
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 15 September 2022
Sec. Multiple Sclerosis and Neuroimmunology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.949843
Objective: Anti-γ-aminobutyric acid-B receptor (GABABR) encephalitis is a rare type of autoimmune encephalitis. There are only a few, small, published studies regarding prognosis, so prediction of prognosis is of limited accuracy. We identified 37 cases of anti-GABABR encephalitis in China. Here, we present these patients' clinical characteristics and long-term outcomes.
Methods: We collected and retrospectively analyzed the clinical data of 37 patients with anti-GABABR encephalitis from Beijing Fengtai You'anmen Hospital.
Results: The study cohort comprised 37 patients of anti-GABABR encephalitis of median age 61 years (range: 11–77), 28 of whom were male. The main clinical manifestations were epilepsy (91.9%, 34/37), psychiatric disorders (94.6%, 35/37) and cognitive impairment (97.3%, 36/37). Tumors were identified in 18 (48.6%) patients. First-line immunotherapy was administered to 34 patients, 31 of whom (90.6%) responded favorably. During a median follow-up of 18 months (range: 1–72 months), 21 patients had good outcomes [Modified Ranking Scale (mRS ≤2)], 16 (43.2%) died (mRS 6), and 7 (18.9%) relapsed. Age (P = 0.005), disturbance of consciousness (P = 0.018), admission to the Neurology Intensive Care Unit (P = 0.003), mechanical ventilation (P = 0.009), more numerous clinical manifestations (P = 0.008), comorbid malignancy (P = 0.008), multiple anti-neuronal antibodies (P = 0.029), and hyponatremia (P = 0.023) differed significantly between patients with good outcomes (mRS 0–2) and those with poor outcomes (mRS 3–6).
Conclusion: Men aged 50–70 years accounted for most of the patients with anti-GABABR encephalitis in our case series. The main clinical manifestations were epilepsy and neuropsychiatric dysfunction. The participants often had concomitant lung cancer, particularly small-cell lung cancer. Patients with lung tumors and/or serious manifestations usually had a poor prognosis with high mortality. Early identification and treatment of tumors improved the poor prognosis to some extent.
Anti-γ-aminobutyric acid-B receptor (GABABR) encephalitis is caused by specific autoimmune antibodies against GABABR on the surfaces of neurons, preventing γ-aminobutyric acid (GABA) from exerting neuroinhibitory effects (1). The main clinical manifestation is evidence of limbic encephalitis such as seizures, memory loss, mental behavioral abnormalities, and disturbance of consciousness (1, 2). Since anti-GABABR encephalitis was first reported in 2010 by Lancaster et al. (2), various cases have been reported in eastern and western countries. Because this type of encephalitis is not commonly encountered in clinical practice (2, 3), few studies have been published on it (1–16). The current study investigated the clinical characteristics, autoantibody profiles, responses to treatment, and prognosis in 37 patients with anti-GABABR encephalitis.
The cohort of this retrospective study comprised consecutive patients diagnosed with anti-GABABR encephalitis [including 21 patients previously reported (17)] at the Department of Neurology of Beijing Fengtai You'anmen Hospital between October 2012 and July 2022. Inclusion criteria were as follows: (i) diagnoses made in accordance with the criteria for autoimmune encephalitis published by Graus et al. in 2016 (18), namely positive anti-GABAB receptor antibody, at least one neurological or psychiatric symptom, at least one abnormal finding on auxiliary examination (examination of cerebrospinal fluid [CSF], neuroimaging or electrophysiological examination), or associated tumors; and (ii) follow-up for more than 6 months. Exclusion criteria were as follows: (i) anti-intracellular antigen-antibody encephalitis and other types of encephalitis of unknown origin; and (ii) follow-up for <6 months or lost to follow-up. This study was approved by the Local Ethics Committee.
The clinical data collected included patient characteristics, clinical manifestations, results of laboratory tests (serum and CSF), findings on electroencephalogram (EEG), brain computed tomography (CT)/magnetic resonance imaging (MRI) and chest CT, treatment protocols, response to treatment, and follow-up data. Anti-neuronal antibodies detected in serum and/or CSF samples by indirect immunofluorescence assay (Euroimmun AG, Luebeck, Germany) included anti-GABAB receptor (neuronal surface antigen) and anti-Yo, -Hu, -Ri, -CV2/CRMP5, -amphiphysin, -Ma2/Ta and -GAD 65 antibodies (intracellular antigens).
The treatment protocols were mainly immunotherapy and supportive treatment such as anti-epilepsy, anti-infection, and tranquilizing medications. First-line immunotherapy included glucocorticoids and intravenous immunoglobulin (IVIg). Second-line immunotherapy, including mycophenolate mofetil, cyclophosphamide, or rituximab, was often used in patients who failed first-line immunotherapy or recurrence. The patients with tumors received chemotherapy alone or in combination with radiotherapy and/or surgery. Surgery could be performed on those patients with Limited-stage SCLC patients (T1-2, N0). All patients were followed up every 3 months at clinic visits or by telephone interviews. The following factors were evaluated during follow-up: (i) changes in symptoms; (ii) relapse, death, and cause of death; and (iii) modified Rankin Scale (mRS) scores (19). These factors were evaluated on admission, one week after immunotherapy, and every 3 months after the end of immunotherapy. The follow-up period was defined as the interval from discharge to the patient's death or last follow-up. Patients were divided into two groups (favorable vs. poor outcomes) on the basis of their mRS scores at the last follow-up. Good outcomes were defined as mRS scores of 0–2 and poor outcomes as mRS scores 3–6. Relapse was defined as recurrence of symptoms after disease improvement or stabilization for more than 2 months or exacerbation of symptoms (mRS score increased by more than one point).
Statistical analysis was performed using SPSS version 23.0 (IBM Corp., Armonk, NY, USA). Descriptive statistics were used to analyze clinical data; these are expressed as median or mean values and percentages. Continuous variables that were normally distributed are expressed as mean ± standard deviation and were compared using Student's t-test.
Continuous variables that were not normally distributed are expressed as the median (m) and were compared using the Mann–Whitney U-test. Fisher's exact test was used to compare categorical variables. Differences were considered statistically significant when P < 0.05.
We identified 37 patients who had been diagnosed with anti-GABABR encephalitis. Their median age was 61 years (range: 11–77) and 28 were male. Nine patients (9/37) had prodromal symptoms within the 2 weeks before onset (three had headaches, two colds, one otitis media, two fever, and one fatigue). Additionally, the initial manifestation was numbness and weakness in the left upper limb in one patient, mental and behavioral disorder in two, fever in one, speech impairment in two, and intellectual impairment in three. The initial manifestation in the remaining 28 patients was seizures.
Clinical manifestations included epilepsy, cognitive impairment, psycho-behavioral disturbances, language disorder/aphasia, sleep disorders, disturbance of consciousness, involuntary movements, autonomic nerve dysfunction, and ataxia. The patients' relevant clinical characteristics are summarized in Table 1 and Figure 1. The median interval between first manifestation and diagnosis was 22 days (range: 5–435 days). Lung cancer was diagnosed by pathological examination of biopsy specimens in 18 cases (48.6%); these cancers comprised 17 small-cell lung cancers (SCLC) and one large-cell neuroendocrine carcinoma of the lung. The median interval between first clinical manifestation and diagnosis of a tumor was 4 months (range: −3 to 20 months).
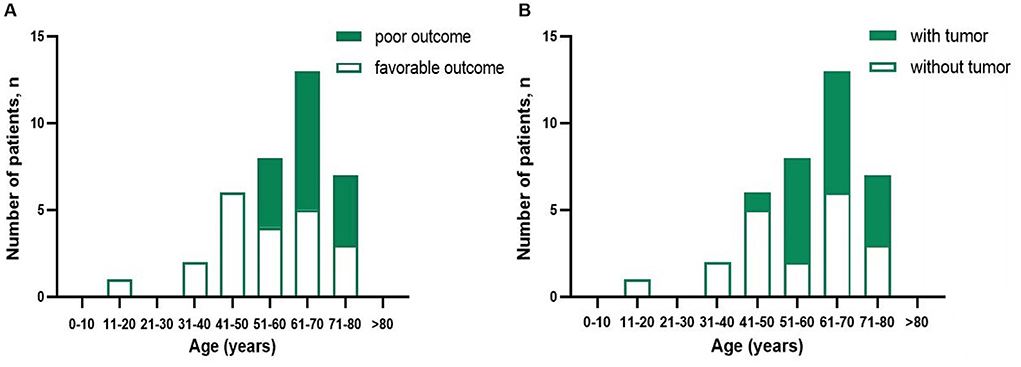
Figure 1. Distribution of prognosis patients with anti-GABABR encephalitis (A) and presence of tumor (B) according to age.
Thirty-seven patients were positive for anti-GABABR antibodies in CSF and serum. Seven patients (18.9%, 7/37) were positive for additional antibodies, including anti-Hu, SOX1, collapsin response mediator protein 5 (CV2), and anti-glutamic acid decarboxylase 65-kD isoform antibody. Anti-herpes simplex virus type 1 (HSV-1) IgG antibody (+) was detected in the CSF in two patients.
Of the 31 patients who underwent EEG examination, 26 showed abnormalities (83.9%), 19 of which were moderate to severe (61.3%). Most of these patients had focal or diffuse slow waves, mostly in the frontotemporal lobe. Additionally, abnormal epileptic discharges were identified in six patients (19.4%).
All patients underwent a cranial MRI. In 26 patients (70.3%), this revealed high signals on T2-weighted imaging (T2WI) and fluid-attenuated inversion recovery sequences that were mainly located in the medial temporal lobe (including hippocampus; 54.1%), followed by the frontoparietal lobe, paraventricular region, occipital lobe, corpus callosum, and semioval center. Pulmonary CT of 33 of the patients (89.2%) was abnormal, findings including pulmonary infection, pulmonary space-occupying lesions, hilar mediastinal lymph node enlargement, pleural effusion, atelectasis, and pulmonary embolism. The results of these auxiliary investigations are shown in Table 1.
After hospitalization, first-line immunotherapy was administered during the acute phase to 34 patients (91.9%), 26 patients (70%) treated with a combination of IVIg and steroids, 8 patients (21.6%) received steroids or IVIg therapy alone, 5 patients (13.5%) received second-line immunotherapy and 3 patients (8.1%) were not given immunotherapy. The median interval between symptoms onset and immunotherapy was 24.5 days (range: 8 days–16 months). Eleven of the 18 patients with lung cancer received chemotherapy alone or in combination with radiotherapy; one patient had attempted surgical resection and postoperative radiotherapy.
During the first 12 months, twenty patients (20/35, 57.1%) had neurological improvement and reached an mRS 0–2 points; five patients (5/35, 14.3%) reached an mRS 3–5 points; ten patients (10/35, 28.6%) deteriorated and eventually died (mRS 6 points). At the last follow-up, twenty-one patients (56.8%) had favorable outcomes (mRS ≤ 2), including six with lung cancer whose median follow-up was 36.5 months (range: 6–72), the main manifestations being a mild cognitive impairment, neuropsychiatric abnormalities, and epilepsy. Sixteen patients had a poor prognosis (mRS 6), including twelve with neoplastic anti-GABABR encephalitis (32.4%), whose median interval between initial manifestations and death was 14.5 months (range: 3–24 months). Neoplastic complications were the leading cause of death; the remaining four patients (10.8%) presented with severe neurological symptoms and multiple organ damage or failure; although they did not diagnose with tumors, two died of pulmonary infection, one died of multiple organ dysfunction syndromes, one was unclear. The median interval between initial manifestations and death was 5 months (2–12 months). The mRS scores in our series before immunotherapy, at the 12-month follow-up, and the last follow-up are shown in Figure 2.
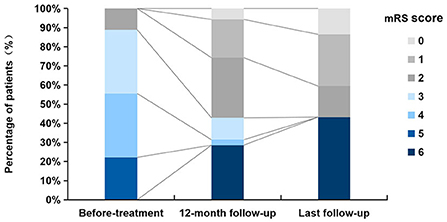
Figure 2. Distribution of mRS scores before treatment, at 12-month follow-up, and at last follow-up.
Recurrence occurred in seven patients (18.9%), all of whom developed frequent and uncontrollable epilepsy, two of them dying of multiple organ dysfunction syndromes secondary to status epilepticus. The median time to recurrence was 6 months (range: 2–32).
Possible prognostic factors in patients with anti-GABABR encephalitis were analyzed. Characteristics of patients with favorable vs. poor outcomes are summarized in Table 1. We found that the poor outcome group was significantly older than the good outcome group (P = 0.005). There were also significant differences between these two groups in rates of disturbance of consciousness (P = 0.018), admission to the intensive care unit (ICU; P = 0.003), mechanical ventilation (P = 0.009), presence of a tumor (P = 0.008), and the number of clinical manifestations (P = 0.008). Additionally, multiple anti-neuronal antibodies (P = 0.029) and hyponatremia (P = 0.023) were associated with a poor prognosis in our study cohort.
We further conducted a survival analysis of patients with (48.6%, 18/37) and without tumor (51.4%, 19/37) to assess the impact of tumors on prognosis (Figure 3). Overall survival (OS) was defined as the interval between diagnosis of anti-GABABR encephalitis and death or last follow-up. The OS rate of patients with tumors was significantly lower than that of patients without them (log-rank P = 0.0163). The estimated median survival time of patients with tumors was 14.5 months and without tumors could not be determined.
The study cohort comprised 37 patients with anti-GABABR encephalitis. Some important characteristics of the current series are as follows: (i) To the best of our knowledge, this is the largest reported, single-center, retrospective, cohort study of anti-GABABR encephalitis and has the greatest age span and longest follow-up of any study in China (3–6, 8, 11–16). (ii) In this group, two patients had anti-GABABR encephalitis secondary to HSV-1 infection. (iii) Multiple anti-neuronal antibodies were detected in seven patients in our series. (iv) We also found that hyponatremia as a prognostic factor in anti-GABABR encephalitis.
A systematic review revealed an average onset age of anti-GABABR encephalitis of 55.2 years (range: 18–76) in Asian patients and that 62.3% of them are male (16). The average age of onset in the present series was 61 years (range: 11–77) and the male: female ratio 28:9, which is consistent with the ages of onset that have been reported in western countries. However, the percentage of male patients is reportedly higher in those countries (1, 2, 7, 9, 10, 20). As previously reported (2–5, 9), in our series older age (>50 years) was associated with a poor prognosis, this being related to the high incidence of malignant tumors in older persons. The rate of concomitant malignant tumors was 60.7% (17/28) in patients aged over 50 years, whereas it was only 11.1% (1/9) in patients younger than 50 years.
There is characteristically no clear history of infection before the onset of anti-GABABR encephalitis (21). However, there may be prodromal symptoms such as headache and influenza-like symptoms; nine of our study patients had headaches and fever before onset. Additionally, there are some novel findings in our cohort: (i) One patient in this cohort was an 11-year-old boy with congenital “language retardation”; this may have denoted a genetic susceptibility to anti-GABABR encephalitis. Further investigation of this possibility is needed. (ii) Two patients in the present series had sustained trauma within the month before the onset of anti-GABABR encephalitis. Whether trauma is linked with this disease is unknown. (iii) two cases had anti-GABABR encephalitis secondary to HSV-1 encephalitis, indicating that HSV-1 infection of the central nervous system can trigger anti-GABABR encephalitis (22). Sometimes multiple factors contributed to the development of anti-GABABR encephalitis. For example, One case had anti-cytomegalovirus antibody (IgG) and HSV-1 antibody IgG positivity in the CSF and meningeal irritability, and lung cancer was diagnosed 4 months later.
Anti-GABABR encephalitis usually starts with epilepsy. Consistent with this, the most prominent features in the early stage are various types of epileptic seizures and neuropsychiatric symptoms (2–7, 9, 10, 12). The latter mostly manifest as personality changes (anxiety, depression, mania), loss of memory for recent events, behavioral abnormalities, disorders of consciousness, and sleep disorders (1–3, 5–7, 9, 10). When the brain stem, cerebellum, and other regions are involved, patients may present with opsoclonus myoclonus syndrome, ataxia, chorea, and brainstem encephalitis (1, 6, 21, 23). In our series, the clinical manifestations were similar to those reported previously. One patient presented with cerebellar ataxia, which was consistent with the distribution of GABABR in the cerebellum (23). We found that the mental and behavioral abnormalities were milder and less long-lasting in this series than has been reported for patients with anti-NMDAR encephalitis. Respiratory complications are common in patients anti-GABABR encephalitis, more than 2/3 of patients having pulmonary infections (4). Pulmonary infection and respiratory failure are reportedly predictors of poor prognosis (3, 5, 24). The incidence of pulmonary infection in the present study was 78.4%; however, we did not find a significant relationship with prognosis. In the present series, impairment of consciousness occurred in 21 patients (56.8%). As a consequence of status epilepticus, pulmonary infection, and sedative drugs, seventeen patients (45.9%) required admission to ICU. The ICU occupancy rate in our group was higher than the 22.2% (4/18) (6) and 10.7% (3/28) (4) previously reported in China, our rate of ICU admission being closer to the 64% reported abroad (10). Disturbance of consciousness, admission to ICU and mechanical ventilation may be important indicators of poor prognosis. Furthermore, in our series patients with poor outcomes had more numerous clinical manifestations than those with good outcomes.
More than half of patients with anti-GABABR encephalitis have concurrent tumors, most of which are SCLC (2–7, 9, 12–15), other associated tumors include thymoma (25), esophageal cancer (1), and melanoma (23), and so on. While only 40/114 (36%) of patients in an Asian series had tumors (13, 16). Eighteen of our patients (48.6%) were diagnosed with lung cancer, comprising one large-cell neuroendocrine carcinoma of the lung and 17 SCLC. The low prevalence of tumors in some Chinese studies may be attributable to more youthful enrolled cases, small series, insufficient screening for tumors, and relatively short follow-up times (12).
Consistent with previous studies (4, 6, 7), we found strong evidence for tumors being critical prognostic factors in patients with anti-GABABR encephalitis. Such tumors are usually identified within 6 months of the onset of that encephalitis (1, 2). One patient in our cohort was diagnosed as having lung cancer 3 months before the onset of encephalitis-related symptoms, the remaining tumors being diagnosed after the onset: 14 within 6 months, two after 12 months, and one after 20 months. Therefore, all patients with anti-GABABR encephalitis should be followed up and encouraged to undergo regular screening for tumors, especially lung cancer. It is recommended that such screening be performed at 3-monthly intervals for at least 2 years. SCLC is often associated with paraneoplastic neurological syndromes, one of which is inappropriate secretion of anti-diuretic hormone, leading to intractable hyponatremia (26). Anti-GABABR encephalitis patients with tumor were more likely to develop hyponatremia (13). In the present study, significantly more patients with (50.0%, 9/18) than without (21.1%, 4/19) tumors developed hyponatremia. Our finding suggests hyponatremia maybe one presentation of paraneoplastic syndrome and was also associated with poor prognosis. However, because ours was a small study, further studies are needed to confirm this finding.
Various anti-neuronal antibodies in addition to anti-GABABR are reportedly found in 7%−40% of patients with anti-GABABR encephalitis (5, 6). It has been found that the anti-Hu antibody is the commonest additional antibody, this antibody being strongly associated with SCLC (9, 21, 23). Anti-SOX1 (27) and anti-CV2 (28) antibodies are also associated with SCLC. In our study cohort, 7/37 (18.9%) patients had one or more above antibodies. Six patients who were positive for additional anti-neuronal antibodies had concomitant lung cancer. The malignant tumor was not detected in the last patient during six-month follow-up. The presence of additional anti-neuronal antibodies predicts a poor prognosis in patients with anti-GABABR encephalitis. One patient in our cohort had three different anti-neuronal antibodies, namely anti-GABABR, anti-GAD65, and anti-CV2 antibodies; this combination of antibodies is rare. The prevalence of anti-CV2 antibody in patients with autoimmune encephalitis is reportedly only 0.7/100,000 (28). This patient was characterized by SCLC, intractable epilepsy, refractory hyponatremia and cognitive impairment.
Studies have shown that neurological deficits improve in about 90% of patients with anti-GABABR encephalitis receiving immunotherapy: indeed, in 50% of them neuropsychiatric manifestations resolve completely (25). Most patients in this group who received first-line immunotherapy responded well to the treatment, regardless of whether or not they had a tumor. The choice of immunotherapeutic agents mainly considers the severity of the disease, the patient's economic situation, and the tolerance of steroids. In this group, three patients gave up immunotherapy due to economic constraints; the first one was critical and deteriorated rapidly to death; the second one died of unclear cause within 14 months with the likelihood of an underlying tumor; the neurological status of the last one improved after receiving chemotherapy, while eventually died of neoplastic complications within 23 months. Our results were consistent with the previous findings that there was no significant correlation between the time of initial immunotherapy and the prognosis (5, 13). It was not rare to be misdiagnosed as epilepsy, viral encephalitis, and psychosis for patients, partly due to autoimmune encephalitis (AE)-related antibodies detection having not been widely popularized; in addition, some patients were easy to be ignored as their symptoms were mild or atypical. In the current study, six patients with malignant tumors had favorable prognoses, all of whom were diagnosed with limited-stage SCLC and responded well to the tumor treatment. Early diagnosis and active treatment of tumors may be vital to improving the prognosis of anti-GABABR encephalitis (10, 13). About 10.1%–21.0% of patients relapsed (4, 5, 24). Seven patients in our group had a relapse, five with malignant tumors, which indicated that concurrent malignancies might be a risk factor for disease recurrence. The possibility of tumor recurrence or potential tumor during the initial episode should be considered in patients with a relapse. However, we did not identify significant relationships between recurrence and tumor or prognosis.
The prognosis of patients with anti-GABABR encephalitis varies greatly, mainly depending on whether there is an associated tumor (4, 6). In this study, most patients without tumors (15/19) had good outcomes with no obvious or mild sequelae, whereas most of the patients with tumors (12/18) had serious manifestations, a poor prognosis, and high mortality. The mortality of our cohort (51.4%) was slightly higher than the 9.1%−32.1% previously reported in China (4, 6, 8, 11, 12), and closer to that reported abroad (2, 7, 10). This discrepancy may at least in part be attributable to the longer duration of follow-up in our study. During the 1-year follow-up, the patients with non-neoplastic anti-GABABR encephalitis reached a relatively stable stage, while some neoplastic patients still died in the second year. Consistent with previous reports (4), the most prolonged interval from initial symptoms to death was 24 months indicating the disease seems to reach a plateau two years later. We found that patients older than 50 years with intractable hyponatremia and additional anti-neuronal antibodies constituted a high-risk subgroup of patients of anti-GABABR encephalitis with underlying malignant tumors. We also found that patients with serious manifestations of anti-GABABR encephalitis usually had a poor prognosis. These patients usually had multiple clinical manifestations, concurrent malignancies, disturbance of consciousness, admission to NICU, and mechanical ventilation. Additionally, some patients with anti-GABABR encephalitis secondary to HSV-1 encephalitis can occur severe and persistent brain damage and even multiple organ dysfunction syndrome, which can lead to poor prognosis. So far, to our knowledge no researchers have reported a link between HSV-1 infection and a poor prognosis of anti-GABABR encephalitis.
Our study had several limitations. Firstly, it was a relatively small, single-center, retrospective study. Secondly, some patients had a short follow-up period or incomplete data. Thirdly, we could not perform multiple regression analysis because of the small sample size. Therefore, large-scale, prospective, multicenter research is needed to further explore our findings.
In conclusion, in our cases series anti-GABABR encephalitis occurred more frequently in men aged 50–70 years. The main clinical manifestations were epilepsy and neuropsychiatric dysfunction. About half the patients had concomitant lung cancer, particularly SCLC. Most patients responded well to first-line immunotherapy. Most patients with anti-GABABR encephalitis and a poor prognosis had an associated complicating malignancy and/or serious manifestations or their disease was secondary to HSV encephalitis. Early identification and treatment of tumors can improve the poor prognosis to some extent. It is recommended that patients with anti-GABABR encephalitis be screened at 2–3 month intervals for at least 2 years.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The study was conducted with the approval of the Ethics Committee of Beijing Fengtai You'anmen Hospital (bjftyamyyll2021-8). Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
XF designed and conducted of the study. YZ and XF drafted and revised the manuscript. YG, JZ, and SY collected and analyzed the clinical data. JL and YZ performed the statistical analyses. LW and XW revised the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (NSFC) (Grant No. 81901225).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Mundiyanapurath S, Jarius S, Probst C, Stöcker W, Wildemann B, Bösel J. GABA-B-receptor antibodies in paraneoplastic brainstem encephalitis. J Neuroimmunol. (2013) 259:88–91. doi: 10.1016/j.jneuroim.2013.04.004
2. Lancaster E, Lai M, Peng X, Hughes E, Constantinescu R, Raizer J, et al. Antibodies to the GABA(B) receptor in limbic encephalitis with seizures: case series and characterization of the antigen. Lancet Neurol. (2010) 9:67–76. doi: 10.1016/S1474-4422(09)70324-2
3. Wen XC, Wang BJ, Wang CJ, Han CL, Guo SG, A. retrospective study of patients with GABABR encephalitis: therapy, disease activity and prognostic factors. Neuropsychiatr Dis Treat. (2021) 17:99–110. doi: 10.2147/NDT.S289942
4. Lin JF Li C, Li AQ, Liu X, Wang R, Chen C, et al. Encephalitis with antibodies against the GABAB receptor: high mortality and risk factors. Front Neurol. (2019) 10:1030. doi: 10.3389/fneur.2019.01030
5. Zhang XY, Lang Y, Sun LC, Zhang WGL, Lin WH, Cui L. Clinical characteristics and prognostic analysis of anti-gamma-aminobutyric acid-B (GABA-B) receptor encephalitis in Northeast China. BMC Neurol. (2020) 20:1. doi: 10.1186/s12883-019-1585-y
6. Guan HZ, Ren HT, Yang XZ, Lu Q, Peng B, Zhu YC, et al. Limbic encephalitis associated with anti-γ-aminobutyric Acid B receptor antibodies: a case series from China. Chin Med J. (2015) 128:3023–8. doi: 10.4103/0366-6999.168989
7. Höftberger R, Titulaer MJ, Sabater L, Dome B, Rozsas A, Hegedus B, et al. Encephalitis and GABAB receptor antibodies: novel findings in a new case series of 20 patients. Neurology. (2013) 81:1500–6. doi: 10.1212/WNL.0b013e3182a9585f
8. Cui JZ, Bu H, He JY, Zhao ZY, Han WX, Gao R, et al. The gamma-aminobutyricacid-B receptor (GABAB) encephalitis: clinical manifestations and response to immunotherapy. Int J Neurosci. (2018) 128:627–33. doi: 10.1080/00207454.2017.1408618
9. Dogan Onugoren M, Deuretzbacher D, Haensch CA, Hagedorn HJ, Halve S, Isenmann S, et al. Limbic encephalitis due to GABAB and AMPA receptor antibodies: a case series. J Neurol Neurosurg Psychiatry. (2015) 86:965–72. doi: 10.1136/jnnp-2014-308814
10. Maureille A, Fenouil T, Joubert B, Picard G, Rogemond V, Pinto AL, et al. Isolated seizures are a common early feature of paraneoplastic anti-GABAB receptor encephalitis. J Neurol. (2019) 266:195–206. doi: 10.1007/s00415-018-9132-0
11. Chen XP, Liu F, Li JM, Xie XQ, Wang Q, Zhou D, et al. Encephalitis with antibodies against the GABAB receptor: seizures as the most common presentation at admission. Neurol Res. (2017) 39:973–80. doi: 10.1080/01616412.2017.1351062
12. Wu H, Wang Y, Wei K, Qiao S, Liu L, Zhang R, et al. Clinical characteristics and elevated ProGRP and positive oligoclonal bands of 13 Chinese cases with anti-GABABR encephalitis. Int J Dev Neurosci. (2021) 81:492–501. doi: 10.1002/jdn.10121
13. Chen W, Wang Y, Guo X, Gao L, Huang Z, Lin Y, et al. A prognostic analysis of the outcomes in patients with anti-γ-aminobutyric acid B receptor encephalitis. Front Immunol. (2022) 13:847494. doi: 10.3389/fimmu.2022.847494
14. Zhao XH, Yang X, Liu XW, Wang SJ. Clinical features and outcomes of Chinese patients with anti-γ-aminobutyric acid B receptor encephalitis. Exp Ther Med. (2020) 20:617–22. doi: 10.3892/etm.2020.8684
15. Zhu F, Shan W, Lv R, Li Z, Wang Q. Clinical characteristics of anti-GABA-B receptor encephalitis. Front Neurol. (2020) 21:403. doi: 10.3389/fneur.2020.00403
16. Ghimire P, Khanal UP, Gajurel BP, Karn R, Ojha R. Anti-LGI1, anti-GABABR, and anti-CASPR2 encephalitides in Asia: a systematic review. Brain Behav. (2020) 10:10. doi: 10.1002/brb3.1793
17. Feng XD Yu SS, Li Y, Zhang YJ, Wang HL. Clinical characteristic of 21 cases series with anti-γ-aminobutyric acid B receptor encephalitis. J Apoplexy and Nervous Diseases. (2020) 37:801–5.
18. Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. (2016) 15:391–404. doi: 10.1016/S1474-4422(15)00401-9
19. Singh TD, Fugate JE, Rabinstein AA. The spectrum of acute encephalitis: causes, management, and predictors of outcome. Neurology. (2015) 84:359–66. doi: 10.1212/WNL.0000000000001190
20. Boronat A, Sabater L, Saiz A, Dalmau J, Graus F. GABA(B) receptor antibodies in limbic encephalitis and anti-GAD-associated neurologic disorders. Neurology. (2011) 76:795–800. doi: 10.1212/WNL.0b013e31820e7b8d
21. Kruer MC, Hoeftberger R, Lim KY, Coryell JC, Svoboda MD, Woltjer RL, et al. Aggressive course in encephalitis with opsoclonus, ataxia, chorea, and seizures: the first pediatric case of γ-aminobutyric acid type B receptor autoimmunity. JAMA Neurol. (2014) 71:620–3. doi: 10.1001/jamaneurol.2013.4786
22. Alexopoulos H, Akrivou S, Mastroyanni S, Antonopoulou M, Dinopoulos A, Giorgi M, et al. Postherpes simplex encephalitis: a case series of viral-triggered autoimmunity, synaptic autoantibodies and response to therapy. Ther Adv Neurol Disord. (2018) 11:1756286418768778. doi: 10.1177/1756286418768778
23. Jarius S, Steinmeyer F, Knobel A, Streiberger K, Hotter B, Horn S, et al. GABAB receptor antibodies in paraneoplastic cerebellar ataxia. J Neuroimmunol. (2013) 256:94–6. doi: 10.1016/j.jneuroim.2012.12.006
24. Qiu XW, Zhang HQ Li DX, Wang J, Jiang ZG, Zhou YZ, et al. Analysis of clinical characteristics and poor prognostic predictors in patients with an initial diagnosis of autoimmune encephalitis. Front Immunol. (2019) 10:1286. doi: 10.3389/fimmu.2019.01286
25. Alexopoulos H, Dagklis IE, Akrivou S, Bostantjopoulu S, Dalakas MC. Autoimmune encephalitis with GABAantibodies, thymoma, and GABA receptor thymic expression. Neurol Neuroimmunol Neuroinflamm. (2014) 1:e39. doi: 10.1212/NXI.0000000000000039
26. Kosuda A, Shirahata T, Kudo N, Uehara Y, Miyawaki M, Hagiwara A, et al. Long-term survival of a patient with small cell lung cancer secreting ADH and ACTH simultaneously, following the prolonged use of amrubicin. Intern Med. (2020) 59:107–12. doi: 10.2169/internalmedicine.2838-19
27. Qin W, Wang X, Yang J, Hu WL. Coexistence of anti-SOX1 and anti-GABAB receptor antibodies with autoimmune encephalitis in small cell lung cancer: a case report. Clin Interv Aging. (2020) 15:171–5. doi: 10.2147/CIA.S234660
Keywords: anti-γ-aminobutyric acid-B receptor encephalitis, autoantibody, lung cancer, seizure, prognosis
Citation: Feng X, Zhang Y, Gao Y, Zhang J, Yu S, Lv J, Zu Y, Wang L and Wang X (2022) Clinical characteristics and prognosis of anti-γ-aminobutyric acid-B receptor encephalitis: A single-center, longitudinal study in China. Front. Neurol. 13:949843. doi: 10.3389/fneur.2022.949843
Received: 21 May 2022; Accepted: 15 August 2022;
Published: 15 September 2022.
Edited by:
Jorge Matias-Guiu, Complutense University of Madrid, SpainReviewed by:
Alina Gonzalez-Quevedo, Instituto de Neurología y Neurocirugía, CubaCopyright © 2022 Feng, Zhang, Gao, Zhang, Yu, Lv, Zu, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuedan Feng, eGQuZmVuZ0Bmb3htYWlsLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.