- 1Department of Neurology and Institute of Neurology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 2Department of Neurology, Hainan General Hospital, Hainan Affiliated Hospital of Hainan Medical University, Haikou, China
- 3Department of Pathology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Neuropsychiatric systemic lupus erythematosus (NPSLE) has been considered to have high morbidity and mortality. Thus, earlier recognition and treatment are of great importance. However, the rapid progression of cognitive dysfunction with leukoencephalopathy as an initial presentation in SLE is rarely described. We report a case in which an elderly man experienced rapidly progressive cognitive impairment with bilateral, symmetric, and diffuse leukoencephalopathy with lasting diffusion-weighted image hyperintensity. An immunological workup showed low complement levels and positivity for antinuclear antibody -speckle and Coombs tests in the patient's serum samples. He had an appropriate improvement in cognitive function after receiving a combination of various immunotherapies. Long-term follow-up showed clinical improvement, including rheumatological labs and neuroimaging. A review of the literature on NPSLE with leukoencephalopathy and a summary of all reported cases to date are also presented. Our case indicated that isolated leukoencephalopathy in NPSLE, as an indicator of severe NPSLE, can be recognized early. Immunotherapy is warranted given the possibility of clinical improvement.
Introduction
Systemic lupus erythematosus (SLE) is a chronic inflammatory autoimmune disorder characterized by multiorgan involvement, the vast majority of whom are females of childbearing age (1). It presents clinically heterogeneous, usually involving in renal, dermatological, neuropsychiatric, musculo-skeletal and cardiovascular symptoms (1). Approximately 50 to 80% of patients have neuropsychiatric involvement in SLE (NPSLE) (2). NPSLE has been associated with an increase in the mortality rate and a lower quality of life (3, 4). Neuropsychiatric involvement occurs in the early stage of SLE and presents a variety of symptoms (4, 5). These include psychiatric manifestations (mood disorders, cognitive impairment, and psychosis), stroke, seizures, myelopathy, chorea, and headaches (5). While neuropsychiatric events as initial symptoms in SLE patients are rare (4). Moreover, neuropsychiatric manifestations were classified into focal and diffuse neuropsychological syndromes (6). In the diffuse neuropsychological syndromes, isolated diffuse leukoencephalopathy with acute confusional state or cognitive dysfunction in lupus has rarely been reported and presents benign or malignant outcomes (7). Given the heterogeneity of the clinical manifestations, it is extremely difficult to recognize and treat early.
Here, our case report illustrates neuropsychiatric symptoms as an initial and only manifestation of SLE, with bilateral, symmetric, and diffuse leukoencephalopathy with lasting hyperintense diffusion-weighted images (DWI). We reviewed the literature on the clinical characteristics of and treatment responses for this condition. These characteristics are rare and make diagnosis challenging. Such a presentation represents a severe variant of NPSLE requiring aggressive immunosuppressive therapies.
Methods and Results
Patient Data
A 58-year-old male initially presented to our hospital with cognitive dysfunction and gait disturbance. He started to have memory decline, impaired attention, and deficits in processing speed 2 months prior to his admission. Subsequently, he presented dysphagia and aphasia. His movement gradually became sluggish and even led to him being bedridden long-term. Meanwhile, he had never suffered from the malar, or butterfly rash, glomerulonephritis, arthralgias, and anemia etc.
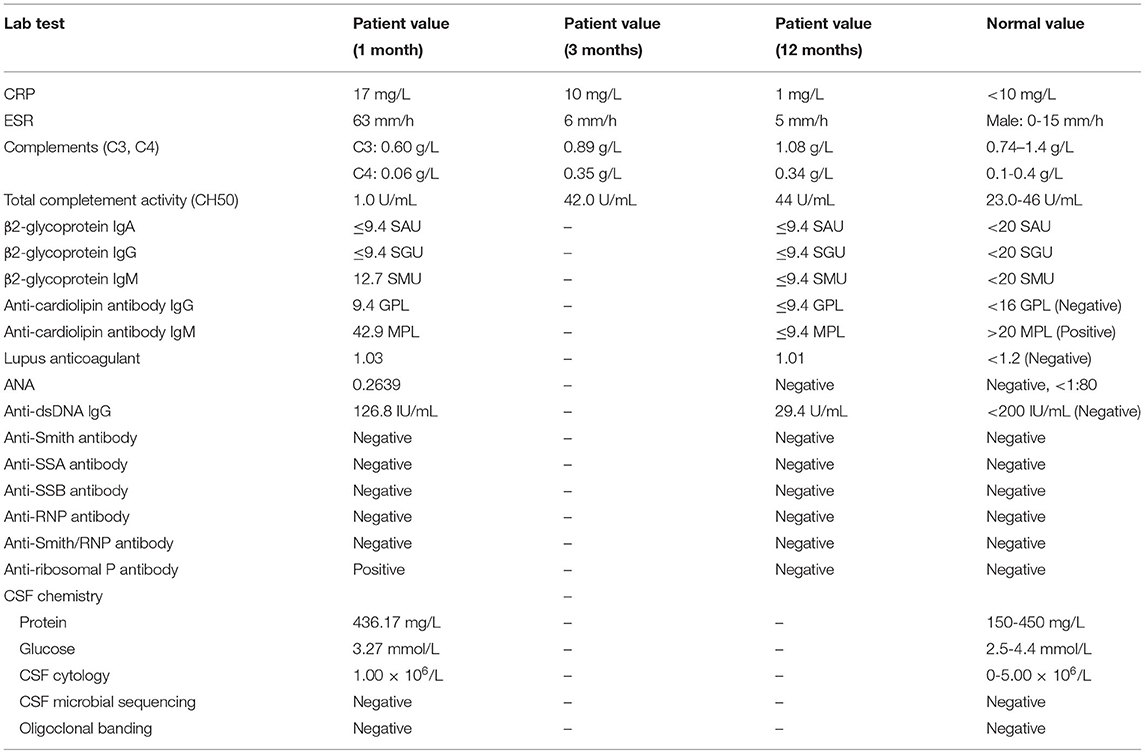
The serological workup of immunology (Table 1) showed an increase in the erythrocyte sedimentation rate (ESR) ( 63 mm/h), anti-cardiolipin antibody IgM (42.9 MPL) level and C-reactive protein (CRP) (17 mg/L) level, a homogeneous antinuclear antibody-speckle (ANA) 320X test, Coombs test and anti-ribosomal P positivity and a decrease in C3 complement (C3) (0.60 g/L), C4 complement (C4) (0.06 g/L) and total completement activity (CH50) (1.0 U/mL) levels. Cerebral spinal fluid (CSF) analysis (Table 1) was negative under normal pressure, including for paraneoplastic antibodies (hu, ri, CV2, ANNA-3, PCA-2, YO, MA2, Amphiphysin, Tr, GAD) and autoimmunity encephalitis-associated antibodies (NMDA, AMPA1, AMPA2, IgLON5, GABA, LGI, CASPR2, MBP, MOG, AQP4). The leukodystrophy-associated gene panel testing results and exercise testing on serum lactate were negative.
Brain magnetic resonance imaging (MRI) (Figure 1) demonstrated symmetric abnormalities on fluid-attenuated inversion recovery (FLAIR) hyperintensity within the white matter of the corona radiata, brachium pontis, and basal ganglia, with T1-weighted associated hypointensity (T1WI), without enhancing lesions but with correlating areas of hyperintensity on DWI. MR spectroscopy showed increased choline and a decreased N-acetyl aspartate (NAA) peak, and positron emission computed tomography (PET-CT) showed decreased fluorodeoxyglucose (FDG) uptake of the corona radiata, suggesting demyelination of the white matter. Accordingly, a stereotactic biopsy for corona radiata excluded the possibility of lymphomas. The pathology showed hydropic degeneration, gliocyte proliferation, and perivascular lymphocyte infiltration in the white matter (Figure 2).
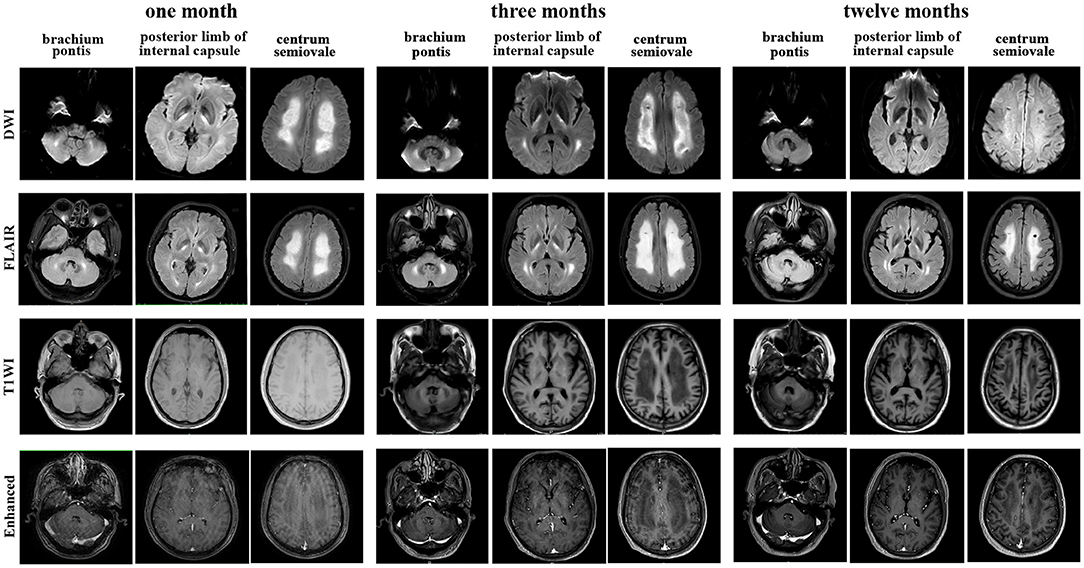
Figure 1. Brain magnetic resonance imaging (MRI) performed at the 1-month, 3-month, and 12-month follow-ups showed T1-weighted, diffusion-weighted images (DWI), fluid-attenuated inversion recovery (FLAIR) and enhanced T1 MRI axial images of the centrum semiovale, brachium pontis and internal capsule lesions.
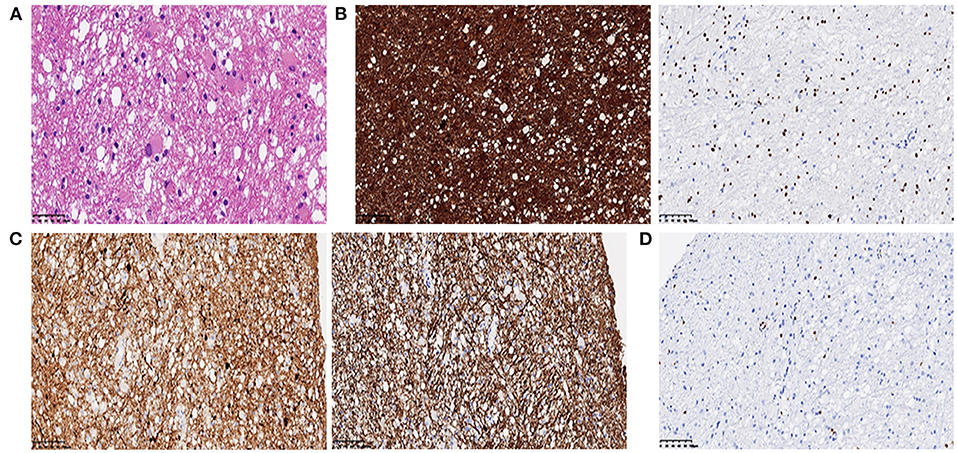
Figure 2. (A) Histopathology showed mild hydropic degeneration and gliocyte proliferation of the lesion (H & E, 400×); (B) GFAP (left panel) and Olig2 (right panel) immunohistochemistry showed positive staining of reactive gliocytes (200×); (C) SYN (left panel) and NF (right panel) immunohistochemistry showed positive staining of neurofilaments. (D) Lymphocytes highlighted by CD3 showed a perivascular infiltration pattern.
He received methylprednisolone (500 mg over 4 days) and had transient cognitive improvement. Due to progressive confusion, low-dose methylprednisolone (80 mg) combined with immunoglobulin G (10 g), plasma exchange (one cycle) and rituximab (500 mg, four cycles) was administered. His consciousness improved, and he could respond with simple body language to the doctor's orders after therapy. However, he remained dysphagic, aphasic and disabled at the time of discharge. The SLE disease activity index (Table 1) was improved throughout the course of immunosuppressive therapy. Repeated MRI showed a stable lesion without new changes after 3 months (Figure 1).
At his one-year follow-up visit, he could perform oral intake and communicate verbally but had difficulty walking alone. Meanwhile, the disease remained stable, without recurrence. He was maintained on 40 mg of methylprednisolone daily. His rheumatological lab results had recovered to a normal status (Table 1). Subsequently, he was treated with rituximab (500 mg, 1 cycles, every year) to prevent relapse. Repeat brain MRI at 1 year showed minimal residual hyperintensity on DWI and a small resolution of T2/FLAIR abnormalities, such as in the brachium pontis (Figure 1).
Literature Review
Twelve cases of leukoencephalopathy in NPSLE patients from 1991 to 2021 was searched by the online database PubMed utilizing the search strategies “NPSLE” and “leukoencephalopathy”. Further searches were undertaken to identify articles by searching string “white matter”, “intracranial hypertension”, and “cerebral oedema”. Additional cases not captured by the initial search method were found in the reference lists of identified cases. All cases that were selected presented leukoencephalopathy in the MRI. Data extracted from these case were age, sex, initial neurological symptoms, other previous organs involved, imaging findings-DWI, CSF, clinical treatment (outcomes), and follow up (Table 2). Our case has been rarely reported until now from the literature review. Most patient experienced aggressive treatment strategies earlier and had benign outcome.
Discussion
This case report describes a male patient with a diagnosis of SLE with the initial and only onset of diffuse leukoencephalopathy on imaging, characterized symptomatically only by the rapid progression of cognitive dysfunction. He had a gradual, but incomplete, recovery with a stronger immunosuppression treatment. To the best of our knowledge, no case reports have been made associating these entities.
SLE affects women more frequently than men, with the preponderance of occurrence around childbearing age (15–44) (19). Similarly, 12 patients with leukoencephalopathy and NPSLE have been reported, largely involving women around a mean age of 34 years (Table 2). NPSLE is more frequent in the juvenile-onset SLE than in adults with SLE (20, 21). Moreover, nearly half of children with SLE will exhibit CNS involvement in the first year after initial diagnosis (20, 21). As shown in Table 2, three cases that were children has been reported with leukoencephalopathy as the first manifestation of juvenile-onset NPSLE. Additionally, men with SLE often have a more rapid progression in the clinical course with severe organ damage, resulting in a poorer prognosis than women (19).
Many patients already had a diagnosis of SLE and/or other systemic symptoms related to their disease prior to CNS involvement (22). In contrast, our patient made a diagnosis of SLE charactered by isolated CNS involvement. Lab testing reveals ANA at a titer of ≥1:80 and then SLE classification requires at least one clinical criterion and ≥10 points according to the 2019 EULAR/ACR criteria for SLE (23). Thus, our case shown ANA at a titer of 1:320, rapid progression of cognitive dysfunction (2 points), positive Coomb's test (4 points), an increase in anti-cardiolipin antibody IgM level (2 points), and low complement (C3 and C4) (4 points). On the above findings, a diagnosis of SLE was highly suspected. However, NPSLE was not specific, and a careful process of exclusion of causes was necessary. In the literature review about leukoencephalopathy, etiologies were warranted to be excluded, involving in metabolism, leukodystrophies and infection. Evidence suggests that SLE is strongly associated with B cell lymphoma (24, 25). In our case, an extensive workup was performed and these results were normal, including autoantibodies and sequencing of CSF, tandem mass spectrometry of blood and urine, exercise testing on serum lactate, cerebral MR spectroscopy, plasma ammonium, thyroid studies, folate, vitamin B12 and leukodystrophy-associated gene panel testing. Ultimately, our case ruled out a CNS infection, metabolism, tumors and genetics etc.
Most SLE patients with cognitive dysfunction have subtle or subclinical disease with a stable, improving or fluctuating course and rarely have a rapid progression to dementia (2). In addition, most SLE-related cognitive dysfunction occurs in the absence of active systemic lupus or major NPSLE events (2). MRI has shown lower hippocampal volumes in SLE patients with cognitive dysfunction than in those without cognitive dysfunction (26). Interestingly, our patient presented dementia with a rapid course as the initial manifestation of active SLE with diffuse leukoencephalopathy, unlike two patients who had gradual cognitive decline and other organs involved, as shown in Table 2. This characteristic is extremely rare and makes early diagnosis difficult.
Common MRI changes in NPSLE patients include small punctate focal lesions in periventricular and subcortical white matter hyperintensity, brain atrophy, and infarcts (27). Indeed, there have been case reports of patients with T2/FLAIR abnormalities with associated leukoencephalopathy, as shown in Table 2. However, we were unable to find an instance of a patient with a lasting DWI abnormality in leukoencephalopathy as the presenting sign of lupus upon initial diagnosis. Furthermore, patients have shown near-complete resolution of FLAIR abnormalities after immunosuppressive treatment. In contrast to these cases, our patient had a unique feature of hyperintense DWI and no reversible lesions at 3 months following a series of immunosuppressive therapies. There was a disappearance of hyperintensity on DWI after 1 year, while the FLAIR abnormalities remained. Hyperintensity on DWI MRI indicates that cytotoxic oedema has occurred and the damage is irreversible. This may be an indicator of severe NPSLE. What is more, inconsistent with most common findings, such as microinfarction and vasculitis in NPSLE (28), the pathology showed hydropic degeneration, gliocyte proliferation in our patient. Based on the relatively severe pathologic injury in brain, we might make an explanation on severe cognitive dysfunction in our patient.
Diagnosis of NPSLE is the lack of specific and sensitive CSF testing (6). As previous studies have shown, some autoantibodies have been suggested as a potential biomarker for diagnosis and therapeutic decision, such as antineuronal, anti-ribosomal P, and ant-NR2 antibodies (6). Autoantibodies may be detected in the CSF as a result of the transfer of peripherally produced autoantibodies across a breached blood brain barrier or increased intrathecal production (2). In addition, these autoantibodies could be synthesized intrathecally, supported by studies reporting that CSF lgG index/oligoclonal bands (OB) are frequently elevated in patients with NPSLE (29). Nevertheless, three NPSLE patients with leukoencephalopathy (Table 2), besides our case, presented negative, and these mechanisms will be warranted to further explore. Idiopathic intracranial hypertension (IIH), defined by an increased intracranial pressure without hydrocephalus or lesions on the MRI and with normal CSF composition, has been reported in a few patients with NPSLE (30). IIH usually indicates a favorable outcome (30). According to Table 2 findings, the increased intracranial pressure was a common manifestation in NPSLE patients with diffuse leukoencephalopathy. Therefore, difference from IIH, this subtype could be considered as vasogenic oedema and requires treatment aggressively to avoid death, especially combined immunotherapy with dehydrant usage, including acetazolamide, mannitol and furosemide.
High dose steroids have been a unifying treatment choice for neuropsychiatric lupus (31). In addition, due to patients' significant autoantibody loads and severe symptoms, rituximab and cyclophosphamide have been used to treat the severity of CNS involvement (32, 33). As Table 2 shows, most patients with acute SLE leukoencephalopathy had a full clinical recovery after alone high-dose steroid therapy. Of course, others had experienced incomplete or no clinical response and combined immunotherapy was warranted, including rituximab and cyclophosphamide. Overall, there have been reports that a large of patients experienced a gradual or marked improvement of clinical symptoms after treatment at period of acute course. In the follow-up visit, oral prednisone was used to maintain therapy and, whether examination or symptom, some patients were under a stable state. But, in the patients with lupus relapse, the aggressive immunosuppressants, such as rituximab, immunoglobulin G, plasmapheresis and even autologous stem cell transplantation, is warranted to avoid death as soon as possible (34). In our patient, given the low response to steroids, various methods were used for treatment, including immunoglobulin G, rituximab, and plasma exchange. Meanwhile, his SLE disease activity index recovered to baseline, and his orientation was improved (Table 1), confirming the response to treatment. At his one-year follow-up visit, his symptoms had improved further without recurrence following low-dose oral prednisone.
Conclusion
NPSLE diagnosis is a challenge for clinicians, both at the diagnostic and therapeutic levels. Isolated leukoencephalopathy with hyperintense DWI on MRI in SLE, an indicator of severe NPSLE, has rarely been reported in the literature. Taken together, as a subtype of NPSLE, it is necessary to recognize severe NPSLE and provide aggressive immunosuppression therapy as soon as possible.
Data Availability Statement
The datasets presented in this article are not readily available because of ethical and privacy restrictions. Requests to access the datasets should be directed to the corresponding author/s.
Ethics Statement
The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
YF, QX, and XY wrote the paper. TY implemented tissue staining. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
SLE, Systemic lupus erythematosus; NPSLE, neuropsychiatric involvement in SLE; DWI, diffusion-weighted images; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; ANA, antinuclear antibody -speckle; CH50, total completement activity; CSF, Cerebral spinal fluid; MRI, Brain magnetic resonance imaging; FLAIR, fluid-attenuated inversion recovery; T1WI, T1-weighted associated hypointensity; NAA, N-acetyl aspartate; PET-CT, positron emission computed tomography; FDG, fluorodeoxyglucose.
References
1. Fava A, Petri M. Systemic lupus erythematosus: diagnosis and clinical management. J Autoimmun. (2019) 96:1–13. doi: 10.1016/j.jaut.2018.11.001
2. Seet D, Allameen NA, Tay SH, Cho J, Mak A. Cognitive dysfunction in systemic lupus erythematosus: immunopathology, clinical manifestations, neuroimaging and management. Rheumatol Ther. (2021) 8:651–79. doi: 10.1007/s40744-021-00312-0
3. Yu HH, Lee JH, Wang LC, Yang YH, Chiang BL. Neuropsychiatric manifestations in pediatric systemic lupus erythematosus: a 20-year study. Lupus. (2006) 15:651–7. doi: 10.1177/0961203306070990
4. Hanly JG, Urowitz MB, Sanchez-Guerrero J, Bae SC, Gordon C, Wallace DJ, et al. Neuropsychiatric events at the time of diagnosis of systemic lupus erythematosus: an international inception cohort study. Arthritis Rheum. (2007) 56:265–73. doi: 10.1002/art.22305
5. Govoni M, Bortoluzzi A, Padovan M, Silvagni E, Borrelli M, Donelli F, et al. The diagnosis and clinical management of the neuropsychiatric manifestations of lupus. J Autoimmun. (2016) 74:41–72. doi: 10.1016/j.jaut.2016.06.013
6. Sarwar S, Mohamed AS, Rogers S, Sarmast ST, Kataria S, Mohamed KH, et al. Neuropsychiatric systemic lupus erythematosus: a 2021 update on diagnosis. Manage Curr Chall Cureus. (2021) 13:e17969. doi: 10.7759/cureus.17969
7. Prabhakaran S, Bramlage M, Edgar MA, Diamond B, Hardin JA, Volpe BT. Overwhelming leukoencephalopathy as the only sign of neuropsychiatric lupus. J Rheumatol. (2005) 32:1843–5.
8. Shibata M, Kibe T, Fujimoto S, Ishikawa T, Murakami M, Ichiki T, et al. Diffuse central nervous system lupus involving white matter, basal ganglia, thalami and brainstem. Brain Dev. (1999) 21:337–40. doi: 10.1016/S0387-7604(99)00027-3
9. Ogawa M, Ishimaru K, Shiroto T, Baba M, Matsunaga M. [A case of benign intracranial hypertension associated with systemic lupus erythematosus (SLE) showing diffuse white matter lesions on MRI]. Rinsho Shinkeigaku. (1994) 34:577–81.
10. Koffman L, Prayson R, Manno EM. Malignant cerebral edema related to systemic lupus erythematosus. J Neurol Sci. (2016) 364:180–2. doi: 10.1016/j.jns.2016.03.040
11. Kirk A, Kertesz A, Polk MJ. Dementia with leukoencephalopathy in systemic lupus erythematosus. Can J Neurol Sci. (1991) 18:344–8. doi: 10.1017/S0317167100031929
12. Ogura N, Atsumi T, Sagawa A, Jodo S, Amasaki Y, Nakabayashi T, et al. [Systemic lupus erythematosus associated with benign intracranial hypertension: a case report]. Ryumachi. (1992) 32:66–72.
13. Chaves-Carballo E, Dabbagh O, Bahabri S. Pseudotumor cerebri and leukoencephalopathy in childhood lupus. Lupus. (1999) 8:81–4. doi: 10.1191/096120399678847317
14. Aydin N, Utku U, Huseyin OT. An uncommon central nervous system manifestation in systemic lupus erythematosus: the diffuse and symmetrical lesions of white matter. Eur J Neurol. (2000) 7:585. doi: 10.1046/j.1468-1331.2000.t01-1-00118.x
15. Isshi K, Hirohata S, Hashimoto T, Miyashita H. Systemic lupus erythematosus presenting with diffuse low density lesions in the cerebral white matter on computed axial tomography scans: its implication in the pathogenesis of diffuse central nervous system lupus. J Rheumatol. (1994) 21:1758–62.
16. Theisen A, Bose P, Knight C, Oliver M. Leukoencephalopathy and cerebral edema as the presenting manifestations of SLE in an ANA-negative adolescent female: a case report and review of literature. Pediatr Rheumatol Online J. (2020) 18:58. doi: 10.1186/s12969-020-00449-2
17. Shintani S, Ono K, Hinoshitd H, Shiigai T, Tsuruoka S. Unusual neuroradiological findings in systemic lupus erythematosus. Eur Neurol. (1993) 33:13–6. doi: 10.1159/000116891
18. Laassila S, Aboulem G, Chtaou N, Belahsen MF. Intracranial hypertension with reversible cerebral edema: atypical presentation of neuropsychiatric systemic lupus erythematosus. Radiol Case Rep. (2022) 17:1416–20. doi: 10.1016/j.radcr.2022.02.018
19. Nusbaum JS, Mirza I, Shum J, Freilich RW, Cohen RE, Pillinger MH, et al. Sex differences in systemic lupus erythematosus: epidemiology, clinical considerations, and disease pathogenesis. Mayo Clin Proc. (2020) 95:384–94. doi: 10.1016/j.mayocp.2019.09.012
20. Levy DM, Kamphuis S. Systemic lupus erythematosus in children and adolescents. Pediatr Clin North Am. (2012) 59:345–64. doi: 10.1016/j.pcl.2012.03.007
21. Giani T, Smith EM, Al-Abadi E, Armon K, Bailey K, Ciurtin C, et al. Neuropsychiatric involvement in juvenile-onset systemic lupus erythematosus: Data from the UK Juvenile-onset systemic lupus erythematosus cohort study. Lupus. (2021) 30:1955–65. doi: 10.1177/09612033211045050
22. Hermosillo-Romo D, Brey RL. Diagnosis and management of patients with neuropsychiatric systemic lupus erythematosus (NPSLE). Best Pract Res Clin Rheumatol. (2002) 16:229–44. doi: 10.1053/berh.2001.0223
23. Aringer M. EULAR/ACR classification criteria for SLE. Semin Arthritis Rheum. (2019) 49:S14–7. doi: 10.1016/j.semarthrit.2019.09.009
24. Dias C, Isenberg DA. Susceptibility of patients with rheumatic diseases to B-cell non-Hodgkin lymphoma. Nat Rev Rheumatol. (2011) 7:360–8. doi: 10.1038/nrrheum.2011.62
25. Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of lymphoma development in autoimmune diseases: a meta-analysis. Arch Intern Med. (2005) 165:2337–44. doi: 10.1001/archinte.165.20.2337
26. Kello N, Anderson E, Diamond B. Cognitive dysfunction in systemic lupus erythematosus: a case for initiating trials. Arthritis Rheumatol. (2019) 71:1413–25. doi: 10.1002/art.40933
27. Jeong HW, Her M, Bae JS, Kim SK, Lee SW, Kim HK, et al. Brain MRI in neuropsychiatric lupus: associations with the 1999 ACR case definitions. Rheumatol Int. (2015) 35:861–9. doi: 10.1007/s00296-014-3150-8
28. Thirunavukkarasu B, Gupta K, Nada R, Rathi M, Dhir V, Ahuja CK, et al. Neuropathological spectrum in systemic lupus erythematosus: A single institute autopsy experience. J Neuroimmunol. (2021) 353:577518. doi: 10.1016/j.jneuroim.2021.577518
29. Sterling G, West MACW. Neuropsychiatric lupus erythematosus: a lo-year prospective study on the value of diagnostic tests. Am J Med. (1995) 8:153–63. doi: 10.1016/S0002-9343(99)80135-1
30. Rajasekharan C, Renjith SW, Marzook A, Parvathy R. Idiopathic intracranial hypertension as the initial presentation of systemic lupus erythematosus. Case Reports. (2013) 2013:r2012007886. doi: 10.1136/bcr-2012-007886
31. Fanouriakis A, Kostopoulou M, Alunno A, Aringer M, Bajema I, Boletis JN, et al. 2019 update of the EULAR recommendations for the management of systemic lupus erythematosus. Ann Rheum Dis. (2019) 78:736–45. doi: 10.1136/annrheumdis-2019-215089
32. Narvaez J, Rios-Rodriguez V., de la Fuente D, Estrada P, Lopez-Vives L, Gomez-Vaquero C, et al. Rituximab therapy in refractory neuropsychiatric lupus: current clinical evidence. Semin Arthritis Rheum. (2011) 41:364–72. doi: 10.1016/j.semarthrit.2011.06.004
33. Fanouriakis A, Pamfil C, Sidiropoulos P, Damian L, Flestea A, Gusetu G, et al. Cyclophosphamide in combination with glucocorticoids for severe neuropsychiatric systemic lupus erythematosus: a retrospective, observational two-centre study. Lupus. (2016) 25:627–36. doi: 10.1177/0961203315622821
Keywords: neuropsychiatric systemic lupus erythematosus (NPSLE), leukoencephalopathy syndrome, rapidly progressive dementia (RPD), diffusion-weighted image (DWI), MRI
Citation: Feng Y, Yu T, Xiao Q and Yang X (2022) Case Report: Rapid Progression of Cognitive Dysfunction as an Initial Feature of Systemic Lupus Erythematosus With Leukoencephalopathy: A Case Report and Literature Review. Front. Neurol. 13:934335. doi: 10.3389/fneur.2022.934335
Received: 02 May 2022; Accepted: 23 June 2022;
Published: 11 July 2022.
Edited by:
Robert Weissert, University of Regensburg, GermanyReviewed by:
Yoshiyuki Arinuma, Kitasato University, JapanAmra Adrovic, Koç University Hospital, Turkey
Grace Yoonheekim Gombolay, Emory University, United States
Copyright © 2022 Feng, Yu, Xiao and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qin Xiao, eHExMDUzN0ByamguY29tLmNu; Xiaodong Yang, eWFuZ3hpYW9kb25nMjAxMUB5ZWFoLm5ldA==
†These authors have contributed equally to this work
 Yukun Feng1,2†
Yukun Feng1,2† Qin Xiao
Qin Xiao Xiaodong Yang
Xiaodong Yang