
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 20 September 2022
Sec. Stroke
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.934051
 Wenting Guo1
Wenting Guo1 Jiali Xu1
Jiali Xu1 Wenbo Zhao1,2
Wenbo Zhao1,2 Mengke Zhang1
Mengke Zhang1 Jin Ma1
Jin Ma1 Jian Chen3
Jian Chen3 Jiangang Duan4
Jiangang Duan4 Qingfeng Ma1
Qingfeng Ma1 Haiqing Song1
Haiqing Song1 Sijie Li2,4*
Sijie Li2,4* Xunming Ji1,3,5*
Xunming Ji1,3,5*Objective: This study aimed to develop and validate a nomogram to predict malignant cerebral artery infarction (MMI) after endovascular treatment (EVT) in patients with acute ischemic stroke (AIS) in the modern thrombectomy era.
Methods: We retrospectively analyzed data from a prospective cohort of consecutive patients with AIS who underwent EVT at Xuanwu hospital between January 2013 and June 2021. A multivariable logistic regression model was employed to construct the nomogram for predicting MMI after EVT. The discrimination and calibration of the nomogram were assessed both in the derivation and validation cohorts.
Results: A total of 605 patients were enrolled in this study, with 425 in the derivation cohort and 180 in the validation cohort. The nomogram was developed based on admission systolic blood pressure (SBP), the National Institute of Health Stroke Score (NIHSS), the Alberta Stroke Program Early Computed Tomography Score (ASPECTS), vessel occlusion site, EVT time window, and recanalization status. The nomogram displayed good discrimination with the area under the receiver operating characteristics (ROCs) curve (AUC) of 0.783 [95% confidence interval (CI), 0.726–0.840] in the derivation cohort and 0.806 (95% CI, 0.738–0.874) in the validation cohort. The calibration of the nomogram was good as well, with the Hosmer–Lemeshow test of p = 0.857 in the derivation cohort and p = 0.275 in the validation cohort.
Conclusion: In the modern thrombectomy era, a nomogram containing admission SBP, NIHSS, ASPECTS, vessel occlusion site, EVT time window, and recanalization status may predict the risk of MMI after EVT in patients with AIS.
Malignant middle cerebral artery infarction (MMI) accounts for 30–50% of strokes caused by large vessel occlusion with a mortality of nearly 80% (1–3). Early decompressive hemicraniectomy (DHC) is the only effective treatment that can reduce mortality and improve clinical outcomes for patients with MMI (4). Thus, the rapid recognition of patients at high risk of MMI is helpful for timely clinical decisions.
Endovascular therapy (EVT) has shown overwhelming efficacy for AIS secondary to large vessel occlusion in the anterior circulation. This includes both early EVT (within 6 h) and late EVT (within 6–24 h) (5–7). However, MMI is common in patients undergoing EVT with an incidence of 19.2–44%, which lessens the benefits of EVT (8–14). Therefore, it is crucial to identify risk factors mediating the development of MMI after EVT and construct a predictive model specifically for this population.
Several studies have explored predictors of MMI in patients with AIS who underwent EVT in the early time window, two of which constructed a nomogram to predict MMI after early EVT (8–12, 14). However, these studies scarcely include patients selected by rigorous imaging examination who received EVT in the late time window. Therefore, this study intends to construct and validate an applicable nomogram predictive model to rapidly and accurately recognize patients at high risk of MMI after EVT in the modern thrombectomy era.
We retrospectively analyzed data from a prospective cohort of consecutive patients with AIS who underwent EVT at Xuanwu Hospital between January 2013 and June 2021. This prospective cohort was approved by the Ethics Committee of Capital Medical University Xuanwu Hospital (No. [2017]030), and written informed consent was obtained from all patients or their legal representatives.
The following criteria were required for participation in this study: (1) age ≥18 years; (2) AIS caused by occlusion of the internal carotid artery (ICA) or M1 and M2 segments of the middle cerebral artery (MCA); (3) EVT performed within 24 h from symptom onset; (4) confirmation of the diagnosis of MMI or non-MMI within 5 days after EVT; and (5) the absence of parenchymal hemorrhage type 2. All EVT procedures and management of patients followed the recommendation of the current guideline (15).
Demographics data (age and sex), vascular risk factors (hypertension, diabetes, hyperlipidemia, atrial fibrillation, and previous stroke), baseline characteristics [admission systolic blood pressure (SBP) and diastolic blood pressure (DBP)], admission stroke severity [the National Institute of Health Stroke Scale (NIHSS) and the Alberta Stroke Program Early Computed Tomography Score (ASPECTS)], vessel occlusion site (ICA occlusion or MCA occlusion), admission laboratory tests [low-density lipoprotein (LDL), fast blood glucose (FBG), and white blood cells (WBC)], stroke etiology [according to the Trial of Org 10,172 in Acute Stroke Treatment (TOAST) classification], treatment information [anesthesia, time interval from symptoms onset to puncture (OTP), time interval from symptoms onset to recanalization (OTR), passes of retriever, and recanalization status], and clinical outcomes at 90 days were collected from the database. Endovascular treatment initiated within 6 h from stroke symptom OTP was defined as the early time window (early EVT) and that within 6–24 h as the late time window (late EVT). Successful recanalization was defined as a modified Thrombolysis in Cerebral Infarction (mTICI) scale of 2b or 3. Clinical outcomes were assessed using the modified Rankin Scale (mRS) at 90 days through the telephone by trained staff, and a favorable outcome was defined as an mRS of 0–2.
Malignant middle cerebral artery infarction or non-MMI was diagnosed within 5 days after the EVT procedure in combination with clinical and imaging characteristics. Patients with the following characteristics can be diagnosed as MMI (4): (1) NIHSS > 15 with a decreased level of consciousness (Item 1a ≥1) either on admission or after secondary deterioration, (2) infarction size exceeding at least two-thirds of the MCA territory on one of the follow-up head computed tomography or magnetic resonance imaging (MRI, within 5 days after EVT) with compression of the ventricles or midline shift, and (3) no other cause of neurological deterioration. The diagnosis of MMI was reviewed by two trained neurologists independently, and disagreement was resolved by discussion or consultation with a third neurologist.
Statistical analyses were performed using SPSS (Version 26; IBM Corp, Armonk, NY) and R Project for Statistical Computing (Version 4.1.2). The p-values of <0.05 (two-sided) were considered significant. Patients were randomly divided at a ratio of 6:4 into the derivation cohort and the validation cohort. Differences between the derivation cohort and the validation cohort were analyzed. Categorical variables are presented as numbers (percentages) and continuous variables are expressed as mean ± standard deviation (SD) or median [interquartile range (IQR)]. Differences between the two groups were explored using the chi-square test for categorical variables and the independent t-test or the Mann–Whitney U-test for continuous variables. Patients with missing data for the baseline characteristics were excluded from the study.
To construct a nomogram, we compared the differences between patients with and without MMI in the derivation cohort, then we implemented multivariable logistic regression analysis to determine independent predictors of MMI, including variables with a p-value of <0.1 in univariate analyses. Variance inflation factors (VIF) were calculated to assess the collinearity assumption (VIF <5 being considered non-significant). The odds ratio (OR) and 95% confidence interval (CI) of each significant risk factor in the final logistic regression model were calculated. Performances of the nomogram were assessed in the derivation and validation cohorts. Discrimination of the nomogram was assessed using the area under the receiver operating characteristics (ROCs) curve (AUC). An AUC of 0.51–0.7 indicates low accuracy, 0.71–0.9 indicates moderate accuracy, and 0.91–1.0 indicates high accuracy to discriminate between patients with MMI and without MMI. The calibration of the nomogram was evaluated by the Hosmer–Lemeshow goodness-of-fit test (p > 0.05 indicates good calibration).
Between January 2013 and June 2021, this cohort registered a total of 1,103 patients. After excluding those patients who only received angiography (n = 95) and had incomplete baseline data (n = 48), 960 patients with AIS who underwent EVT were retrospectively screened (2 with age <18 years old, 279 with posterior circulation AIS, 4 with OTP >24 h, 1 absent of follow-up CT or MRI, and 55 with parenchymal hemorrhage type 2). Finally, 605 patients were included in the present study with 372 patients in the derivation cohort and 247 patients in the validation cohort (Figure 1). There was no significant difference in characteristics between the two cohorts except for the probability of successful recanalization (Table 1).
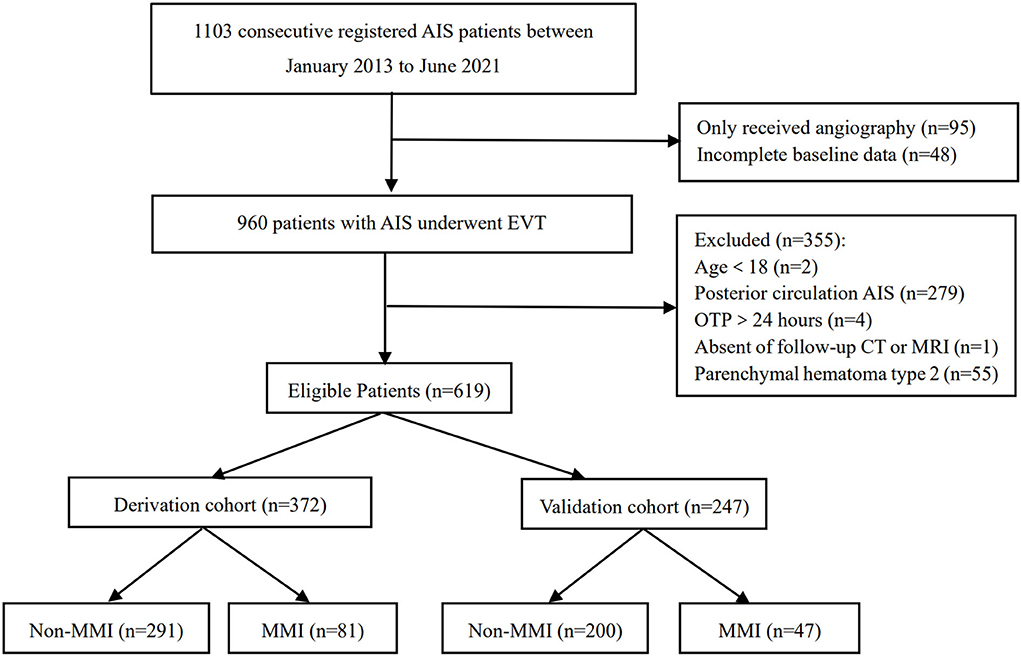
Figure 1. A flowchart of the patients enrolled in the study. AIS, acute ischemic stroke; EVT, endovascular therapy; OTP, time interval from symptoms onset to puncture; MMI, malignant middle cerebral artery infarction.
In the derivation cohort, 81 patients developed MMI, and 291 did not. Univariate analyses of the derivation cohort (Table 2) showed that patients in the MMI group had higher admission SBP (152.9 ± 24.3 vs. 144.5 ± 24.3 mmHg, p = 0.006), had higher admission NIHSS (median 17 vs. 14, p < 0.001) and lower admission ASPECTS (median 9 vs. 9, p = 0.036), had higher WBC count [(9.5 ± 3.5) ×106/L vs. (8.8 ± 2.7) ×106/L, p = 0.060] and FBG (median, 7.9 vs. 7.1 mmol/L, p = 0.017) compared with the non-MMI group. Patients in the MMI group also had a higher proportion of ICA occlusion (67.9 vs. 38.1%, p < 0.001), had a higher proportion of general anesthesia (43.2 vs. 26.1%, p = 0.003), had passes of retriever ≥3 (23.5 vs. 10.3%, p = 0.002), and had a lower proportion of recanalization (70.4 vs. 90.4%, p < 0.001). Furthermore, the median OTP was 344 min in the MMI group and 364 min in the non-MMI group (p = 0.037), and patients with MMI were more likely to receive EVT in the early time window (58.0 vs. 48.1%, p = 0.114).
Predictors in the final multivariable logistic regression model (Table 3) included admission SBP (OR, 1.012; 95% CI, 1.001–1.023; p = 0.037), admission NIHSS (OR, 1.094; 95% CI, 1.047–1.143; p < 0.001), admission ASPECTS (OR, 0.720; 95% CI, 0.599–0.866; p < 0.001), ICA occlusion (OR, 2.996; 95% CI, 1.704–5.269; p < 0.001), late EVT (OR, 0.556; 95% CI, 0.308–1.001; p = 0.050), and recanalization (OR, 0.339; 95% CI, 0.170–0.673; p = 0.002).
The nomogram was constructed based on admission SBP, NIHSS, ASPECTS, vessel occlusion site, EVT time window, and recanalization status (Figure 2). The discriminative ability of the nomogram was good with an AUC of 0.783 (95% CI, 0.726–0.840, Figure 3A) in the derivation cohort and 0.806 (95% CI, 0.738–0.874, Figure 3B) in the validation cohort. The calibration of this predictive nomogram was satisfactory both in the derivation cohort (the Hosmer–Lemeshow test, p = 0.857) and the validation cohort (the Hosmer–Lemeshow test, p = 0.257).
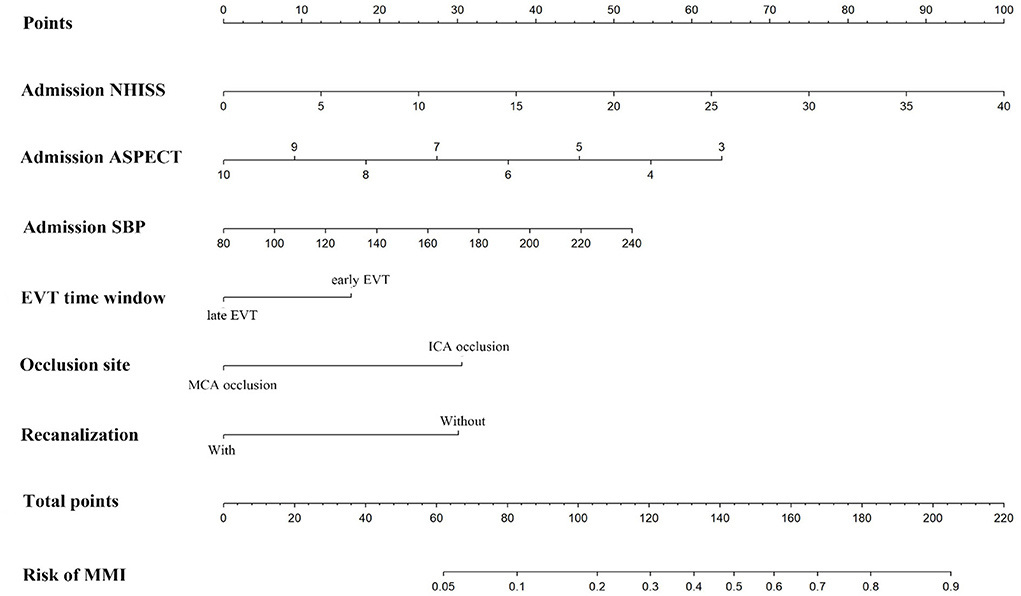
Figure 2. The nomogram for predicting the probability of malignant middle cerebral artery infarction. NIHSS, National Institute of Health Stroke Scale; ASPECTS, Alberta Stroke Program Early Computed Tomography Score; SBP, systolic blood pressure; EVT, endovascular therapy; ICA, internal carotid artery; MCA, middle cerebral artery; MMI, malignant middle cerebral artery infarction.
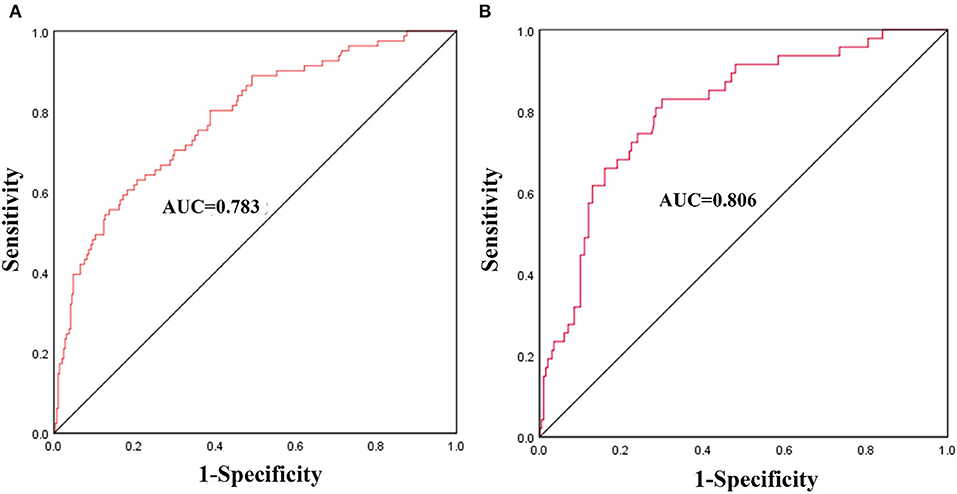
Figure 3. The receiver operating characteristics (ROCs) curve of the nomogram model to predict malignant middle cerebral artery infarction in the derivation cohort (A) and validation cohort (B).
In the derivation cohort, DHC was implemented in 27 (33.3%) patients in the MMI group and 1 (0.3%) patient in the non-MMI group. Data of clinical outcomes at 90 days were available for 360 out of 372 patients, 283 were in the non-MMI group and 77 were in the MMI group. Patients with MMI had a significantly higher proportion of mortality (51.9 vs. 8.1%, p < 0.001) and a lower proportion of favorable functional outcomes (5.2 vs. 55.1%, p < 0.001).
This study constructed and validated a practical nomogram based on admission SBP, NIHSS, ASPECTS, vessel occlusion site, EVT time window, and recanalization status, which displayed good discrimination and calibration to predict MMI after EVT in the modern thrombectomy era.
Predictive factors for MMI have been widely discussed. Consistent with previous studies, our study found that higher admission SBP, NIHSS, and lower ASPECTS predicted the occurrence of MMI (16, 17). In addition, we found that ICA occlusion was an independent predictor of MMI. Internal carotid artery occlusion implies fewer collaterals supplied from the anterior cerebral artery or anterior communication artery compared with MCA occlusion (10), and poor collaterals may augment the progression of infarct volume and lead to severe brain edema (18). Therefore, MMI occurred more frequently in patients with ICA occlusion. Successful recanalization has been demonstrated to decrease the incidence of MMI (10, 12, 17). Similarly, our study found that patients in the MMI group had a lower proportion of recanalization. Unexpectedly, we found that late EVT was associated with a lower risk of MMI. This can be explained by the rigorously selected patients for late EVT, namely, only those patients who had small or moderate infarct core and large salvageable brain tissue were eligible for late EVT. However, hyperglycemia and age, which were found to be strongly associated with MMI in some studies, were not independent predictors of MMI after EVT in our multivariable logistic regression model (8, 9).
Timely DHC can reduce mortality and improve functional outcomes for patients with MMI (4). However, patients with MMI had a lower proportion to receive DHC in real-world studies, because the decision on DHC was finally at the discretion of families of the patients (5, 13, 14). Furthermore, this study had a low percentage of DHC (33.3%) despite being higher than the percentage in previous similar studies (25.4, 16.7, and 9.7%) (5, 13, 14), which can be attributed to the observational design of the study. Thus, an accurate and simple predictive tool for MMI is crucial to facilitate the families of patients to understand the imperative of DHC for patients with MMI, thereby helping to enhance the proportion of DHC and to enable timely intervention. Previous studies have established several predictive models for MMI among patients with AIS, such as the EDEMA score (19), the DASH score (2), and the MBE score (20). However, these scoring systems were designed to distinguish potential MMI in patients with large hemisphere stroke, which were not specifically for patients who underwent EVT. Given that EVT could significantly decrease the incidence of malignant brain edema (10, 12, 17, 21), predictive models specifically designed for patients with EVT are imperative.
Du et al. established a nomogram that included age, baseline NIHSS, FBG, collateral circulation, and mTICI to predict malignant brain edema in patients undergoing EVT (9). Chen et al. developed a nomogram based on ASPECTS, hypertension, cisternal effacement, and recanalization status to predict the risk of malignant brain edema after EVT (14). However, these two studies only enrolled patients with AIS who underwent EVT within 6–8 h, and none of them has been validated externally. In the present study, we constructed and validated a nomogram consisting of admission SBP, NIHSS, ASPECTS, vessel occlusion site, EVT time window, and recanalization status. This nomogram had a moderate discriminative ability to predict MMI after EVT in the modern thrombectomy era as supported by the AUC value of 0.783 in the derivation cohort and 0.806 in the validation cohort. Besides, the calibration of this nomogram was good as well with p = 0.857 in the derivation cohort and p = 0.275 in the validation cohort. The performance of our nomogram model is not inferior to the nomogram of Du et al. and Chen et al., whose AUC was 0.805 and 0.874, respectively (9, 14). What is important, risk factors in our predictive model can be easily assessed as part of the routine clinical assessments for patients undergoing EVT.
Our study also has limitations. First, the results of our study were based on a single-center prospective registry, which might generate systematic bias and prevent the generalization of our predictive model. Second, some advanced predictors of MMI found in other studies, such as baseline infarct volume and collateral circulation, were not available in our study. Third, we excluded patients with parenchymal hemorrhage type 2, which might lead to selection biases due to the coexistence of malignant brain edema and hemorrhage in some patients. A multicenter study is necessary to confirm the performance of our nomogram model before its application in clinical practice. Finally, our study found that patients rigorously selected by imaging treated in the late time window had a lower risk of MMI, however, our cohort did not include data on perfusion mismatch volume that can be used for the exploration of the association between perfusion CT mismatch volume in the late therapeutic time window and the development of MMI.
The nomogram composed of admission SBP, NIHSS, ASPECTS, vessel occlusion site, EVT time window, and recanalization status may predict the risk of MMI after EVT.
The raw data supporting the conclusions of this article will be available from the corresponding authors on reasonable request.
The studies involving human participants were reviewed and approved by Ethics Committee of Capital Medical University Xuanwu Hospital (No. [2017]030). The patients/participants provided their written informed consent to participate in this study.
WG conceived of the study idea, collected and analyzed the data, and drafted the manuscript. JX, MZ, and JM participated in the data collection. WZ, SL, and XJ helped to modify the manuscript. XJ, JC, JD, QM, and HS participated in the coordination of the study. All authors read and approved the final manuscript.
This study was supported by the Beijing Nova Program (No. Z201100006820143), the National Natural Science Foundation of China (Nos. 82001257, 81801313, and 81971114), and the General Project of Science and Technology of Beijing Municipal Education Commission (No. KM202110025018).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Foerch C, Otto B, Singer O, Neumann-Haefelin T, Yan B, Berkefeld J, et al. Serum S100B predicts a malignant course of infarction in patients with acute middle cerebral artery occlusion. Stroke. (2004) 35:2160–4. doi: 10.1161/01.STR.0000138730.03264.ac
2. Shimoyama T, Kimura K, Uemura J, Yamashita S, Saji N, Shibazaki K, et al. The DASH score: a simple score to assess risk for development of malignant middle cerebral artery infarction. J Neurol Sci. (2014) 338:102–6. doi: 10.1016/j.jns.2013.12.024
3. Berrouschot J, Sterker M, Bettin S, Köster J, Schneider D. Mortality of space-occupying ('malignant') middle cerebral artery infarction under conservative intensive care. Intensive Care Med. (1998) 24:620–3. doi: 10.1007/s001340050625
4. Vahedi K, Hofmeijer J, Juettler E, Vicaut E, George B, Algra A, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. (2007) 6:215–22. doi: 10.1016/S1474-4422(07)70036-4
5. Albers G, Marks M, Kemp S, Christensen S, Tsai J, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. (2018) 378:708–18. doi: 10.1056/NEJMoa1713973
6. Goyal M, Menon B, van Zwam W, Dippel D, Mitchell P, Demchuk A, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. (2016) 387:1723–31. doi: 10.1016/S0140-6736(16)00163-X
7. Nogueira R, Jadhav A, Haussen D, Bonafe A, Budzik R, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. (2018) 378:11–21. doi: 10.1056/NEJMoa1706442
8. Davoli A, Motta C, Koch G, Diomedi M, Napolitano S, Giordano A, et al. Pretreatment predictors of malignant evolution in patients with ischemic stroke undergoing mechanical thrombectomy. J Neurointerv Surg. (2018) 10:340–4. doi: 10.1136/neurintsurg-2017-013224
9. Du M, Huang X, Li S, Xu L, Yan B, Zhang Y, et al. A nomogram model to predict malignant cerebral edema in ischemic stroke patients treated with endovascular thrombectomy: an observational study. Neuropsychiatr Dis Treat. (2020) 16:2913–20. doi: 10.2147/NDT.S279303
10. Huang X, Yang Q, Shi X, Xu X, Ge L, Ding X, et al. Predictors of malignant brain edema after mechanical thrombectomy for acute ischemic stroke. J Neurointerv Surg. (2019) 11:994–8. doi: 10.1136/neurintsurg-2018-014650
11. Tracol C, Vannier S, Hurel C, Tuffier S, Eugene F, Le Reste PJ. Predictors of malignant middle cerebral artery infarction after mechanical thrombectomy. Rev Neurol. (2020) 176:619–25. doi: 10.1016/j.neurol.2020.01.352
12. Kimberly W, Dutra B, Boers A, Alves H, Berkhemer O, van den Berg L, et al. Association of reperfusion with brain edema in patients with acute ischemic stroke: a secondary analysis of the MR CLEAN trial. JAMA Neurol. (2018) 75:453–61. doi: 10.1001/jamaneurol.2017.5162
13. Jiang QM, Yu S, Dong XF, Wang HS, Hou J, Huang ZC, et al. Predictors and dynamic nomogram to determine the individual risk of malignant brain edema after endovascular thrombectomy in acute ischemic stroke. J Clin Neurol. (2022) 18:298–307. doi: 10.3988/jcn.2022.18.3.298
14. Chen X, Huang Q, Deng Q, Shen R, Liu Y, Lu M, et al. A prediction model of brain edema after endovascular treatment in patients with acute ischemic stroke. J Neurol Sci. (2019) 407:116507. doi: 10.1016/j.jns.2019.116507
15. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: a Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. (2019). 50:e344–418. doi: 10.1161/STR.0000000000000211
16. Wu S, Yuan R, Wang Y, Wei C, Zhang S, Yang X, et al. Early prediction of malignant brain edema after ischemic stroke. Stroke. (2018) 49:2918–27. doi: 10.1161/STROKEAHA.118.022001
17. Miao J, Song X, Sun W, Qiu X, Lan Y, Zhu Z. Predictors of malignant cerebral edema in cerebral artery infarction: a meta-analysis. J Neurol Sci. (2020) 409:116607. doi: 10.1016/j.jns.2019.116607
18. Elijovich L, Goyal N, Mainali S, Hoit D, Arthur AS, Whitehead M, et al. CTA collateral score predicts infarct volume and clinical outcome after endovascular therapy for acute ischemic stroke: a retrospective chart review. J Neurointerv Surg. (2016) 8:559–62. doi: 10.1136/neurintsurg-2015-011731
19. Ong CJ, Gluckstein J, Laurido-Soto O, Yan Y, Dhar R, Lee JM. Enhanced detection of edema in malignant anterior circulation stroke (EDEMA) score: a risk prediction tool. Stroke. (2017) 48:1969–72. doi: 10.1161/STROKEAHA.117.016733
20. Jo K, Bajgur SS, Kim H, Choi HA, Huh PW, Lee K. A simple prediction score system for malignant brain edema progression in large hemispheric infarction. PLoS ONE. (2017) 12:e0171425. doi: 10.1371/journal.pone.0171425
Keywords: ischemic stroke, thrombectomy, malignant, brain edema, nomogram
Citation: Guo W, Xu J, Zhao W, Zhang M, Ma J, Chen J, Duan J, Ma Q, Song H, Li S and Ji X (2022) A nomogram for predicting malignant cerebral artery infarction in the modern thrombectomy era. Front. Neurol. 13:934051. doi: 10.3389/fneur.2022.934051
Received: 02 May 2022; Accepted: 12 August 2022;
Published: 20 September 2022.
Edited by:
Thanh G. Phan, Monash Health, AustraliaReviewed by:
Nikoloz Tsiskaridze, Pineo Medical Ecosystem, GeorgiaCopyright © 2022 Guo, Xu, Zhao, Zhang, Ma, Chen, Duan, Ma, Song, Li and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sijie Li, bGlzaWppZUB4d2guY2NtdS5lZHUuY24=; Xunming Ji, aml4bUBjY211LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.