2021) 52:2026–34. doi: 10.1161/STROKEAHA.120.032380
Keywords: ischemic stroke, transient ischemic attack, post-stroke depression, Patient Health Questionnaire-9, functional outcome
Citation: Baik M, Lee H, Lee IH, Heo J, Nam HS, Lee HS and Kim YD (2022) Early depression screening and short-term functional outcome in hospitalized patients for acute ischemic stroke. Front. Neurol. 13:950045. doi: 10.3389/fneur.2022.950045
Received: 22 May 2022; Accepted: 18 July 2022;
Published: 05 August 2022.
Edited by:
Zilong Hao, Sichuan University, ChinaCopyright © 2022 Baik, Lee, Lee, Heo, Nam, Lee and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Young Dae Kim, bmV1cm8wNUB5dWhzLmFj
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.