- 1Department of Neurology, University of Virginia School of Medicine, Charlottesville, VA, United States
- 2Department of Neurology, University of Florida College of Medicine, Jacksonville, FL, United States
- 3Department of Epidemiology, University of Florida College of Medicine, Jacksonville, FL, United States
Introduction: Hereditary transthyretin amyloidosis (hATTR) can cause multisystem organ disorders including polyneuropathy and cardiomyopathy. Amongst the many known pathologic mutations of the transthyretin (TTR) gene, the Val122Ile (V122I) mutation can be found in 3–4% of African Americans. Up to 47% of patients with the V122I hATTR cardiomyopathy had a history of carpal tunnel syndrome (CTS). This raises the question should we screen for this mutation in African Americans with bilateral CTS for the purpose of preventing advanced disease associated with hATTR. This is a prospective pilot study to determine the likelihood of African Americans with bilateral CTS having the V122I mutation and whether various clinical factors contribute to that probability.
Methodology: Adult African American patients without prior history of amyloidosis diagnosed with bilateral CTS were recruited for the study. They received genetic testing to screen for a TTR mutation. They also completed questionnaires to screen for symptoms of cardiomyopathy and neuropathy, other risk factors for CTS, and family history of CTS and cardiomyopathy.
Result: Two of the sixteen patients (12.5%) in this cohort were found to have the V122I mutation. The absence of polyneuropathy and cardiomyopathy symptoms, presence of other CTS risk factors, and absence of family history of CTS and cardiomyopathy did not decrease the likelihood of V122I mutation in this cohort.
Conclusion: The frequency of V122I transthyretin mutation in African Americans with bilateral CTS may be higher than 3–4%. The presence of bilateral CTS alone may be a justification to screen for TTR mutation in this population.
Introduction
Hereditary transthyretin amyloidosis (hATTR) is an inheritable autosomal dominant disorder. Normal transthyretin (TTR) proteins form tetramers that serve as carrier proteins for thyroid hormone and retinol. Mutant TTR proteins form unstable tetramers that tend to dissociate into monomers, which can form pathologic amyloid fibrils in various organ systems. The disease manifestations of hATTR include cardiomyopathy, polyneuropathy, autonomic insufficiency, carpal tunnel syndrome (CTS), and more. Many different TTR mutations have been described worldwide (1). Among them, the Val30Met mutation is more prevalent in the Portuguese population. It is associated with progressive polyneuropathy more than cardiomyopathy and can have disease manifestation as early as the third decade of life. The Thr60Ala mutation is more prevalent in those with Irish ancestry and often presents with a mixed phenotype of neuropathy and cardiomyopathy. The Val122Ile (V122I) mutation often results in cardiomyopathy, although polyneuropathy can also occur (1).
According to the Transthyretin Amyloid Outcome Survey (THAOS) study, the most prevalent TTR mutation in the United States is the V122I mutation. It makes up about 23% of TTR amyloidosis cases, with more than 85% of those individuals being African American (2). Previous epidemiology studies, including newborn genetic screening studies, found that 3–4% of the African American population carry at least one copy of the V122I mutation (3–5). When individuals with the V122I mutation develop cardiomyopathy, the diagnosis of hATTR amyloidosis is often not made until the seventh decade, although the onset of cardiac symptoms may precede the diagnosis by a decade (2, 6). The THAOS study also showed that 29.1% of its cohort with V122I hATTR had a history of CTS release surgery (2). Similarly, another retrospective study reported that up to 47% of their cohort with V122I hATTR cardiomyopathy also had CTS (6).
Currently, the presence of a TTR mutation with only CTS is not an indication for treatment if there is no evidence of hATTR polyneuropathy or cardiomyopathy. Still, bilateral CTS is one of the so-called Red Flag symptoms that justifies a diagnostic investigation for hATTR, in the appropriate clinical context (7). In addition to peripheral neuropathy and cardiomyopathy, other Red Flag symptoms/signs include autonomic insufficiency, gastrointestinal (GI) symptoms, proteinuria, and vitreous opacity. However, there are no prospective studies examining the predictability of these Red Flag symptoms for hATTR. Whether the presence of bilateral CTS alone justifies screening for hATTR, especially for the purpose of early identification of those at risk for development of cardiomyopathy, remains a topic of interest (8).
Given that the prevalence of the V122I mutation in the African American population is 3–4%, and that up to 47% of these individuals could have had CTS by the time hATTR cardiomyopathy is diagnosed, it raises the question as to whether we should routinely screen for the V122I mutation in African Americans diagnosed with bilateral CTS. Of course, CTS is relatively common, and there can be many other risk factors associated with it. Should we screen for TTR mutation in those with bilateral CTS when there are no other risk factors for CTS? Similarly, considering there are other Red Flag symptoms associated with hATTR, should we only screen for TTR mutations in those with bilateral CTS in the presence of other Red Flag symptoms? Furthermore, hATTR is an inherited disease, so should a family history of CTS and cardiomyopathy factor into the screening decision as well? Considering that we now have RNA-interference silencing and RNA antisense oligonucleotide drugs for treatment of hATTR neuropathy (9), as well as TTR tetramer stabilizer treatment for TTR cardiomyopathy (10), it is even more relevant than before, to determine what clinical factors should be used to efficiently identify hATTR before it progresses to advanced disease.
The main objective of this pilot study was to investigate whether the frequency of the V122I mutation in African Americans with bilateral CTS is significantly higher than the rate of 3–4% in the general African American population. In addition, this study explores whether certain Red Flag symptoms, other risk factors for CTS, and family history of CTS or cardiomyopathy affect the probability of this patient population having the V122I mutation.
Method
Patient Selection
Patients self-identified as (Black) African American seen in the neuromuscular clinic or EMG lab of University of Florida in Jacksonville, diagnosed with bilateral CTS, were at least 18 years of age, did not have an existing diagnosis of amyloidosis, and could give informed consent were recruited for the study. The study protocol was approved by the University of Florida Institutional Review Board.
Diagnosis of Carpal Tunnel Syndrome
Patients were given a clinical diagnosis of CTS if they reported intermittent or persistent numbness/tingling in digits 1, 2, or 3 for at least 1 month (11), and had electrophysiological features of median neuropathy at the wrist (12). Those electrophysiological features included significantly prolonged median latency in a mixed transcarpal comparison study between the median and ulnar nerves, significantly prolonged median sensory latency of the palm to digit segment compared with the palm to wrist segment, significantly prolonged median distal latency in the lumbrical-interosseous comparison study, or significantly prolonged median distal motor latency in the appropriate context (i.e., when the aforementioned comparison study responses are unobtainable and without evidence of proximal median motor conduction slowing).
Genetic Testing
Patients were de-identified prior to their blood or saliva specimen being sent for genetic testing for mutation of the TTR gene. Genetic testing was performed through Invitae (sponsored by Alnylam pharmaceuticals), a College of American Pathologists-accredited, Clinical Laboratory Improvement Amendments-certified laboratory. Patients who were found to have a TTR mutation were given genetic counseling, referred to appropriate providers, and managed according to current practice standards.
Questionnaires
Each patient's age, gender, Red Flag symptoms, any risk factors of CTS, and family history of CTS and cardiomyopathy were collected through a set of questionnaires.
Statistical Analysis
Data was summarized using counts and frequencies (percentages). Associations were assessed using the Fisher's Exact test. The level of significance was set at 5%. All analyses were done in SAS® for Windows Version 9.4.
Results
Two men and 14 women participated in the study. The average age was 55.75 years, ranging from 38 to 73 years. The results of genetic testing, presence of co-existing Red Flag symptoms, risk factors for CTS, and relevant family history for each patient are presented in Table 1.
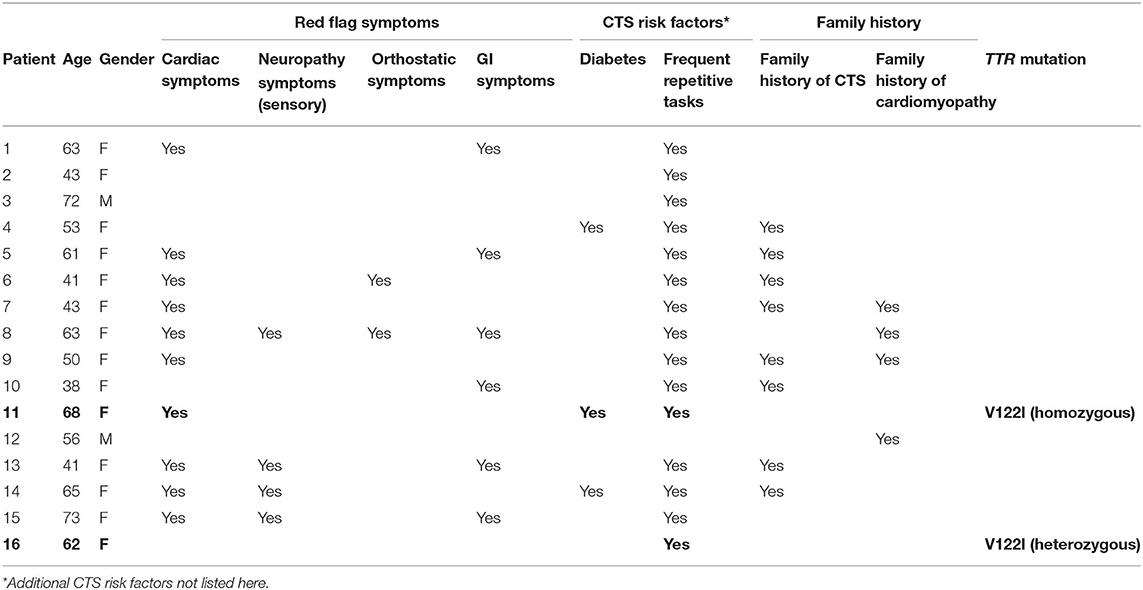
Table 1. Results of TTR mutation screening, with red flag symptom, CTS risk factors, and family history of CTS and cardiomyopathy.
Frequency of the V122I Mutation
Two of the 16 patients (12.5%) had the V122I TTR mutation. Both were women and in the sixth decade of life. One was heterozygous, and the other was homozygous, for the mutation. The other 14 patients did not have a TTR mutation. The percentages of patients with and without TTR mutation, with Red Flag symptoms, other risk factors of CTS, family history of CTS, and cardiomyopathy, are summarized in Table 2.
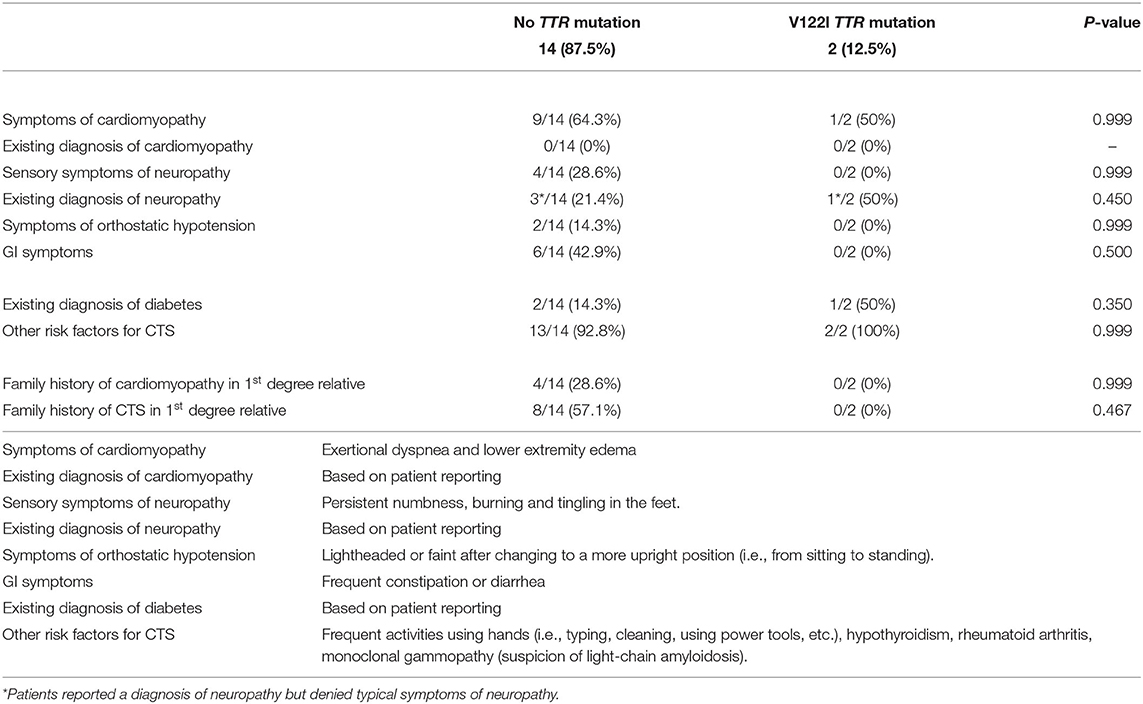
Table 2. Association of red flag symptoms, CTS risk factors, and family history of CTS and cardiomyopathy with V122I TTR mutation vs. no mutation.
Red Flag Symptoms
None of the patients had an existing diagnosis of cardiomyopathy. Symptoms of cardiomyopathy were reported in nine of the patients without the TTR mutation. Of the two patients with the V122I mutation, the one with homozygous mutation reported symptoms of cardiomyopathy but had no specific diagnosis.
Three patients without a TTR mutation and one of the two patients with V122I mutation reported a prior diagnosis of neuropathy, although they denied the typical sensory symptoms of polyneuropathy.
In those without a TTR mutation, two endorsed orthostatic symptoms, and six endorsed GI symptoms. The two patients with the V122I mutation denied orthostatic and GI symptoms.
Risk Factors and Clinical Features Related to CTS
Two of the patients without a TTR mutation and one with the V122I mutation had an existing diagnosis of diabetes. Thirteen of those without a TTR mutation and both with the V122I mutation had other risk factors for CTS (mainly frequent repetitive use of the hands). One of the patients with V122I mutation had a diagnosis of rheumatoid arthritis, and the other had a diagnosis of hypothyroidism. Both patients with the V122I mutation endorsed performed jobs or activities that required frequent repetitive use the hands.
Family History
A family history of cardiomyopathy in one or more first degree relatives was reported in four patients without a TTR mutation, and a family history of CTS in one or more first degree relatives was reported in eight patients without a TTR mutation. None of the two subjects with the V122I mutation knew of any first-degree relatives with cardiomyopathy or CTS.
Discussion
Primary Objective
Our main objective was to examine whether the frequency of the V122I mutation in African Americans who present with bilateral CTS would be greater than the expected 3–4%. We found the V122I mutation in two of the 16 patients (12.5%) who participated in the study. It is interesting to note that both patients with V122I mutation in this cohort were in their 6th decade of life. A previous study showed that for African American patients with V122I hATTR cardiomyopathy, the average age at diagnosis was 70, although the first cardiac symptoms may have preceded the diagnosis by more than a decade (6). In the same study, 47% of those patients had a history of carpal tunnel syndrome. A more recent retrospective study showed for those with TTR amyloidosis (including wild-type and hereditary), the probability of having CTS was the highest during the 5–9 years prior to the diagnosis of cardiac amyloidosis, and that CTS was an independent mortality risk factor in transthyretin amyloidosis (13). These findings may further justify screening for the V122I mutation in African Americans who develop carpal tunnel syndrome after their 5th or 6th decade of life, so that those who are found to have the mutation can be evaluated or monitored for cardiomyopathy.
Explorative Objectives
We also took this opportunity to explore whether some of the Red Flag symptoms, other risk factors for CTS, and family history of CTS and cardiomyopathy would have any predictive value for the V122I mutation in African Americans with bilateral CTS. Prior to the study, it would have been natural to assume that those with co-existing Red Flag symptoms would have an increased likelihood of finding a TTR mutation, thus leading one to think it may be low yield to screen for the mutation if there are no Red Flag symptoms. However, in this study, the absence of Red Flag symptoms did not decrease the likelihood of a V122I mutation. Similarly, when patients have risk factors for CTS, it would have been natural to attribute the cause of their CTS to these existing risk factors. However, in this study, the presence of other CTS risk factors did not decrease the likelihood of finding the mutation. Finally, this study showed that the lack of family history of CTS and cardiomyopathy also did not decrease the likelihood of a V122I mutation in this cohort. One must bear in mind that the questionnaires administered in this study regarding Red Flag symptoms, CTS risk factors, and family history were designed to be applicable for real-life clinical settings, where we can ask simple screening questions but are often unable to verify them. Thus, the validity of these findings is contingent on the accuracy of the self-reported symptoms and history provided by the patients.
Other Findings
This cohort had significantly more women than men. A higher frequency of CTS in women than men has been reported, but whether this difference is due to physiological or occupational risk factors is debated (14, 15). It is uncertain if a patient sample with equal gender representation would have yielded different results.
Of note, two of the patients in the group without a TTR mutation developed CTS during pregnancy, and the patients with the V122I mutation did not report that pregnancy was associated with the onset of their CTS symptoms. One cannot not draw the conclusion that CTS onset associated with pregnancy would make it less likely to be related to hATTR, although this may be explored further in a larger study.
Limitations
This is a small pilot study with major limitations, thus it is uncertain whether these findings would apply to the general African American population. Also, it should be emphasized that all self-reported components of this study (i.e., symptoms onset, co-existing diagnoses, family history, etc.) are subject to recall error, since they were not verified. All patients in this cohort had self-identified as (Black) African American, and from an ethical standpoint, their self-reported racial identity is indisputable. However, from a Mendelian inheritance standpoint, when studying the prevalence of a gene mutation in a racial cohort, individuals of mixed racial origin could affect the carrier frequency of that mutation. The possibility of multi-racial origin in any of the individuals of this cohort was not explored.
The so-called Red Flag symptoms are often found in patients diagnosed with hATTR, and these symptoms (and signs) can justify diagnostic investigations in the clinical setting. However, many of these symptoms are non-specific, and their predictive value for hATTR or TTR mutation is unknown. We explored the predictive value of some of the major Red Flag symptom for a TTR mutation, such as symptoms of cardiomyopathy, orthostatic hypotension, or GI complaints, and they did not seem to increase the likelihood of a TTR mutation in our African American cohort with bilateral CTS. However, the predictive value of other Red Flag symptoms, such as renal dysfunction, vitreous opacities, or unexplained weight loss were not explored.
We did not routinely perform ultrasound studies on patients with CTS. However, a recent study using peripheral nerve ultrasound showed the average median nerve cross-sectional area was smaller in those with TTR mutation than those with idiopathic CTS. In that study, increased median nerve cross-sectional area correlated with increased severity of idiopathic CTS, but this correlation was not apparent in those with TTR mutation (16). Whether ultrasound features can serve as a predictive factor for the likelihood of having TTR mutation in patients with CTS remains an interesting area of exploration.
It must be emphasized that those with the V122I mutation in this study were not confirmed to have amyloidosis. Confirmation of hATTR causing CTS would require tenosynovial biopsy during CTS surgery, which was not a part of this study. It is also possible to have a false negative biopsy in an individual with amyloidosis simply due to sampling error. Not every case of CTS requires surgery and carpal tunnel release surgery is often performed for more severe cases or those refractory to non-surgical management. A nationwide Danish registry study that included 56,032 patients showed CTS surgery was associated with higher risk of amyloidosis and heart failure compared to control subjects, although this study did not distinguish the type of amyloidosis (17). In a prospective study examining men ages ≥50 and women ages ≥60 undergoing CTS surgery (85% of those with bilateral CTS), 10 out of the 98 patients (10.2%) were found to have amyloid deposits, although only seven had TTR amyloid deposition and only two of those patients had TTR mutations (Leu58His and Ala81Thr) (18). In comparison, our prospective study screening African American patients with bilateral CTS identified two out of 16 (12.5%) patients with V122I TTR mutation. If this probability is reproducible in a larger study, it could be clinically impactful. Biopsy confirmation of ATTR deposition in patients with bilateral carpal tunnel syndrome with V122I TTR mutation may be possible in a future study with long term follow up as well.
Ultimately, whether identification of the V122I mutation based on CTS prior to the onset of cardiomyopathy, polyneuropathy, or dysautonomia will ultimately impact mortality and morbidity associated with hATTR, remains to be determined.
Justification for Future Studies
This was a prospective evaluation of the predictability of bilateral CTS for the V122I mutation in an African American population, and we found that 12.5% of our cohort had the mutation. We focused on the African American population, because carrier rate of the V122I mutation in this population was already established to be 3–4%, which serves as a benchmark, in that any screening approach that identifies a higher percentage of the mutation can be clinically impactful. The greater purpose of this effort is to determine efficient ways to identify TTR mutations with the aim of preventing advanced disease associated with hATTR. Despite the limitations of this study, the findings here could suggest that the presence of bilateral CTS in African American individuals, especially in their 5th or 6th decade of life, may be an adequate justification to screen for a TTR mutation, regardless of Red Flag symptoms, other risk factors for CTS, or family history of CTS or cardiomyopathy. Of course, a larger study would help to verify these findings. Ideally, a future study would address the limitations of this study. Furthermore, in individuals found to have a TTR mutation on the basis of bilateral CTS, it would be valuable to determine what proportion of them may already have cardiomyopathy. Ultimately, if screening for a TTR mutation based on a diagnosis of bilateral CTS leads to identification of otherwise overlooked hATTR cardiomyopathy or prevention of advanced hATTR-associated disease in a significant number of African American individuals, it may be justified to incorporate this screening approach into a practice guideline.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by University of Florida, Jacksonville, Florida, Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JS designed and carried out the study, wrote the abstract, introduction, much of the methodology, results and discussion of the manuscript. MB contributed to the design of a database for the study, carried out the study, and contributed to the methodology section of the manuscript. CS contributed to the design of the database, performed statistical analysis, and contributed to the methodology section of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
University of Florida internal funding.
Conflict of Interest
JS has participated on the Alnylam Pharmaceuticals advisory board in 2020, given a disease awareness talk in 2021 for Alnylam, and received financial compensations for those activities.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Special thanks to Michael Pulley and Shannon Laboy for their contribution in patient recruitment, Larry Phillips and Howard Goodkin for their guidance, and Andrea Denton for her assistance in manuscript formatting.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.949401/full#supplementary-material
Abbreviations
CTS, carpal tunnel syndrome; hATTR, hereditary transthyretin amyloidosis; THAOS, transthyretin amyloid outcome survey; TTR, transthyretin; V122I, Val122Ile.
References
1. Adams D, Koike H, Slama M, Coelho T. Hereditary transthyretin amyloidosis: a model of medical progress for a fatal disease. Nat Rev Neurol. (2019) 15:387–404. doi: 10.1038/s41582-019-0210-4
2. Maurer MS, Hanna M, Grogan M, Dispenzieri A, Witteles R, Drachman B, et al. Genotype and phenotype of transthyretin cardiac amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey). J Am Coll Cardiol. (2016) 68:161–72. doi: 10.1016/j.jacc.2016.03.596
3. Jacobson DR, Alexander AA, Tagoe C, Buxbaum JN. Prevalence of the amyloidogenic transthyretin (TTR) V122I allele in 14 333 African–Americans. Amyloid. (2015) 22:171–4. doi: 10.3109/13506129.2015.1051219
4. Jacobson DR, Pastore R, Pool S, Malendowicz S, Kane I, Shivji A, et al. Revised transthyretin Ile 122 allele frequency in African-Americans. Hum Genet. (1996) 98:236–8. doi: 10.1007/s004390050199
5. Yamashita T, Asl KH, Yazaki M, Benson MD. A prospective evaluation of the transthyretin Ile122 allele frequency in an African-American population. Amyloid. (2005) 12:127–30. doi: 10.1080/13506120500107162
6. Connors LH, Prokaeva T, Lim A, Théberge R, Falk RH, Doros G, et al. Cardiac amyloidosis in African Americans: comparison of clinical and laboratory features of transthyretin V122I amyloidosis and immunoglobulin light chain amyloidosis. Am Heart J. (2009) 158:607–14. doi: 10.1016/j.ahj.2009.08.006
7. Conceição I, González-Duarte A, Obici L, Schmidt HHJ, Simoneau D, Ong M-L, et al. “Red-flag” symptom clusters in transthyretin familial amyloid polyneuropathy. J Peripher Nerv Syst. (2016) 21:5–9. doi: 10.1111/jns.12153
8. Ton V-K, Patel S, Gottlieb SS. Carpal Tunnel Syndrome and Future Amyloidosis. J Am Coll Cardiol. (2019) 74:24–5. doi: 10.1016/j.jacc.2019.04.055
9. Buxbaum JN. Oligonucleotide drugs for transthyretin amyloidosis. N Engl J Med. (2018) 379:82–5. doi: 10.1056/NEJMe1805499
10. Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med. (2018) 379:1007–16. doi: 10.1056/NEJMoa1805689
11. Rempel D, Evanoff B, Amadio PC, de Krom M, Franklin G, Franzblau A, et al. Consensus criteria for the classification of carpal tunnel syndrome in epidemiologic studies. Am J Public Health. (1998) 88:1447–51. doi: 10.2105/AJPH.88.10.1447
12. Jablecki CK, Andary MT, So YT, Wilkins DE, Williams FH. Literature review of the usefulness of nerve conduction studies and electromyography for the evaluation of patients with carpal tunnel syndrome. AAEM Quality Assurance Committee. Muscle Nerve. (1993) 16:1392–414. doi: 10.1002/mus.880161220
13. Milandri A, Farioli A, Gagliardi C, Longhi S, Salvi F, Curti S, et al. Carpal tunnel syndrome in cardiac amyloidosis: implications for early diagnosis and prognostic role across the spectrum of aetiologies. Eur J Heart Fail. (2020) 22:507–15. doi: 10.1002/ejhf.1742
14. Farioli A, Curti S, Bonfiglioli R, Baldasseroni A, Spatari G, Mattioli S, et al. Observed differences between males and females in surgically treated carpal tunnel syndrome among non-manual workers: a sensitivity analysis of findings from a large population study. Ann Work Expo Health. (2018) 62:505–15. doi: 10.1093/annweh/wxy015
15. McDiarmid M, Oliver M, Ruser J, Gucer P. Male and female rate differences in carpal tunnel syndrome injuries: personal attributes or job tasks? Environ Res. (2000) 83:23–32. doi: 10.1006/enrs.2000.4042
16. Salvalaggio A, Coraci D, Cacciavillani M, Obici L, Mazzeo A, Luigetti M, et al. Nerve ultrasound in hereditary transthyretin amyloidosis: red flags and possible progression biomarkers. J Neurol. (2021) 268:189–98. doi: 10.1007/s00415-020-10127-8
17. Fosbol E, Rorth R, Leicht BP, Schou M, Maurer M, Kristensen SL, et al. Association of carpal tunnel syndrome with amyloidosis, heart failure, and adverse cardiovascular outcomes. J Am Coll Cardiol. (2019) 74:15–23. doi: 10.1016/j.jacc.2019.04.054
Keywords: hereditary transthyretin amyloidosis (hATTR), V122I TTR mutation, carpal tunnel syndrome, African American health, amyloid neuropathies
Citation: Shije JZ, Bautista MAB and Smotherman C (2022) The Frequency of V122I Transthyretin Mutation in a Cohort of African American Individuals With Bilateral Carpal Tunnel Syndrome. Front. Neurol. 13:949401. doi: 10.3389/fneur.2022.949401
Received: 20 May 2022; Accepted: 23 June 2022;
Published: 26 July 2022.
Edited by:
Giuseppe Vita, University of Messina, ItalyCopyright © 2022 Shije, Bautista and Smotherman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jeffrey Z. Shije, c2hpamVqQGdtYWlsLmNvbQ==; YXZ5NXFmQHZpcmdpbmlhLmVkdQ==
 Jeffrey Z. Shije
Jeffrey Z. Shije Maria A. B. Bautista
Maria A. B. Bautista Carmen Smotherman
Carmen Smotherman