- 1Neuromedicine Center, University of Hong Kong Shenzhen Hospital, Shenzhen, China
- 2Physiotherapy Department, University of Hong Kong Shenzhen Hospital, Shenzhen, China
- 3Core Laboratory, University of Hong Kong Shenzhen Hospital, Shenzhen, China
Objective: Using rhythmic auditory stimulation (RAS) to improve gait disturbance in Parkinson's disease (PD) is an available treatment option, yet a consensus on its effectiveness remains controversial. We summarized the effects of RAS on gait, functional activity and quality of life in PD patients through a systematic review and meta-analysis.
Methods: PubMed, Embase, Web of Science, Medline, and Cochrane Library databases were initially searched to identify relevant literature up to August 2021. Next, the methodological quality of eligible comparative studies was assessed by the Physiotherapy Evidence Database Scale. The treatment effects to clinical outcome in relation to gait, motor activities, and quality of life were analyzed.
Results: A total of 18 studies consisted of 774 subjects were included in this meta-analysis. Comparing with the control group, RAS had significantly increased stride length (p < 0.001), accelerated gait speed (p < 0.001), reduced the occurrence of freezing events during walking (P = 0.009), achieved an improvement in Unified Parkinson's Disease Rating Scale (UPDRS) II (P = 0.030), UPDRS-III (P < 0.001) and Parkinson's Disease Quality of Life Questionnaire (PDQL) (p = 0.009) scores over an interval of 1–26 months.
Conclusion: In this meta-analysis of 18 randomized controlled trials, we have demonstrated that RAS improves the general motor functions (UPDRS-III), particularly in gait, mobility and quality of life, in patients with Parkinson's disease.
Introduction
Parkinson's disease (PD) is a common age-related neurodegenerative disease after Alzheimer's disease, affecting 1% of the world's population over the age of 60 years (1). With an aging population, the number PD patients are expected to reach 13 million by 2024, doubling in the next 10 years by 2034 (2–4). PD patients often present with tremor, rigidity, bradykinesia, gait disturbance, balance and coordination disorders, accompanied by non-motor-related symptoms such as cognitive and psychological impairment, neurobehavioral abnormalities, and sleep disturbances (5–7). Motor symptoms are caused by the loss and degeneration of dopaminergic neurons in the dense part of substantia nigra. As there is no curative treatment for Parkinson's disease, symptomatic relief by medications and the Deep Brain Stimulation are regarded as the main management modalities (8). Pharmacological interventions are primarily to increase dopamine levels via the use of dopaminergic drugs. However, long-term use of dopaminergic drugs can have serious side effects on patients, such as loss of efficacy and accumulation of toxicity (9, 10). Besides, the axial symptoms of gait disturbances do not respond to pharmacotherapy and deep brain stimulation (11–14). 25–60% of patients experience freezing of gait usually after several years from disease-onset. As Gait disturbances respond poorly to treatments, physical rehabilitation techniques are gaining interest as an adjunct in the management of these patients when the combined therapies of medication and surgery are failing (5, 15, 16).
Physical activity has a positive impact on gait, cognitive function, and quality of life in patients with Parkinson's disease (17, 18). The joy of an independent functional mobility does generate a positive motivation in these patients (19). Music is an effective emotional relaxant that helps relieve anxiety and pain (20). Rhythmic auditory and visual cues can improve all types of freezing of gait, dopamine-responsive or dopamine-resistant, according to the literature, a Level B evidence (4). At present, there is no systematic review or meta-analysis using high quality randomized double-blinded controlled trials of sufficient size and power to lead to a definitive study in the future (4). Therefore, combining music with physical activity is a feasible, enjoyable, and probably sustainable option. Studies have shown that gait training accompanied by music and rhythmic auditory stimulation (RAS) can significantly increase patients' stride length and speed (21). Compared with treadmill gait training alone, treadmill gait training with rhythmic auditory stimulation can significantly improve gait and quality of life (22, 23). Several systematic reviews and meta-analyses have reported the effectiveness of RAS on gait in patients with Parkinson's disease (24, 25). In addition to the retrospective cohort studies, there have been several recent randomized controlled trials (RCTs) in the field. We have updated the published RCTs with a more comprehensive meta-analysis.
Methods
Study design
This systematic review and meta-analysis were carried out under the statement of Preferred Reporting Items for Systematic Reviews, PRISMA (26).
Retrieval strategy and literature selection
PubMed, Embase, Web of Science, Medline, and Cochrane Library databases were thoroughly searched, to obtain studies published between January 2000 and August 2021. The searching keywords included (“tread,” “gait,” “train,” “exercise,” “rehabilitation” or “treatment”) and (“Rhythmic,” “auditory stimulation,” “musical stimulation,” “music” or “acoustic”) and (“Parkinson's disease”).
All eligible studies in this meta-analysis had to meet the following criteria: (1) patients had idiopathic Parkinson's disease; (2) patients in the intervention group received a course of music or rhythmic auditory stimulation during physical therapy whereas the control group received conventional physical therapy; (3) the effects of the intervention on gait, mobility, and quality of life were reported; (4) patients participating in study ≥ 10; (5) the study should be publish in English and peer-reviewed journals; (6) the study should be randomized controlled trials.
Exclusion criteria: (1) patients should not be too frail to receive physical therapy. They should not be cognitively impaired to follow instructions of physical therapy; (2) case reports, reviews, letters, comments and abstracts were not included; (3) studies where assessment outcome was unavailable.
Quality evaluation
The Physiotherapy Evidence Database (PEDro) scale was used to assess the quality of included studies (27). The PEDro scale consists of 11 items, including random assignment, undercover assignment, baseline comparability, subject blinding, therapist blinding, assessor blinding, adequate follow-up, intention-to-treat analysis, between-group comparisons, point measures, and variance measures. The maximum PEDro score is 10. The quality of the study was classified as “excellent” (9–10 points), “good” (6–8 points), and “fair” (≤ 5 points) based on the PEDro score (25). Studies with a PEDro score ≥ 6 will be included in this meta-analysis. The quality assessment was performed independently by two researchers (LL and HR). When any disagreements arose, the two researchers resolved them in discussion with a third researcher (YXF).
Data extraction
Two researchers (LL and HR) independently extracted primary data from eligible studies using a standardized form. The following relevant variables would be extracted: (1) study characteristics: first author, year of publication, region of study, and PEDro score; (2) subject characteristics: sample size, age, disease duration, and Hoehn and Yahr staging; (3) gait kinematic parameters: stride length, stride duration, gait speed, stride frequency, swing, and timed up-and-go test (TUG); and (4) clinical parameters: Unified Parkinson's Disease Rating Scale (UPDRS), Parkinson's Disease Quality of Life Questionnaire (PDQL) score, Berg Balance Scale (BBS), Falls Efficacy Scale (FES), and Freezing of Gait Questionnaire (FOGQ). UPDRS-III is the most popular assessment tool for motor function impairment for patients with Parkinson's disease. We have therefore chosen it as the primary outcome for our study.
Both freezing of gait (FoG) and Speed are regarded as refractory symptoms in advanced Parkinson's disease. We have selected them for secondary outcomes (FOGQ and Speed). If the corresponding data could not be extracted directly from the study, it would need to be reanalyzed. Where there were disagreements between the above two researchers, a third researcher (YXF) was asked to review literatures until a consensus was reached. The data management and statistical analysis were performed by YXF and reviewed by statistician JYZ from the core laboratory.
Data analysis
Analyses were conducted using STATA 16.0 SE. As the outcomes investigated were continuous variables and scale of measurement, we used mean difference (MD) and corresponding 95% confidence interval (95% CI) for assessment. When the same scale and units were used for all study outcomes, the weighted mean difference (WMD) and its corresponding 95% CI were used as the pooled statistic in the meta-analysis. Forest plots were used to display the pooled results of the meta-analysis. Between-study heterogeneity was assessed using I2 and two-tailed p-values (28). No statistically significant heterogeneity was considered when I2 < 50%, p > 0.05, so a fixed-effects model was adopted. Otherwise, a random-effects model was applied (29). The effect size was significant when the pooled 95% CI excluded 0 and the p-value < 0.05.
Results
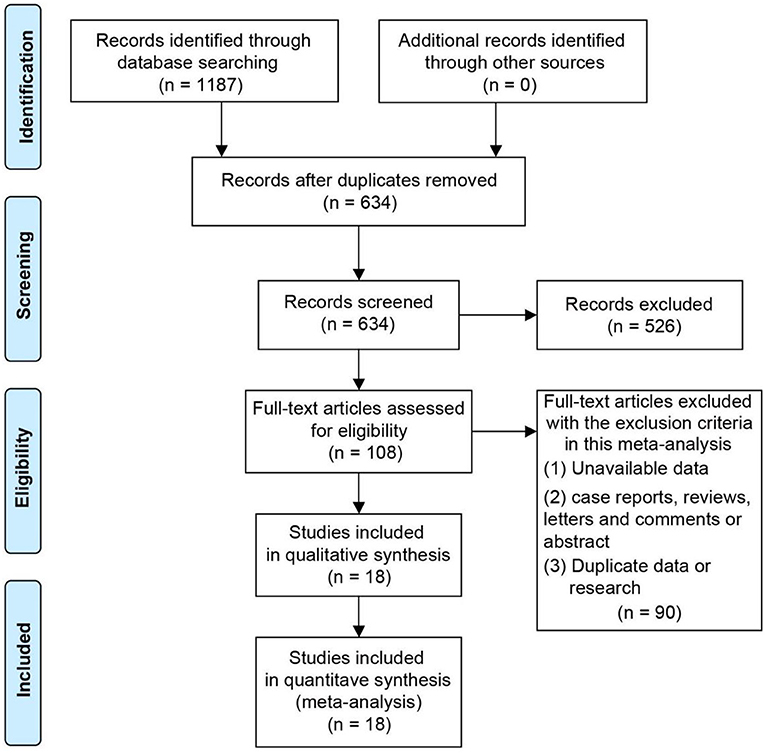
Flow chart Figure 1 showed the process and results of literature screening. By searching the online database, a total of 1,187 studies were obtained. After excluding the duplicate studies, 634 remained. Five hundred and twenty six studies were removed as they did not meet the eligibility criteria. Finally, 18 studies were included after the full text of 108 literature were read (22, 23, 30–45).
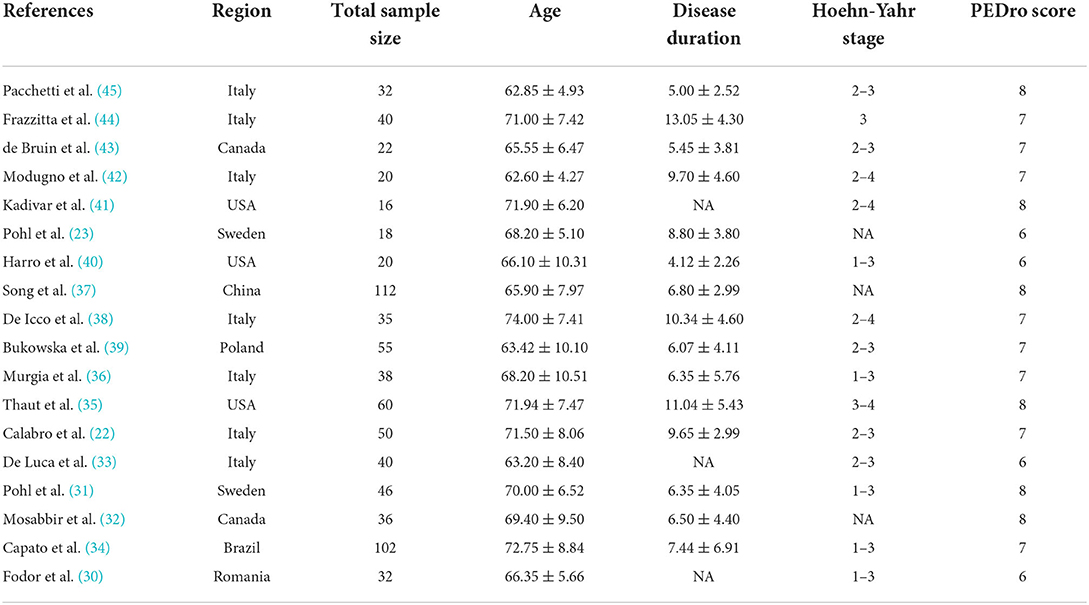
A total of 774 subjects were included in these 18 studies, there were a total of 396 patients in the intervention group and 378 patients in the control group. The sample sizes of subjects included in these studies ranged from 16 to 112. The mean age of the participants in each study ranged from 62 to 72 years. The regions of eligible studies included Italy (22, 33, 36, 38, 42, 44, 45), Sweden (23, 31), Poland (39), Brazil (34) and Romania (30), Canada, (32, 43), the United States (35, 40, 41), China (37). The average disease duration of PD patients included in the study ranged from 4 to 13 years. Three papers did not specify the duration of disease in the included subjects. The Hoehn-Yahr staging of the included Parkinson's patients covered stages 1 to 4. Similarly, three studies did not specify the Hoehn-Yahr staging of the included subjects. The PEDro scores of the included studies were all six and above. All eligible studies were RCT studies. Table 1 demonstrates the essential characteristics of all eligible studies.
Effect of RAS on gait parameters
A total of six studies reported the effect of RAS on the stride length of Parkinson's patients. As no significant heterogeneity was found (I2 = 0.0%, p = 0.935), we used a fixed-effects model for analysis. The stride length of patients in the intervention group significantly increased by 5 cm compared with the control group (WMD = 4.64, 95% CI: 3.12–7.69, p < 0.001) (Figure 2A). Three studies with a total of 112 patients reported on the stride duration of patients. The pooled WMD was −0.03 (95% CI: −0.09–0.04, p = 0.426), suggesting no significant effect of RAS on shortening stride duration (Figure 2B). Seven studies of published material on stride speed showed that rhythmic auditory stimulation significantly accelerated speed in patients compared with the control group (WMD = 0.06, 95% CI: 0.03–0.08, p < 0.001) (Figure 2C).
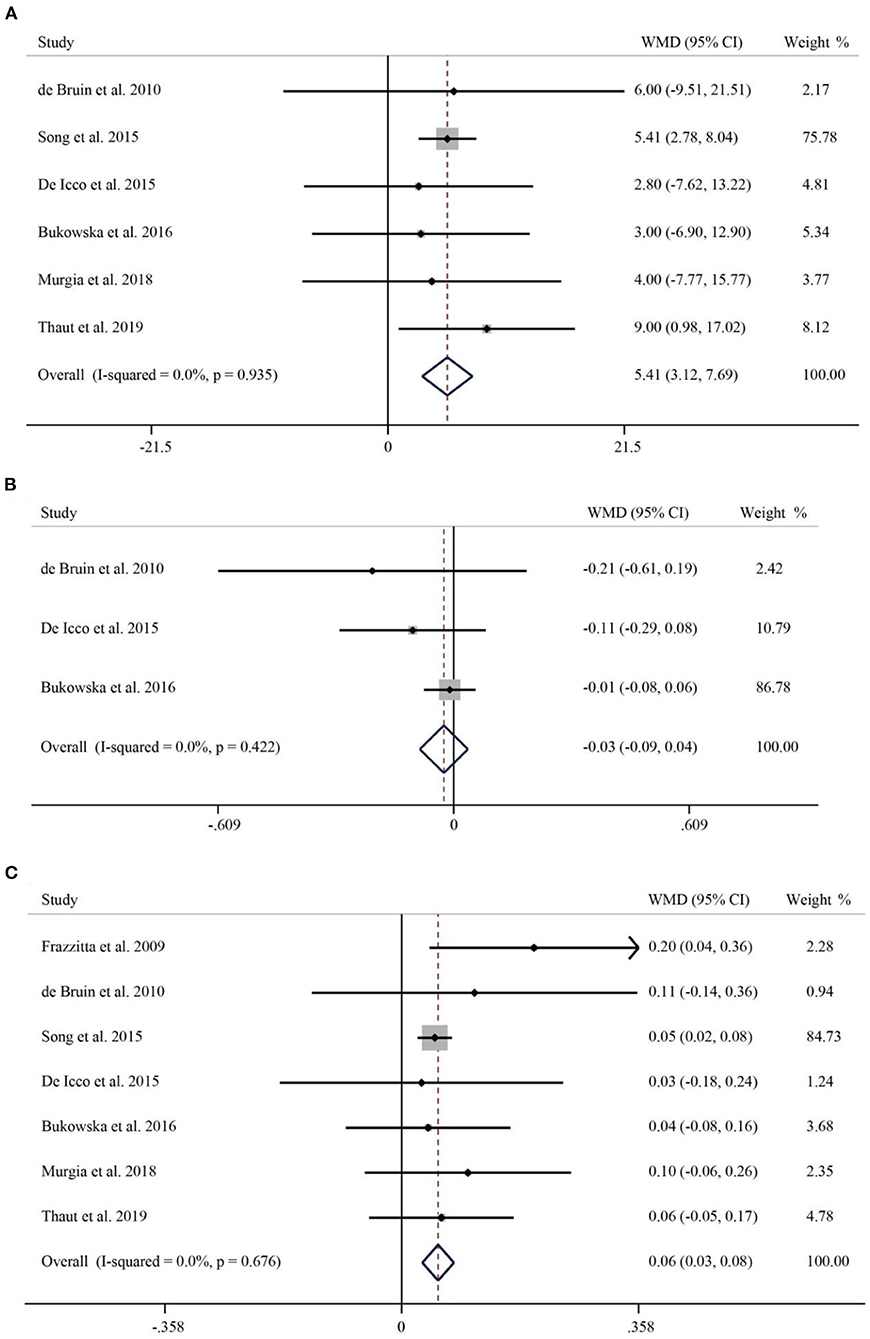
Figure 2. Forest plot of RAS vs. the control group for stride length (A), stride duration (B), and speed (C).
A total of 5 studies with 287 subjects compared patients' step frequency in the intervention and control groups. Due to significant heterogeneity (I2 = 79.4%, p < 0.001), a random-effects model was used to analyze the role of RAS on step frequency. The pooled WMD was 1.57 (95% CI: −4.91–8.05, P = 0.635), suggesting that the effect of RAS on step frequency was not significant (Figure 3A). A total of 3 publications reported the percentage of patients swinging. Pooled results showed no statistically significant difference between the rhythmic stimulation and the swing of patients in the control group (WMD = 0.39, 95% CI: −0.44–1.22, p = 0.468) (Figure 3B). Seven studies involving 332 Parkinson's patients reported TUG. I2 = 87.2%, p < 0.001, suggesting significant heterogeneity, so a random-effects model was used for the pooled analysis of TUG. There was no significant difference in the effect of RAS in reducing TUG compared to the control group (WMD = −0.68, 95% CI: −3.69–2.33, P = 0.658) (Figure 3C).
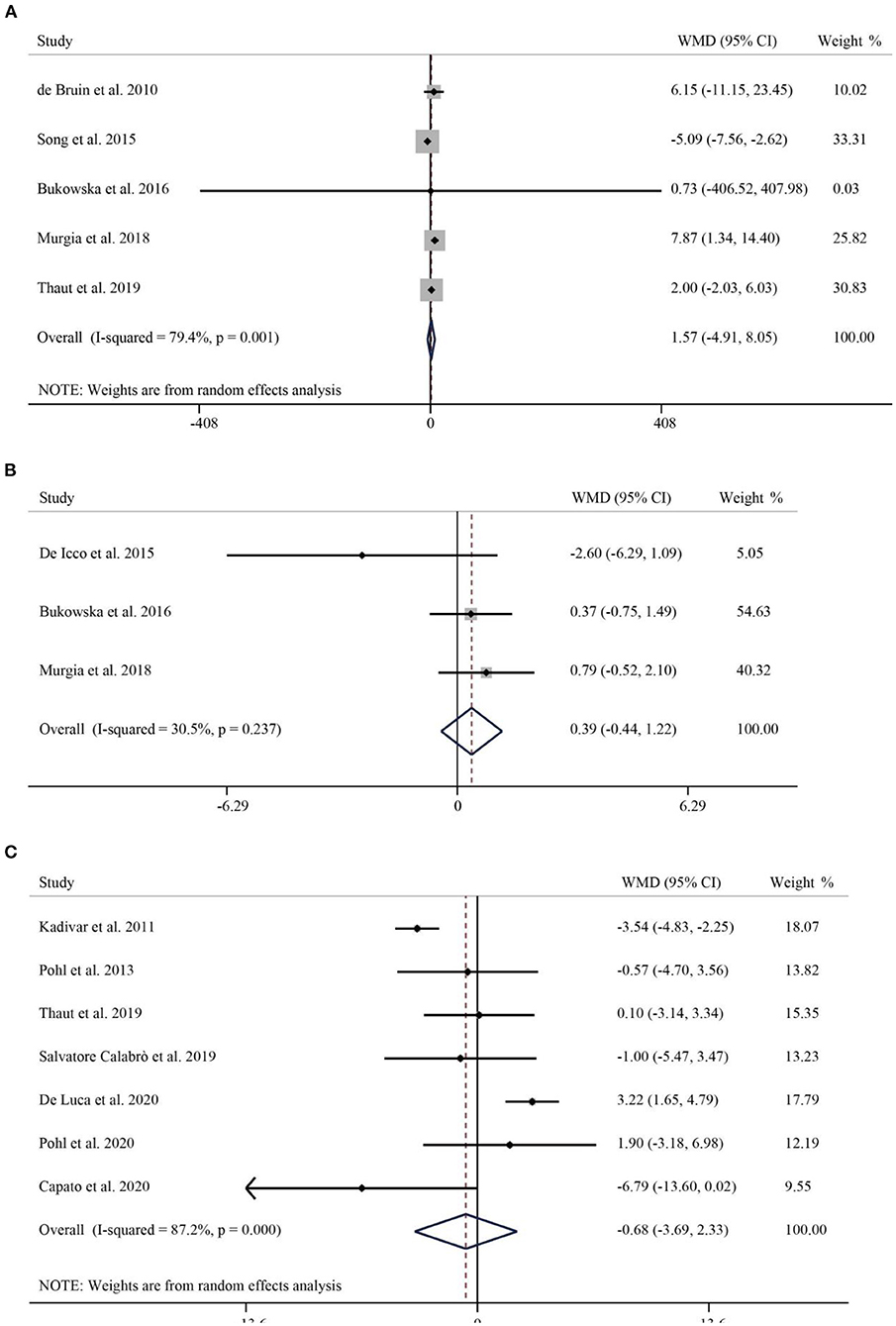
Figure 3. Forest plot of RAS vs. the control group for step frequency (A), swing (B), and TUG (C). TUG, Timed Up-and-Go.
Effect of RAS on clinical parameters
BBS was used to assess balancing capacity of PD patients. A total of 4 studies, including 242 patients, reported the effect of RAS on BBS. The pooled results showed no statistically significant difference between RAS and the control group in improving the balance of patients (WMD = 1.44, 95% CI: −0.53–3.42, p = 0.152) (Figure 4A). Next, we used the FES to assess patients' fear of falling. A total of 3 publications reported FES. The pooled WMD was −1.68 (95% CI: −3.35–0.00, P = 0.05), suggesting that no significant difference emerged between the control and intervention groups in improving the effect of FES (Figure 4B). Finally, the FOGQ was used to assess patients reported freezing events during walking. Five studies follow the effect of RAS on FOGQ in patients with PD showed that RAS significantly reduced the occurrence of freezing events during walking compared with the control group (WMD = −2.06, 95% CI: −3.60–0.53, p = 0.009) (Figure 4C).
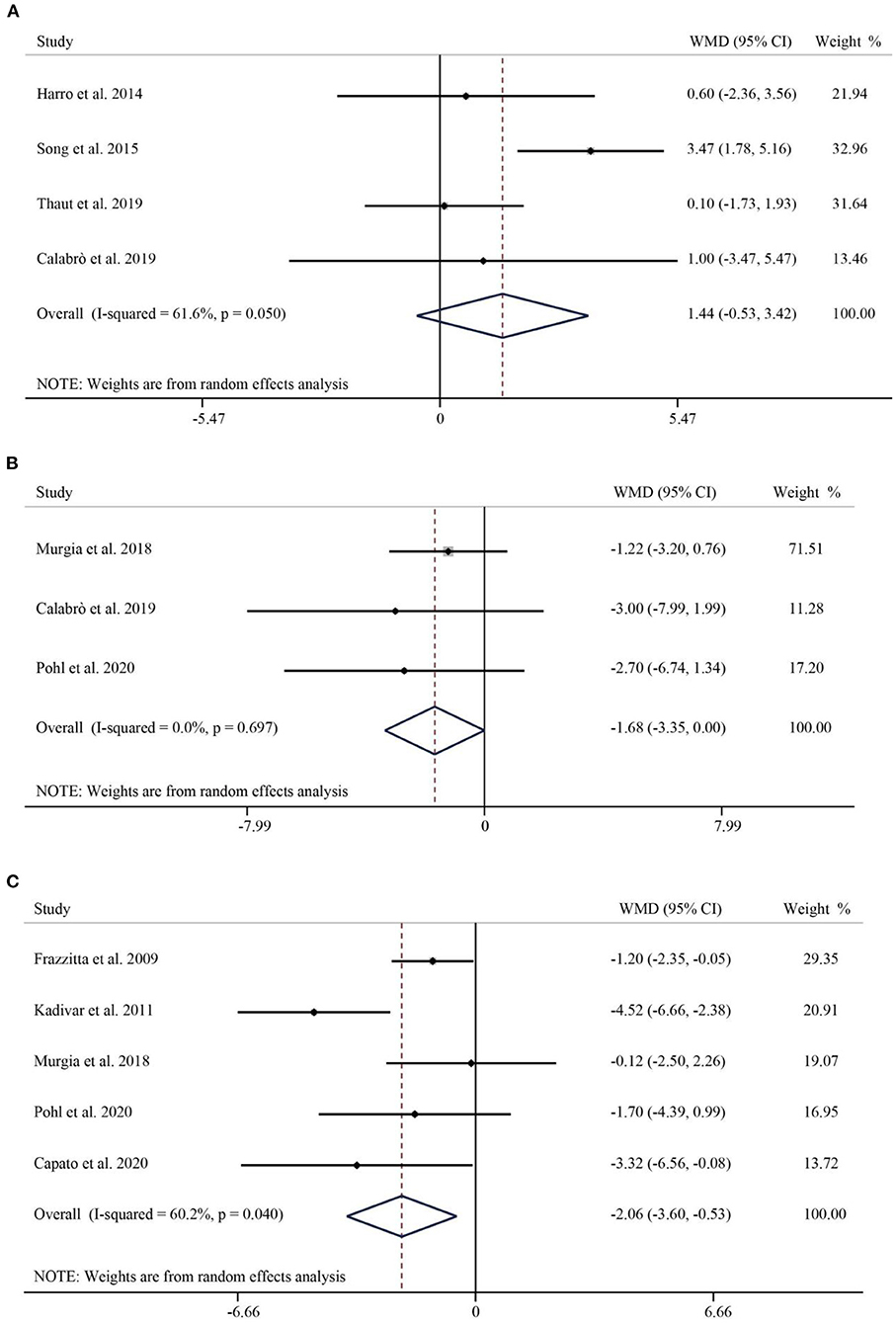
Figure 4. Forest plot of RAS vs. the control group for BBS (A), FES (B), and FOGQ (C). BBS, Berg Balance Scale; FES, Falls Efficacy Scale; FOGQ, Freezing of Gait Questionnaire.
The results obtained in the analysis of the second part of the UPDRS (UPDRS-II) showed that RAS significantly improved impairment in activities of daily living in Parkinson's patients (WMD = −2.76, 95% CI: −5.25 to −0.27, p = 0.030) (Figure 5A). The third part of the UPDRS (UPDRS-III) was used to measure motor impairment. A total of 10 studies containing 403 subjects reported the UPDRS-III. The pooled WMD was −4.74 (95% CI: −6.98–2.51, p < 0.001), indicating that the RAS significantly reduced the occurrence of dyskinesia with significant heterogeneity (I2 = 84.7%, p < 0.001) (Figure 5B). A total of four papers have examined the effect of RAS on PDQL scores. Compared with the control group, RAS had a positive effect in improving PDQL scores without significant heterogeneity (WMD = −4.52, 95% CI: −8.11– −0.94, P = 0.009) (Figure 5C).
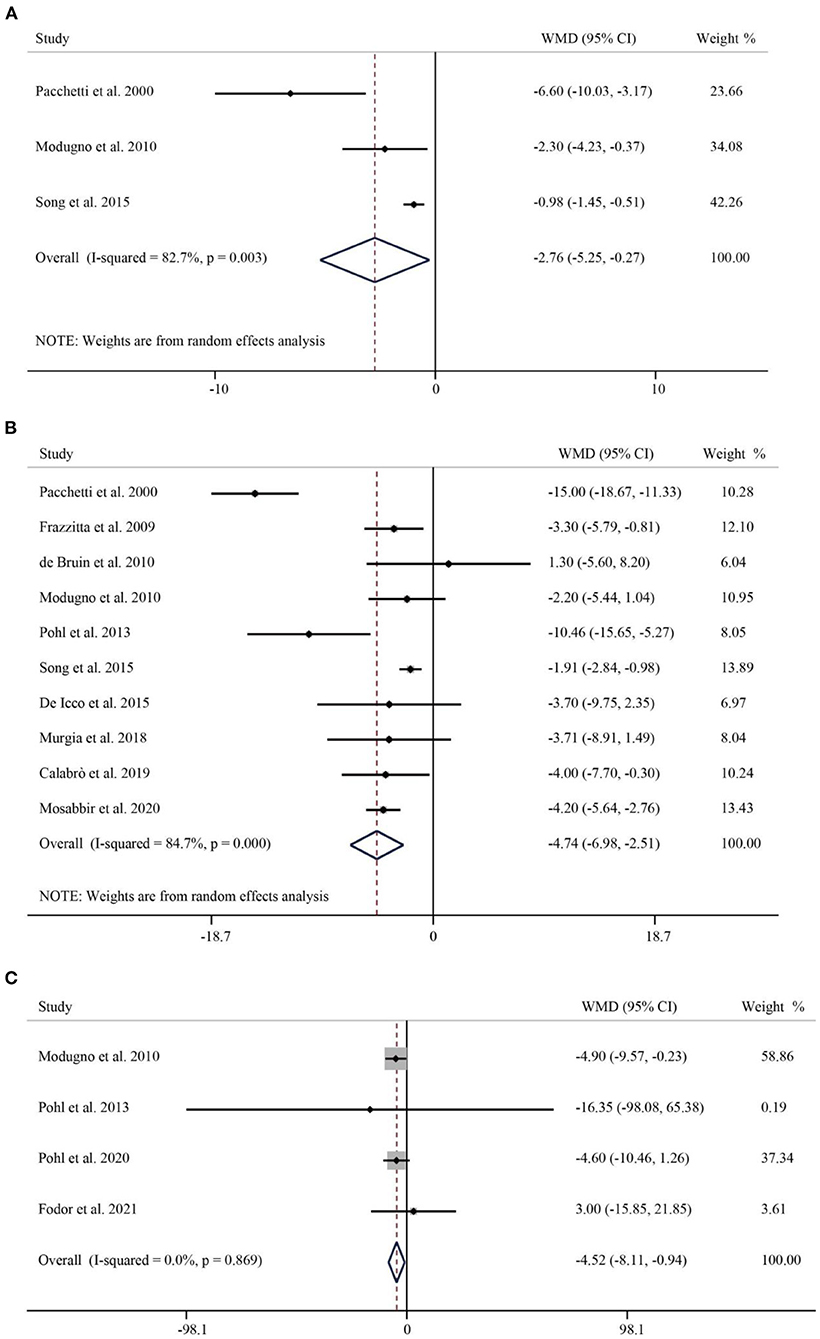
Figure 5. Forest plot of RAS vs. the control group for UPDRS-II (A), UPDRS-III (B), and PDQL (C). UPDRS, Unified Parkinson's Disease Rating Scale; UPDRS-II, UPDRS- Activities of Daily Living; UPDRS-III, UPDRS- Motor Symptoms; PDQL, Parkinson's Disease Quality of Life Questionnaire.
Publication bias
The R software was employed to test the publication bias of one primary and two secondary outcomes. These data points represented by individual studies in a funnel plot (Figure 6) were distributed on both sides of the middle solid line, basically in a symmetrical shape. The funnel plots for UPDRS-III, Speed and FOGQ suggest no significant publication bias.
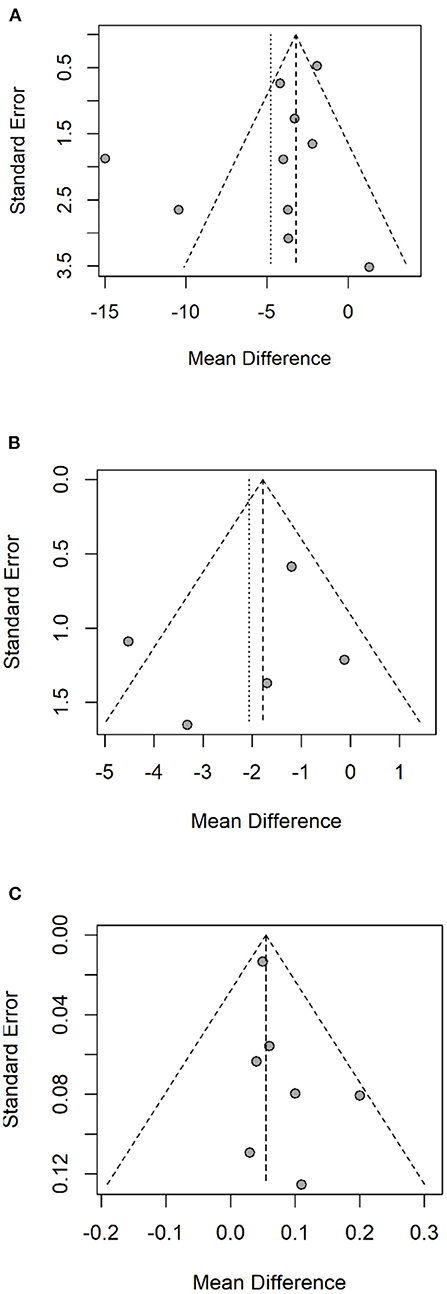
Figure 6. Funnel plot of UPDRS-III (A), FOGQ (B), Speed (C). UPDRS-III, Unified Parkinson's Disease Rating Scale - Motor Symptoms; FOGQ, Freezing of Gait Questionnaire.
Discussion
Parkinson's disease is a common age-related neurodegenerative disease and has remained a challenging health problem. Most patients will develop disabling symptoms such as gait freezing, despite optimal medical and surgical therapies. Gait training represent a potentially effective aid for managing PD symptoms not responding to dopaminergic drugs, as cues seem to be able to access rhythmic entrainment mechanisms even in the absence of dopaminergic stimulation (7, 38). This current systematic review and meta-analysis summarizing the effects of the 18 selected studies that met the inclusion criteria, generated the pooled results of RAS exhibiting a significant improvement for gait disturbances, motor activities, and quality of life. In addition, concurrent RAS during physiotherapy significantly increased stride length, accelerated stride speed, reduced the occurrence of walking freezes, promoted mobility, and improved PDQL scores in Parkinson's patients.
Moreover, external stimuli such as acoustic, visual, and somatosensory stimuli can modulate motor patterns in Parkinson's patients, helping them start physical activity and maintain the motivation for motor tasks (19, 38, 46). PD can severely affect patient's gait parameters, such as stride length, stride duration, speed, and gait frequency. The temporal and spatial parameters of gait are associated with unhealthy events in the elderly, including falls, functional decline, and even death (47). Studies have shown that providing RAS alongside gait training significantly improved patients' overall gait quality index, balance, strides length and number, consistent with the results of our meta-analysis (22). The pooled results indicated that RAS had no significant effect on cadence in PD patients. The increase or decrease in step frequency had different effects on patients at different stages of the disease (48). Studies have shown that in order to maintain gait speed, people's gait frequency increases with age. However, the increase in gait frequency can adversely affect the stability of walking (49).
Freezing of gait (FoG), defined as ”a brief, intermittent absence or significant reduction in the forward progress of the foot despite intentional walking," is the most distinctive features of patients with advanced Parkinson's disease (50, 51). FoG can lead to reduced mobility, increased incidence of falls, and a significant negative impact on quality of life (52, 53). Wroblewska et al. found that Nordic walking has a lasting improvement effect on PD patients (54). Studies have shown that receiving auditory and visual cues during treadmill training has a better effect on improving gait freezing than traditional treatments (44). Capato et al. proved that compared with conventional training, RAS can have a significant improvement in the overall well-being of PD during the 6-month follow-up (34).
UPDRS is used to measure the severity of Parkinson's disease. Although UPDRS-II does not directly evaluate the walking and mobility of PD patients, it covers the evaluation of the patient's motor and non-motor symptoms, such as walking, mobility, and other activities of daily living. The results of existing studies and this meta-analysis show that RAS intervention significantly improves UPDRS-II (37, 42, 45). On the other hand, UPDRS-III is used to assess motor status, including tremor, rigidity, bradykinesia, gait, and postural instability. Duncan et al. concluded that compared with the control group, tango can significantly improve the UPDRS-III score of PD patients, and it still has a lasting improvement effect after 12 months of follow-up (55). Our pooled results align with previous studies that RAS significantly reduces the disability scores of UPDRS-III (22, 37, 38).
Levodopa, dopamine agonists, and type B monoamine oxidase inhibitors are traditional medications for Parkinson's disease (9). However, pharmacotherapy can only alleviate symptoms, not the underlying pathology (56). In addition, adverse effects such as loss of potency and toxicity may occur with long-term use of dopaminergic drugs, which may be due to a decrease in the integrity of dopamine transport in the striatal nerve endings of the substantia nigra associated with levodopa. Also, the progression of the disease may reduce the effectiveness of the drug (10, 57). Therefore, RAS has increasingly received attention to enhance gait performance in patients with Parkinson's disease. Rhythmic changes are associated with various neurophysiological changes, such as increased activation of neurons in the frontal-occipital network and increased excitability of spinal motor neurons by the reticulospinal pathway (58).
Acceptance of RAS can facilitate motor activation patterns by increasing frontal-occipital network connectivity and beta frequency oscillations in the cortex (59). Both the basal ganglia and cerebellum influence cortical movement and movement-related areas via the thalamus (60, 61). Literatures suggest that the cerebellar-thalamocortical motor network can compensate for the deleterious basal ganglia connection-thalamocortical motor network function associated with internal chronotropic processing (62, 63). Stimulating the cerebellum using oscillating transcranial currents delivered at frequencies similar to intrinsic musical rhythms can largely shape the frontal-parietal connections and the sensorimotor rhythms associated with fine adjustment of gait parameters (22, 64). Thus, the cerebellum may participate in internal timing mechanisms when subjected to external rhythmic auditory stimulation.
The literature included in this meta-analysis was all RCTs, which significantly reduced various potential biases and provided high quality evidence. In addition, various parameters and scales assessing gait, mobility, and quality of life in PD patients were included to evaluate the effectiveness of RAS in improving patients' gait and mobility impairment from multiple aspects.
Limitations of this study should be discussed as they may limit the extrapolation of results. First, the majority of the subjects had mild or moderate disease. This lack of information regarding disease severity and their specific deficits have limited their interpretation of outcomes. Second, the number of studies included in the meta-analysis was limited, and the sample size was small. Only two studies had more than 100 subjects, thirteen studies had <50 subjects, making the generalizability of the study survey difficult. Thirdly, by employing the 18 RCTs we had included in this meta-analysis for the PICOs (Population, Intervention, Control and Outcome evaluation), the Table 2 so constructed has demonstrated for each study the specific intervention method: the interventions given to patients in these 18 individual studies were either RAS, (22, 31, 34–39, 41, 44), Rhythm with Musical melody (23, 30, 31, 33, 40, 42, 43, 45) or Physiotherapy on an Acoustic Vibration Chair (32). For control groups, patients would either receive conventional physical therapy, with or without a structured instruction, or an intervention placebo (the vibration chair without rhythm or melody.) These differences in intervention and control could make the comparison's interpretation difficult. Finally, Language bias has always been possible in meta-analysis. Although all 18 RCTs were published in the English language peer-reviewed journals, the minority (5/18) were from native English-speaking countries. Among all these 18 studies: there were 7 studies from Italy, 1 from Poland, 1 from Romania, 2 from Sweden, 3 from the United States, 2 from Canada, 1 from China and 1 from Brazil. Our Funnel Plots using the UPDRS-III, FoG and Speed as the three major outcomes assessments did not exhibit significant publication biases (Figures 6A–C). However, we are reassured by a recent epidemiology paper by Nussbaumer-Streit 2019 (65) using 59 Cochrane Reviews with or without excluding non-English studies to answer this specific question: excluding non-English publication from evidence syntheses does not change conclusions. In summary, the results of this meta-analysis provide more convincing evidence for the effectiveness of RAS in the rehabilitation of PD patients.
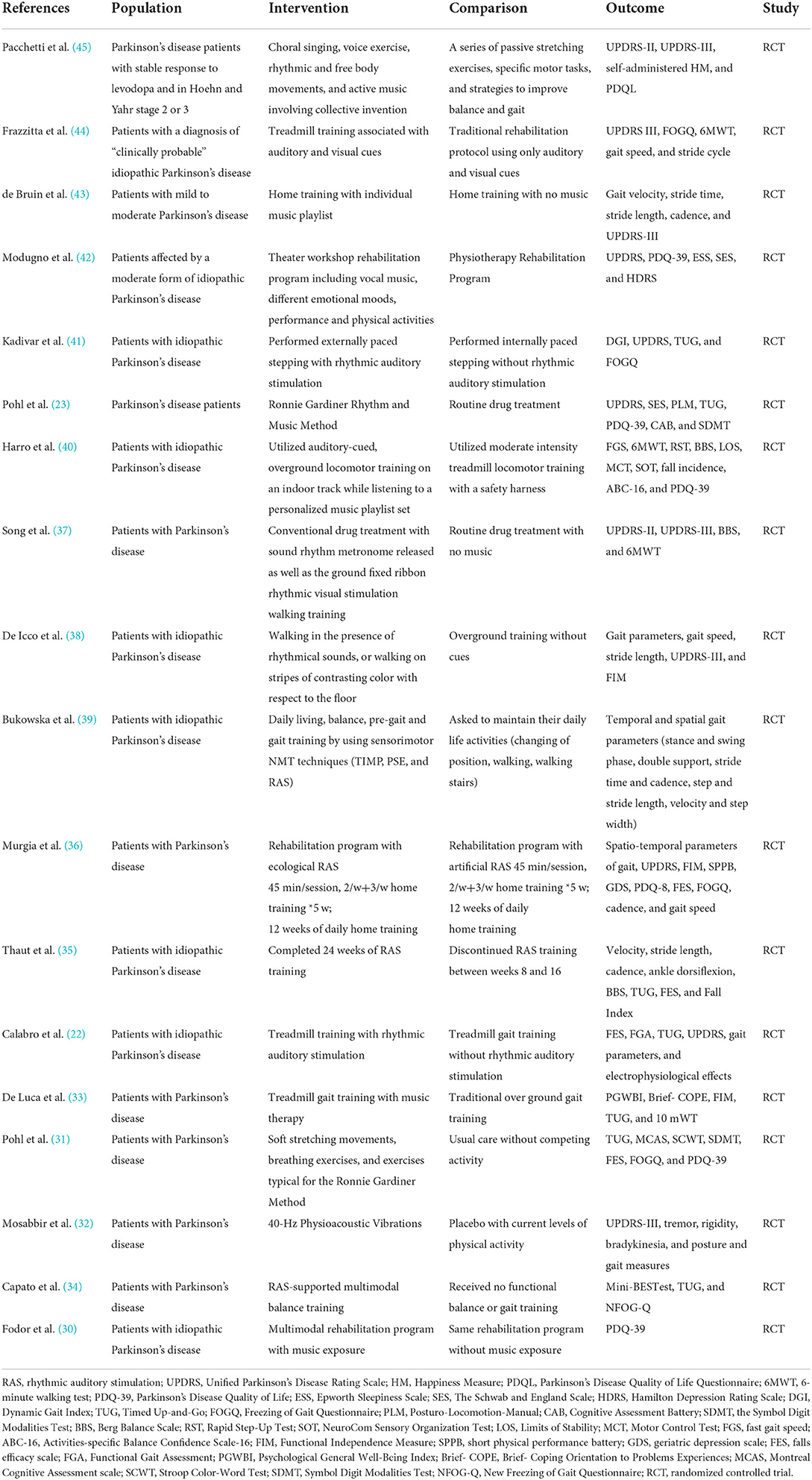
Table 2. PICOs (population, intervention, control, outcome and strategy) characteristics of the studies included in the meta-analysis.
Our study shows the significant efficacy of RAS in improving gait, motor activities and quality of life in Parkinson's patients and suggests its application in clinical practice. However, as the data came from different studies where the sample size, disease severity, stimulation frequency, intervention intensity and functional assessment tools were different. One of the most intriguing examples is the Falls Efficacy Scale (FES). Three out of our 18 studies have independently concluded that rhythmic auditory stimulation can improve gait disturbance (22, 31, 36), however, in combining the original data (a total of 106 patients), the results were of borderline significance (p = 0.05). It is desirable to have a multicenter randomized controlled trial that can simultaneously include the key indicators to further determine the RAS efficacy in gait improvement and quality of life in Parkinson's disease.
Conclusion
In this meta-analysis of 18 randomized controlled trials, we have demonstrated that Rhythmic Auditory Stimulation (RAS) could improve gait, mobility and quality of life in patients with Parkinson's disease. A definitive multi-centre study with a well-defined disease severity, treatment intensity and functional assessment tools should be planned in the future.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
The paper completed at the suggestion and supervised by WP. Two researchers (LL and RH) independently extracted primary data from eligible studies using a standardized form. If the corresponding data could not be extracted directly from the study, it would need to be reanalyzed. Where there were disagreements between above two researchers, a third researcher (XY) was asked to review literatures until a consensus was reached. The data management and statistical analysis were performed by XY and reviewed by statistician YJ from core laboratory. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
RAS, Rhythmic Auditory Stimulation; PD, Parkinson's disease; RCT, Randomized Controlled Trial; PRISMA, Preferred Reporting Items for Systematic Reviews; PEDro, Physiotherapy Evidence Database; TUG, Timed Up-and-Go test; UPDRS, Unified Parkinson's Disease Rating Scale; PDQL, Parkinson's Disease Quality of Life Questionnaire; BBS, Berg Balance Scale; FES, Falls Efficacy Scale; FOGQ, Freezing of Gait Questionnaire; MD, Mean Difference; CI, Confidence Interval; WMD, Weighted Mean Difference; FoG, Freezing of Gait.
References
1. Reeve A, Simcox E, Turnbull D. Ageing and Parkinson's disease: why is advancing age the biggest risk factor? Ageing Res Rev. (2014) 14:19–30. doi: 10.1016/j.arr.2014.01.004
2. de Lau LM, Breteler MM. Epidemiology of Parkinson's disease. Lancet Neurol. (2006) 5:525–35. doi: 10.1016/S1474-4422(06)70471-9
3. Dorsey ER, Bloem BR. The Parkinson pandemic-a call to action. JAMA Neurol. (2018) 75:9–10. doi: 10.1001/jamaneurol.2017.3299
4. Nonnekes J, Snijders AH, Nutt JG, Deuschl G, Giladi N, Bloem BR. Freezing of gait: a practical approach to management. Lancet Neurol. (2015) 14:768–78. doi: 10.1016/S1474-4422(15)00041-1
5. Sveinbjornsdottir S. The clinical symptoms of Parkinson's disease. J Neurochem. (2016) 139 (Suppl. 1):318–24. doi: 10.1111/jnc.13691
6. Peterson DS, Horak FB. Neural control of walking in people with Parkinsonism. Physiology. (2016) 31:95–107. doi: 10.1152/physiol.00034.2015
7. Gazewood JD, Richards DR, Clebak K. Parkinson disease: an update. Am Fam Physician. (2013) 87:267–73.
8. Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, et al. Bilateral deep brain stimulation vs best medical therapy for patients with advanced Parkinson disease: a randomized controlled trial. JAMA. (2009) 301:63–73. doi: 10.1001/jama.2008.929
9. Group PDMC, Gray R, Ives N, Rick C, Patel S, Gray A, et al. Long-term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson's disease (PD MED): a large, open-label, pragmatic randomised trial. Lancet. (2014) 384:1196–205. doi: 10.1016/S0140-6736(14)60683-8
10. Levy G. The relationship of Parkinson disease with aging. Arch Neurol. (2007) 64:1242–6. doi: 10.1001/archneur.64.9.1242
11. Nonnekes J, Nieuwboer A. Towards personalized rehabilitation for gait impairments in Parkinson's disease. J Parkinsons Dis. (2018) 8:S101–6. doi: 10.3233/JPD-181464
12. Bloem BR, de Vries NM, Ebersbach G. Nonpharmacological treatments for patients with Parkinson's disease. Mov Disord. (2015) 30:1504–20. doi: 10.1002/mds.26363
13. Seppi K, Weintraub D, Coelho M, Perez-Lloret S, Fox SH, Katzenschlager R, et al. The movement disorder society evidence-based medicine review update: treatments for the non-motor symptoms of Parkinson's disease. Mov Disord. (2011) 26 (Suppl. 3):S42–80. doi: 10.1002/mds.23884
14. Obeso JA, Rodriguez-Oroz MC, Benitez-Temino B, Blesa FJ, Guridi J, Marin C, et al. Functional organization of the basal ganglia: therapeutic implications for Parkinson's disease. Mov Disord. (2008) 23 (Suppl. 3):S548–59. doi: 10.1002/mds.22062
15. Abbruzzese G, Marchese R, Avanzino L, Pelosin E. Rehabilitation for Parkinson's disease: current outlook and future challenges. Parkinsonism Relat Disord. (2016) 22 (Suppl. 1):S60–4. doi: 10.1016/j.parkreldis.2015.09.005
16. Keus SH, Munneke M, Nijkrake MJ, Kwakkel G, Bloem BR. Physical therapy in Parkinson's disease: evolution and future challenges. Mov Disord. (2009) 24:1–14. doi: 10.1002/mds.22141
17. Porta M, Pilloni G, Pili R, Casula C, Murgia M, Cossu G, et al. Association between objectively measured physical activity and gait patterns in people with Parkinson's disease: results from a 3-month monitoring. Parkinsons Dis. (2018) 2018:7806574. doi: 10.1155/2018/7806574
18. Lauze M, Daneault JF, Duval C. The effects of physical activity in Parkinson's disease: a review. J Parkinsons Dis. (2016) 6:685–98. doi: 10.3233/JPD-160790
19. Urell C, Zetterberg L, Hellstrom K, Anens E. Factors explaining physical activity level in Parkinson s disease: a gender focus. Physiother Theory Pract. (2021) 37:507–16. doi: 10.1080/09593985.2019.1630875
20. Magee WL, Davidson JW. The effect of music therapy on mood states in neurological patients: a pilot study. J Music Ther. (2002) 39:20–9. doi: 10.1093/jmt/39.1.20
21. Bella SD, Benoit CE, Farrugia N, Schwartze M, Kotz SA. Effects of musically cued gait training in Parkinson's disease: beyond a motor benefit. Ann N Y Acad Sci. (2015) 1337:77–85. doi: 10.1111/nyas.12651
22. Calabro RS, Naro A, Filoni S, Pullia M, Billeri L, Tomasello P, et al. Walking to your right music: a randomized controlled trial on the novel use of treadmill plus music in Parkinson's disease. J Neuroeng Rehabil. (2019) 16:68. doi: 10.1186/s12984-019-0533-9
23. Pohl P, Dizdar N, Hallert E. The Ronnie Gardiner rhythm and music method - a feasibility study in Parkinson's disease. Disabil Rehabil. (2013) 35:2197–204. doi: 10.3109/09638288.2013.774060
24. Forte R, Tocci N, De Vito G. The impact of exercise intervention with rhythmic auditory stimulation to improve gait and mobility in Parkinson Disease: an umbrella review. Brain Sci. (2021) 11:685. doi: 10.3390/brainsci11060685
25. Ghai S, Ghai I, Schmitz G, Effenberg AO. Effect of rhythmic auditory cueing on parkinsonian gait: a systematic review and meta-analysis. Sci Rep. (2018) 8:506. doi: 10.1038/s41598-017-16232-5
26. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. (2009) 62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006
27. de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. (2009) 55:129–33. doi: 10.1016/S0004-9514(09)70043-1
28. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
29. Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. (2010) 1:97–111. doi: 10.1002/jrsm.12
30. Fodor DM, Breda XM, Valean D, Marta MM, Perju-Dumbrava L. Music as add-on therapy in the rehabilitation program of Parkinson's disease patients-a Romanian pilot study. Brain Sci. (2021) 11:569. doi: 10.3390/brainsci11050569
31. Pohl P, Wressle E, Lundin F, Enthoven P, Dizdar N. Group-based music intervention in Parkinson's disease - findings from a mixed-methods study. Clin Rehabil. (2020) 34:533–44. doi: 10.1177/0269215520907669
32. Mosabbir A, Almeida QJ, Ahonen H. The effects of long-term 40-Hz physioacoustic vibrations on motor impairments in Parkinson's disease: a double-blinded randomized control trial. Healthcare. (2020) 8:113. doi: 10.3390/healthcare8020113
33. De Luca R, Latella D, Maggio MG, Leonardi S, Sorbera C, Di Lorenzo G, et al. Do patients with PD benefit from music assisted therapy plus treadmill-based gait training? An exploratory study focused on behavioral outcomes. Int J Neurosci. (2020) 130:933–40. doi: 10.1080/00207454.2019.1710147
34. Capato TTC, de Vries NM, IntHout J, Ramjith J, Barbosa ER, Nonnekes J, et al. Multimodal balance training supported by rhythmic auditory stimuli in Parkinson disease: effects in freezers and nonfreezers. Phys Ther. (2020) 100:2023–34. doi: 10.1093/ptj/pzaa146
35. Thaut MH, Rice RR, Braun Janzen T, Hurt-Thaut CP, McIntosh GC. Rhythmic auditory stimulation for reduction of falls in Parkinson's disease: a randomized controlled study. Clin Rehabil. (2019) 33:34–43. doi: 10.1177/0269215518788615
36. Murgia M, Pili R, Corona F, Sors F, Agostini TA, Bernardis P, et al. The use of footstep sounds as rhythmic auditory stimulation for gait rehabilitation in Parkinson's disease: a randomized controlled trial. Front Neurol. (2018) 9:348. doi: 10.3389/fneur.2018.00348
37. Song JH, Zhou PY, Cao ZH, Ding ZG, Chen HX, Zhang GB. Rhythmic auditory stimulation with visual stimuli on motor and balance function of patients with Parkinson's disease. Eur Rev Med Pharmacol Sci. (2015) 19:2001–7.
38. De Icco R, Tassorelli C, Berra E, Bolla M, Pacchetti C, Sandrini G. Acute and chronic effect of acoustic and visual cues on gait training in Parkinson's disease: a randomized, controlled study. Parkinsons Dis. (2015) 2015:978590. doi: 10.1155/2015/978590
39. Bukowska AA, Krezalek P, Mirek E, Bujas P, Marchewka A. Neurologic music therapy training for mobility and stability rehabilitation with Parkinson's disease - a pilot study. Front Hum Neurosci. (2015) 9:710. doi: 10.3389/fnhum.2015.00710
40. Harro CC, Shoemaker MJ, Frey O, Gamble AC, Harring KB, Karl KL, et al. The effects of speed-dependent treadmill training and rhythmic auditory-cued overground walking on balance function, fall incidence, and quality of life in individuals with idiopathic Parkinson's disease: a randomized controlled trial. NeuroRehabilitation. (2014) 34:541–56. doi: 10.3233/NRE-141048
41. Kadivar Z, Corcos DM, Foto J, Hondzinski JM. Effect of step training and rhythmic auditory stimulation on functional performance in Parkinson patients. Neurorehabil Neural Repair. (2011) 25:626–35. doi: 10.1177/1545968311401627
42. Modugno N, Iaconelli S, Fiorlli M, Lena F, Kusch I, Mirabella G. Active theater as a complementary therapy for Parkinson's disease rehabilitation: a pilot study. Sci World J. (2010) 10:2301–13. doi: 10.1100/tsw.2010.221
43. de Bruin N, Doan JB, Turnbull G, Suchowersky O, Bonfield S, Hu B, et al. Walking with music is a safe and viable tool for gait training in Parkinson's disease: the effect of a 13-week feasibility study on single and dual task walking. Parkinsons Dis. (2010) 2010:483530. doi: 10.4061/2010/483530
44. Frazzitta G, Maestri R, Uccellini D, Bertotti G, Abelli P. Rehabilitation treatment of gait in patients with Parkinson's disease with freezing: a comparison between two physical therapy protocols using visual and auditory cues with or without treadmill training. Mov Disord. (2009) 24:1139–43. doi: 10.1002/mds.22491
45. Pacchetti C, Mancini F, Aglieri R, Fundaro C, Martignoni E, Nappi G. Active music therapy in Parkinson's disease: an integrative method for motor and emotional rehabilitation. Psychosom Med. (2000) 62:386–93. doi: 10.1097/00006842-200005000-00012
46. Rocha PA, Porfirio GM, Ferraz HB, Trevisani VF. Effects of external cues on gait parameters of Parkinson's disease patients: a systematic review. Clin Neurol Neurosurg. (2014) 124:127–34. doi: 10.1016/j.clineuro.2014.06.026
47. Rodriguez-Molinero A, Herrero-Larrea A, Minarro A, Narvaiza L, Galvez-Barron C, Gonzalo Leon N, et al. The spatial parameters of gait and their association with falls, functional decline and death in older adults: a prospective study. Sci Rep. (2019) 9:8813. doi: 10.1038/s41598-019-45113-2
48. Mirelman A, Bonato P, Camicioli R, Ellis TD, Giladi N, Hamilton JL, et al. Gait impairments in Parkinson's disease. Lancet Neurol. (2019) 18:697–708. doi: 10.1016/S1474-4422(19)30044-4
49. Herssens N, Verbecque E, Hallemans A, Vereeck L, Van Rompaey V, Saeys W. Do spatiotemporal parameters and gait variability differ across the lifespan of healthy adults? A systematic review. Gait Posture. (2018) 64:181–90. doi: 10.1016/j.gaitpost.2018.06.012
50. Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol. (2011) 10:734–44. doi: 10.1016/S1474-4422(11)70143-0
51. Giladi N, Treves TA, Simon ES, Shabtai H, Orlov Y, Kandinov B, et al. Freezing of gait in patients with advanced Parkinson's disease. J Neural Transm. (2001) 108:53–61. doi: 10.1007/s007020170096
52. Li E, Ruan X, Li Y, Zhang G, Li M, Wei X. Exploring cortical thickness alteration in Parkinson disease patients with freezing of gaits. Neural Plast. (2020) 2020:8874119. doi: 10.1155/2020/8874119
53. Kerr GK, Worringham CJ, Cole MH, Lacherez PF, Wood JM, Silburn PA. Predictors of future falls in Parkinson disease. Neurology. (2010) 75:116–24. doi: 10.1212/WNL.0b013e3181e7b688
54. Wroblewska A, Gajos A, Smyczynska U, Bogucki A. The therapeutic effect of Nordic walking on freezing of gait in Parkinson's disease: a pilot study. Parkinsons Dis. (2019) 2019:3846279. doi: 10.1155/2019/3846279
55. Duncan RP, Earhart GM. Randomized controlled trial of community-based dancing to modify disease progression in Parkinson disease. Neurorehabil Neural Repair. (2012) 26:132–43. doi: 10.1177/1545968311421614
56. Kolk N, Vries N, Kessels R, Joosten H, Bloem BR. Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson's disease: a double-blind, randomised controlled trial. Lancet Neurol. (2019) 18:998–1008. doi: 10.1016/S1474-4422(19)30285-6
57. Fahn S, Parkinson Study Group. Does levodopa slow or hasten the rate of progression of Parkinson's disease? J Neurol. (2005) 252 (Suppl. 4):IV37–42. doi: 10.1007/s00415-005-4008-5
58. Thaut MH, Gardiner JC, Holmberg D, Horwitz J, Kent L, Andrews G, et al. Neurologic music therapy improves executive function and emotional adjustment in traumatic brain injury rehabilitation. Ann N Y Acad Sci. (2009) 1169:406–16. doi: 10.1111/j.1749-6632.2009.04585.x
59. Braunlich K, Seger CA, Jentink KG, Buard I, Kluger BM, Thaut MH. Rhythmic auditory cues shape neural network recruitment in Parkinson's disease during repetitive motor behavior. Eur J Neurosci. (2019) 49:849–58. doi: 10.1111/ejn.14227
60. Bonnefoi-Kyriacou B, Legallet E, Lee RG, Trouche E. Spatio-temporal and kinematic analysis of pointing movements performed by cerebellar patients with limb ataxia. Exp Brain Res. (1998) 119:460–6. doi: 10.1007/s002210050361
61. Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. (1989) 12:366–75. doi: 10.1016/0166-2236(89)90074-X
62. Petter EA, Lusk NA, Hesslow G, Meck WH. Interactive roles of the cerebellum and striatum in sub-second and supra-second timing: support for an initiation, continuation, adjustment, and termination (ICAT) model of temporal processing. Neurosci Biobehav Rev. (2016) 71:739–55. doi: 10.1016/j.neubiorev.2016.10.015
63. Sen S, Kawaguchi A, Truong Y, Lewis MM, Huang X. Dynamic changes in cerebello-thalamo-cortical motor circuitry during progression of Parkinson's disease. Neuroscience. (2010) 166:712–9. doi: 10.1016/j.neuroscience.2009.12.036
64. Naro A, Leo A, Russo M, Cannavo A, Milardi D, Bramanti P, et al. Does transcranial alternating current stimulation induce cerebellum plasticity? Feasibility, safety and efficacy of a novel electrophysiological approach. Brain Stimul. (2016) 9:388–95. doi: 10.1016/j.brs.2016.02.005
Keywords: Parkinson's patients, rhythmic auditory stimulation, gait, mobility, meta-analysis
Citation: Ye X, Li L, He R, Jia Y and Poon W (2022) Rhythmic auditory stimulation promotes gait recovery in Parkinson's patients: A systematic review and meta-analysis. Front. Neurol. 13:940419. doi: 10.3389/fneur.2022.940419
Received: 10 May 2022; Accepted: 24 June 2022;
Published: 28 July 2022.
Edited by:
Hannes Devos, University of Kansas, United StatesReviewed by:
Carlos Zúñiga-Ramírez, Civil Hospital of Guadalajara, MexicoFrancesco Lena, Mediterranean Neurological Institute Neuromed (IRCCS), Italy
Copyright © 2022 Ye, Li, He, Jia and Poon. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Waisang Poon, d3Bvb25Ac3VyZ2VyeS5jdWhrLmVkdS5oaw==
 Xiaofan Ye
Xiaofan Ye Ling Li
Ling Li Rong He2
Rong He2 Yizhen Jia
Yizhen Jia Waisang Poon
Waisang Poon