- 1Department of Neurology, The Affiliated Hospital of Northwest University, Xi'an No.3 Hospital, Xi'an, China
- 2The College of Life Sciences, Northwest University, Xi'an, China
- 3Xi'an Key Laboratory of Cardiovascular and Cerebrovascular Diseases, Medical Research Center, The Affiliated Hospital of Northwest University, Xi'an No.3 Hospital, Xi'an, China
Background: Ischemic stroke (IS) is a complex neurological disease affected by genetics and environment. Matrix metalloproteinase-2 (MMP2) is involved in extracellular matrix (ECM) degradation, inflammation and angiogenesis to regulate the development and recovery of IS.
Purposes: The aim of this study was to explore the association of rs1053605, rs243849 and rs14070 in MMP2 with the risk of IS in Chinese Shaanxi population.
Methods: In this study, 677 IS patients and 681 normal controls were recruited. Rs1053605, rs243849 and rs14070 in MMP2 were genotyped. Logistic regression analysis was applied to evaluate the association of rs1053605, rs243849 and rs14070 in MMP2 with IS susceptibility and the association of environmental factors with MMP2 genetic susceptibility to IS.
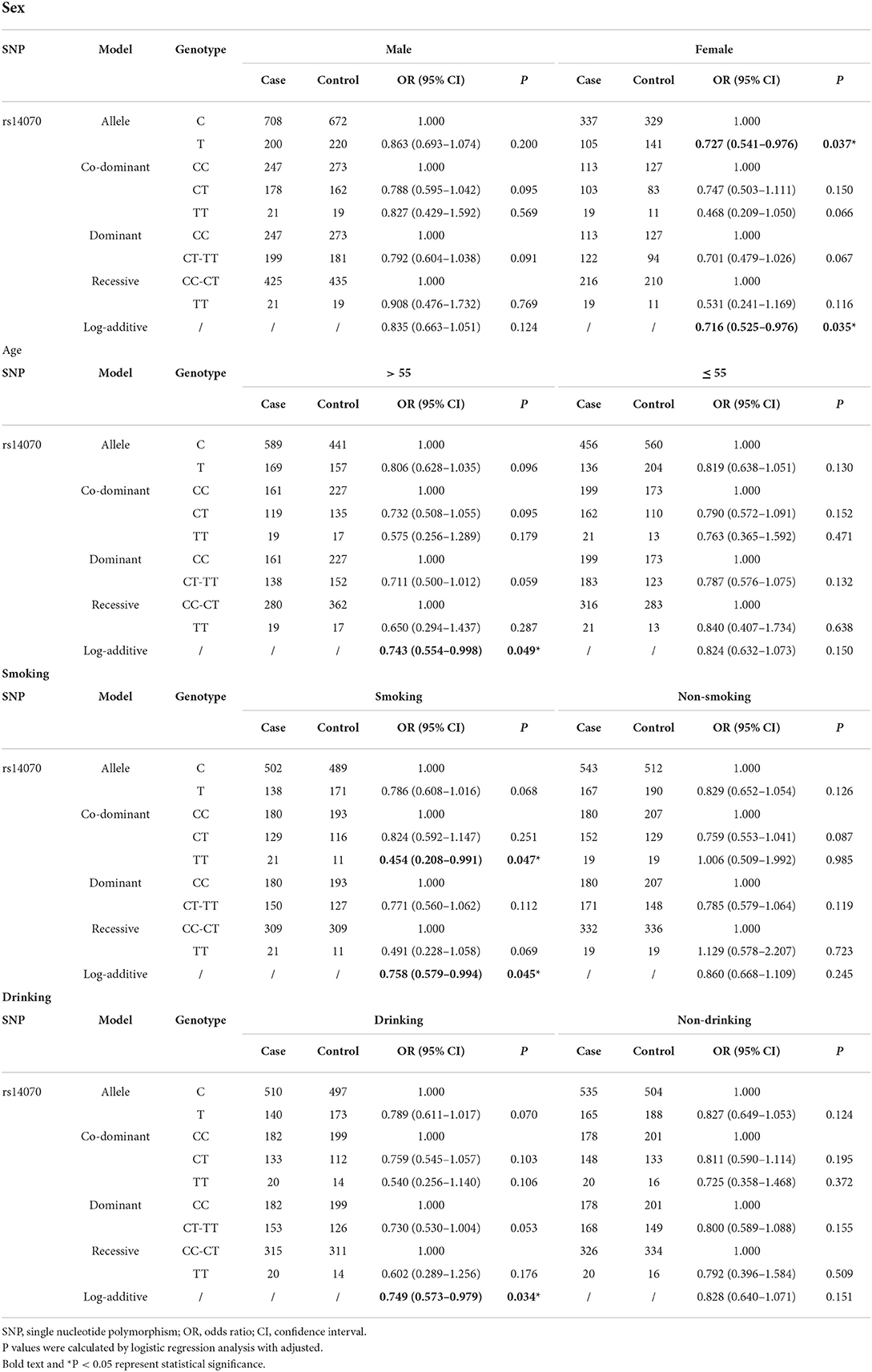
Results: The results of the overall analysis demonstrated that rs14070 in MMP2 significantly reduced the risk of IS in Chinese Shaanxi population (OR = 0.767, 95% CI = 0.619–0.952, P = 0.016). Subgroup analysis illustrated that rs243849 in MMP2 evidently increased the risk of IS among drinkers, while rs14070 in MMP2 apparently reduced IS susceptibility among females, participants with aged >55, smokers and drinkers.
Conclusions: Collectively, rs243849 and rs14070 in MMP2 were significantly associated with the risk of IS in Chinese Shaanxi population, and the effect of MMP2 to IS may be associated with its genetic susceptibility.
Introduction
Stroke is caused by cerebrovascular disorders, including ischemic stroke (IS) and hemorrhagic stroke (HS), of which IS accounts for 70 to 90% (1, 2). High morbidity, high recurrence, high mortality, high disability and low cure rate are the main characteristics of IS (3). As a multifactorial complex neurological disorder, stroke involves clinical, environmental and genetic factors (4). Advanced age, smoking, drinking, hypertension and diabetes are the risk factors to IS (5, 6). Study indicated that the incidence of IS after the age of 40 increased evidently with age, and the susceptibility of IS in male was obviously higher than that in female in the same age group (7). There was a significant dose effect between the numbers of daily cigarettes smoked and IS in young men (8). However, clinical and environmental factors do not adequately explain differences in IS disease progression (9). Epidemiological analysis shows genetic factors play a crucial role in IS susceptibility (2, 6), accounting for 50% (10). Therefore, there is an urgent need to study the genetic variants associated with the occurrence of IS (11).
Vascular inflammation is a key factor in IS (11). Inflammation can not only promote thrombus formation and improve the stability of thrombus, but also damage the blood-brain barrier (BBB) (12). Matrix metalloproteinases (MMPs), as a class of proteolytic zinc-dependent enzymes, can regulate cytokines (13), angiogenesis (10), extracellular matrix (ECM) degradation (14), and cause BBB disorders (4), which affect the pathogenesis of vascular inflammation, stroke and atherosclerosis (11). It has been reported that the MMPs associated with IS were mainly concentrated in MMP2 and MMP9 (15). MMP2 also regulates angiogenesis, vascular inflammation, ECM degradation, and BBB disruption, which are critical to IS occurrence, progression, and recovery (13). Genetic polymorphisms of MMP2 affect its transcription and expression (10), a preliminary study found that rs1132896 and rs243849 of MMP2 evidently reduced the risk of IS in southern Chinese populations (9). However, genetic variation in MMP2 were not notably associated with the risk of IS in the Han Hakka populations (16). Due to the differences in the risk correlation between MMP2 and IS in different populations, the study of MMP2 polymorphisms and IS susceptibility in different populations needs to be explored extensively.
The purpose of this study was to investigate the effect of MMP2 polymorphisms on the occurrence of IS in Chinese Shaanxi population. Three SNPs (rs1053605, rs243849 and rs14070) in MMP2 were selected and logistic regression analysis with OR and 95% CI values was used to evaluate the association between MMP2 polymorphisms and IS susceptibility. Our research results will hopefully provide valuable data support to the early prevention, diagnosis and treatment of IS, and contribute to the development of IS targeted therapy strategies.
Methods
Study participants
The research subjects included 677 patients with IS in the acute phase (within 24 h of onset) and 681 normal individuals, who were recruited from the Affiliated Hospital of Northwest University (Xi'an No.3 Hospital). The inclusion criteria of the IS case group were to use the National Institutes of Health Stroke Scale (NIHSS) to assess the degree of neurological deficit, and to confirm the diagnosis by neurological examination, brain computed tomography (CT) and magnetic resonance imaging (MRI) (16). Patients with tumors, brain trauma, cerebral hemorrhage and cerebrovascular malformations were excluded. The control group was from patients who received physical examinations in the hospital during the same period without any family history of stroke, hypertension, diabetes and cardiovascular disease (3, 17). Trained staff administered questionnaires to participants to collect demographic data (including age, sex, smoking and drinking habits, and disease history), while collecting venous blood samples from recruiters who had fasted for at least 8 h. The protocol was approved by the Ethics Committee of this hospital and complies with the Declaration of Helsinki. Moreover, all participants signed an informed consent form.
SNPs selection and genotyping
Three SNPs in MMP2 (rs1053605, rs243849, and rs14070) were selected to identify MMP2 candidate SNP variants based on NCBI database (https://www.ncbi.nlm.nih.gov/snp), minor allele frequency (MAF) >5%, and SNP was better assessed in previous studies (9, 18–20). After the extraction and purification of the above-mentioned sample DNA, primers for candidate SNPs (rs1053605, rs243849 and rs14070) were designed based on the gene sequence of MMP2 using Primer5.0 primer design software (Supplementary Table 1). AgenaMassArray and AgenaTyper 4.0 were applied for genotyping and data analysis, respectively. Five percent of DNA samples were chosen for repeat testing to control quality, with >99% concordance of typing.
Statistical analysis
The χ2 test and t test were used to perform statistical data processing analysis on demographic characteristics, allele and genotype frequencies of cases and controls. The Hardy-Weinberg Equilibrium (HWE) method was applied to assess the overall representativeness of the sample. Multivariate logistic regression was applied to calculate odds ratios (ORs) and 95% confidence intervals (CIs) after stratification for sex, age, smoking, and drinking. The associations between SNPs and IS risk in various models (allele, co-dominant, dominant, recessive and additive) and the degree of genetic association among the three SNPs were assessed using Plink software (version 1.9). In addition, multivariate dimensionality reduction (MDR) software was used to analyze the interactions among SNPs. In this study, p < 0.05 indicated a statistically significant difference. STRING and Oebiotech databases were adopted to analyze the biological activity and function analysis of MMP2.
Results
Participant characteristics
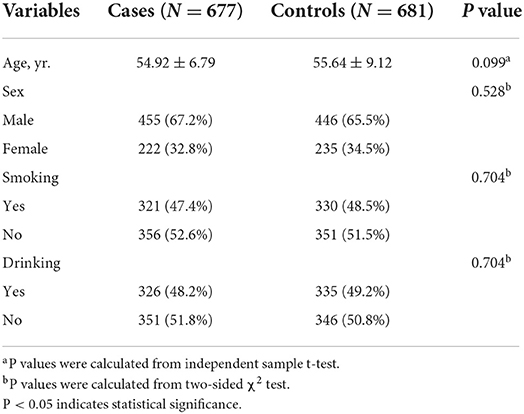
As depicted in Table 1, clinical characteristics of 677 IS patients and 681 normal control individuals included in this study. Notably, the mean age of the cases and the controls were 54.92 ± 6.79 and 55.64 ± 9.12, respectively, and there was no significant difference in age between the two groups (P = 0.099). In the case group, there were 455 males (67.2%) and 222 females (32.8%) with a sex ratio of 2.05:1. The sex ratio of 446 males (65.5%) and 235 females (34.5%) in the control group was 1.90:1. Thus, there was no obvious difference in gender distribution between the two groups (P = 0.528). Moreover, the distributions of smoking and drinking were not significantly different between cases and controls, both P = 0.704. In summary, there was no significant difference in clinical characteristics between the case and control groups, excluding the interference of confounding factors.
Candidate SNPs information and overall susceptibility
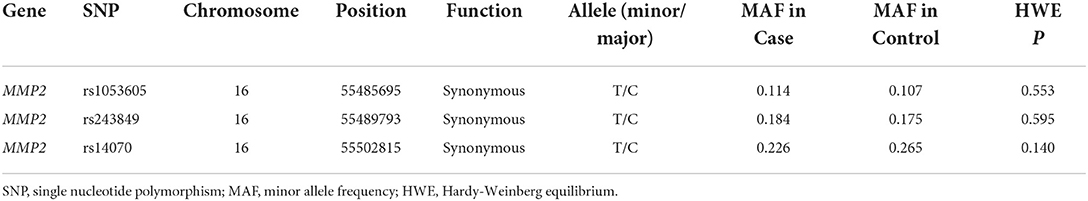
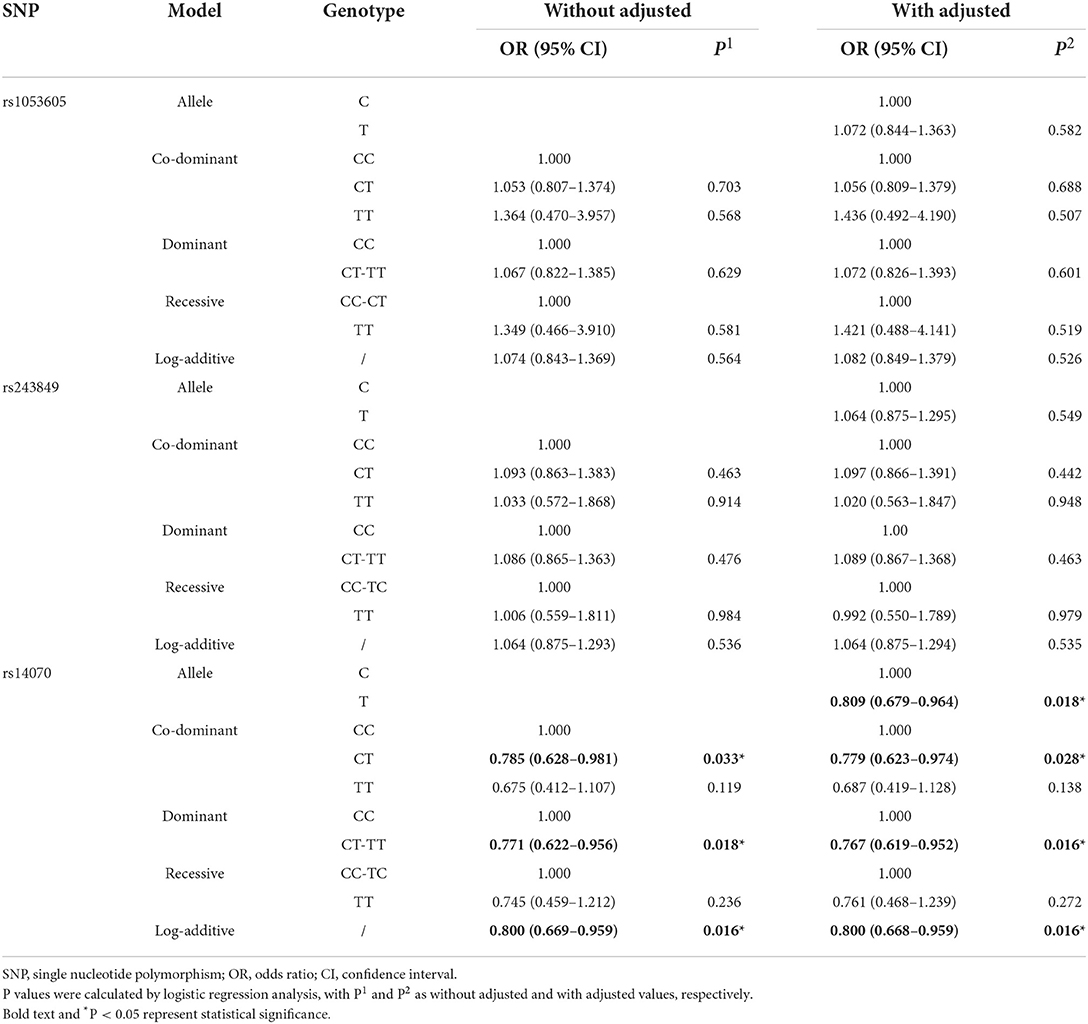
The basic biological information of 3 SNPs (rs1053605, rs243849 and rs14070) in MMP2 was presented in Table 2, which included chromosomes, physical location, function, MAF and HWE. The three SNPs in MMP2 are located on chromosome 16. Both the MAF of cases and controls and the HWE of cases were >0.05, indicating that the selected samples were representative. Overall correlation analysis demonstrated that rs14070 in MMP2 evidently reduced the risk of IS in multiple genetic models (Allele model: OR = 0.809, 95% CI = 0.679–0.964, P = 0.018; Co-dominant model: OR = 0.779, 95% CI = 0.623–0.974, P = 0.028; Dominance model: OR = 0.767, 95% CI = 0.619–0.952, P = 0.016; Log-additive model: OR = 0.800, 95% CI = 0.668–0.959, P = 0.016) (Table 3 and Figure 1A). However, rs1053605 and rs243849 in MMP2 in Chinese Shaanxi population did not show an evident association with IS susceptibility in the overall analysis.
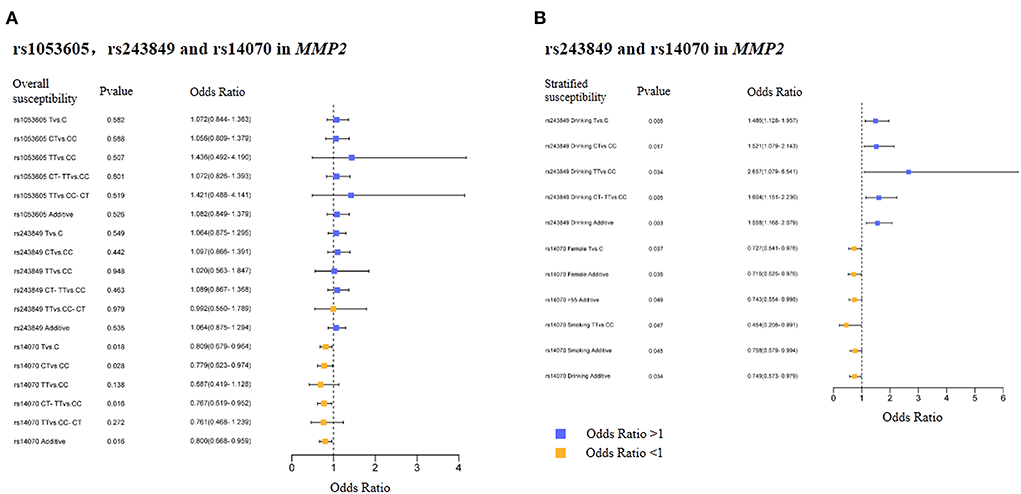
Figure 1. Overall and stratified analysis results of rs1053605, rs243849 and rs14070 in MMP2. (A) Overall results of rs1053605, rs243849, and rs14070 in MMP2. (B) Stratified significant results of rs243849, and rs14070 in MMP2.
Stratified analysis
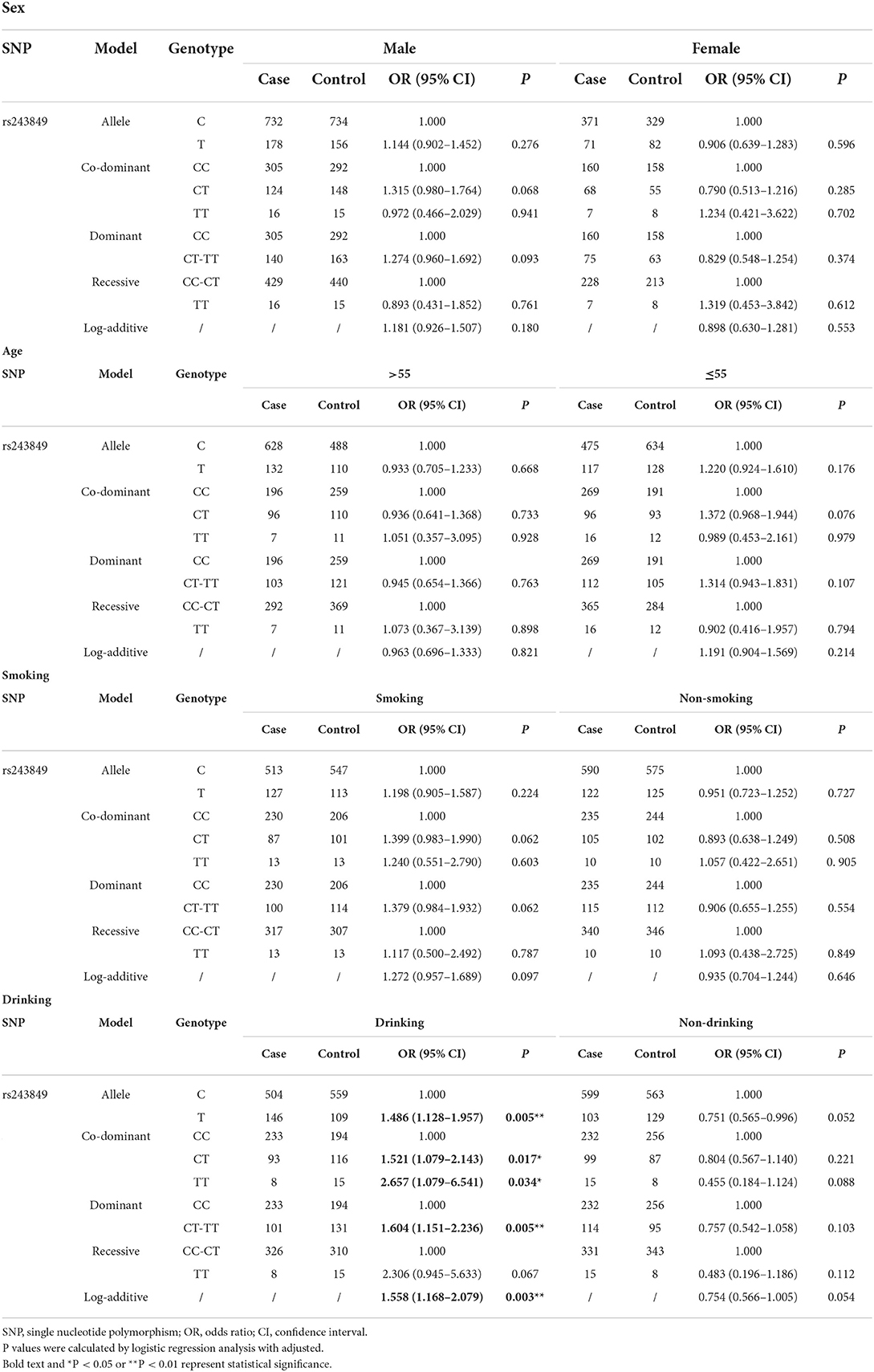
In order to exclude the interference of environmental factors on the reliability of the results, we stratified the recruited samples by gender, age, smoking and drinking (Tables 4, 5 and Supplementary Table 2), with the results of significant correlation shown in Figure 1B. Statistical analysis results revealed that rs243849 in MMP2 was significantly associated with the occurrence and development of IS risk under multiple genetic models of drinkers (Allele model: OR = 1.486, 95% CI = 1.128–1.957, P = 0.005; Co-dominant model: OR = 1.521, 95% CI = 1.079–2.143, P = 0.017; Dominance model: OR = 1.604, 95% CI = 1.151–2.236, P = 0.005; Log-additive model: OR = 1.558, 95% CI = 1.168–2.079, P = 0.003). Stratified results suggested that rs14070 in MMP2 apparently reduced susceptibility to IS in additive model across females (OR = 0.716, 95% CI = 0.525–0.976, P = 0.035), participants with aged >55 (OR = 0.743, 95% CI = 0.554–0.998, P = 0.049), smoker (OR = 0.758, 95% CI = 0.579–0.994, P = 0.045) and drinker (OR = 0.749, 95% CI = 0.573–0.979, P = 0.034). In addition, the allelic model of rs14070 in MMP2 evidently reduced the risk of IS in the female population (OR = 0.727, 95% CI = 0.541–0.976, P = 0.037) and the co-dominant model among smoking participants (OR = 0.454, 95% CI = 0.208–0.991, P = 0.047). Rs1053605 in MMP2 was not associated with the risk of IS in multiple genetic models with different stratifications.
Haplotype and MDR analysis
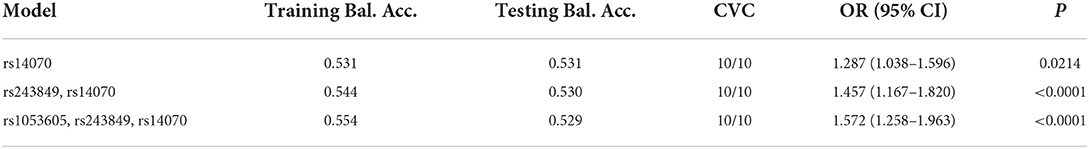
The linkage disequilibrium (LD) results demonstrated that the three candidate SNPs (rs1053605, rs243849 and rs14070) of MMP2 formed an LD block (Figure 2A), which illustrated that there was a strong linkage relationship among the SNPs of MMP2. Additionally, the results of MDR analysis claimed that the single-locus model (rs14070), the two-loci model (rs243849 and rs14070) and the three-loci model (rs1053605, rs243849 and rs14070) all had high test accuracy, high cross-validation consistency (CVC) and P < 0.05, as depicted in Table 6, Figures 2B,C. In conclusion, the three candidate SNPs in MMP2 have strong genetic associations, and the interaction of gene polymorphisms may play an essential role in the genetic susceptibility of IS.
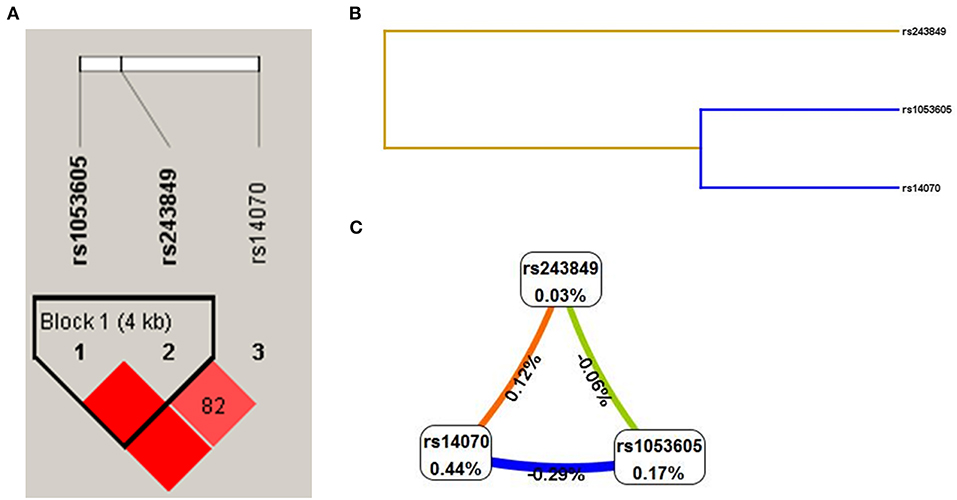
Figure 2. Genetic association between 3 SNPs (rs1053605, rs243849 and rs14070) in MMP2. (A) LD results of 3 SNPs in MMP2. (B) SNP-SNP interaction dendrogram of MDR analysis. (C) Fruchterman-reingold of MDR analysis (The closer to red the stronger the synergy, the closer to the blue the more redundancy).
MMP2 related functions
To further explore the potential function of MMP2 in regulating IS, protein-protein interaction figure and KEGG enrichment were constructed. It was indicated that MMP2 can regulate the metalloproteinase inhibitor family (TIMP1, TIMP2 and TIMP3) and angiogenesis-related targets such as VEGFA and TGF-β (Figure 3A). Pathway enrichment analysis demonstrated that MMP2 and its related proteins mainly regulate relaxin signaling pathway involved in angiogenesis, inflammation and vascular endothelial function (Figures 3B,C). In brief, MMP2 mainly regulated angiogenesis and inflammation and plays a pivotal role in the occurrence, development and recovery of IS.
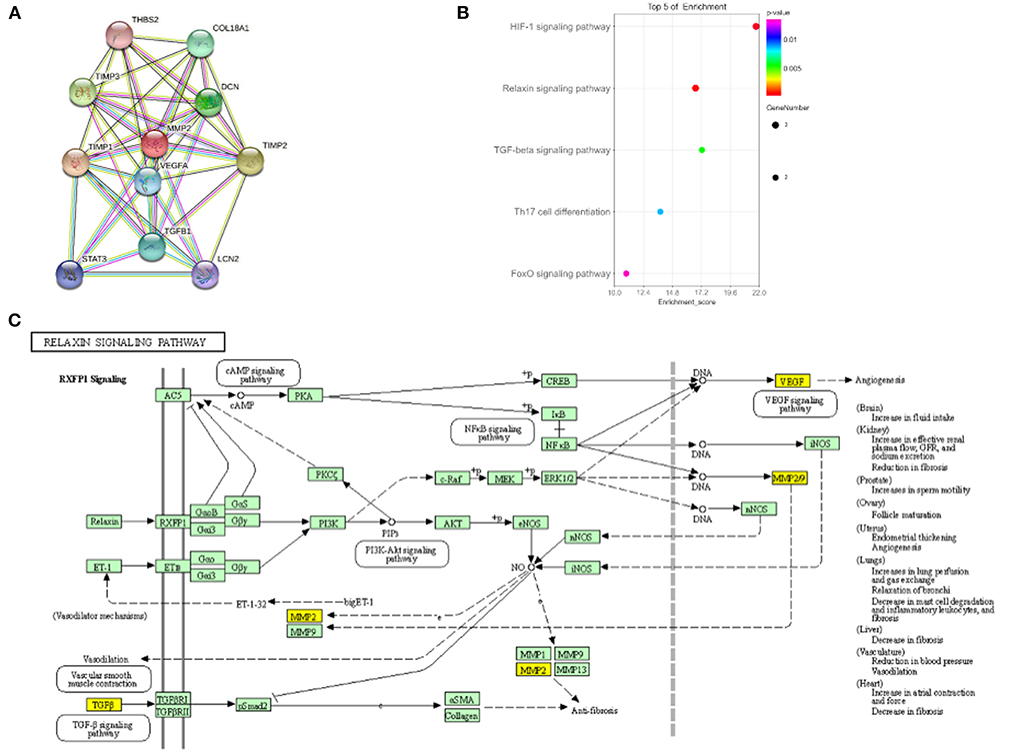
Figure 3. Biological function of MMP2. (A) Protein interaction diagram of MMP2. (B) KEGG enrichment results of MMP2. (C) MMP2 involved in Relaxin signaling pathway.
Discussion
IS is the leading cause of disability and death worldwide (10), a complex neurological disorder involving multiple factors including genetics and environment (4). The International Stroke Genetics Consortium suggested that genetic factors may account for up to 50% of an individual's risk of stroke (10). Previous extensive GWAS analyses have implicated genetic factors as a major contributor to stroke occurrence (21). Twenty-two new stroke risk loci were found to be significantly associated with stroke subtypes and pleiotropic models by GWAS analysis of 520,000 subjects from multiple countries (22). Studies have shown that people with high genetic risk have a 35% higher risk of stroke compared with people with low genetic risk, with a hazard ratio of 1.35 (95% CI: 1.21–1.50, P < 0.001) (23). In many neurological diagnoses, significant individual differences in rehabilitation outcomes may be associated with genetic variation. For example, rs6265 (val66met) on brain-derived neurotrophic factor (BDNF) significantly affected neuroplasticity after stroke in Chinese, Iranian, Korean and East Asian populations (24). In a nutshell, genetic factors are the main contributors to the occurrence, development and recovery of stroke.
As one of the major constituent enzymes in the brain (25), MMP2 is a crucial target in the MMPs family that regulates ECM degradation and blood-brain barrier disruption (26). MMP-2 was involved in the process of stroke injury in the early stage, and its activity and protein expression were obviously increased at this time (26). At the same time, Claudin-5 and occludin were degraded by MMP2, the BBB was destroyed and the size of cerebral infarction increased (27). MMP2 can also promote endogenous repair, especially angiogenesis, cerebral blood flow reconstruction and repair of brain tissue damage in the recovery stage after stroke (25). In general, MMP2 is an important regulatory indicator of stroke occurrence and recovery.
The regulatory role of MMP2 may be related to the regulation of its genetic variation. Li et al. (13) illustrated that compared with the normal control group, the frequency of CC genotype and C allele of MMP2 735C/T in the first and recurrent IS in the Chinese population were significantly increased, and the frequency of IS recurrence was more significant. Previous studies demonstrated that rs243849 in MMP2 evidently reduced the risk of IS in Hainan population, while rs1053605 in MMP2 was not found to be associated with IS susceptibility (9). At present, no study has explored the correlation between rs14070 in MMP2 and IS susceptibility, only found that rs14070 was positively correlated with the incidence of hypertension caused by urinary cadmium (20). In our study, the results showed that rs1053605 was not significantly associated with IS susceptibility in Chinese Shaanxi population, which was consistent with the results reported in the literature. This study indicated that rs243849 in MMP2 obviously increase the risk of IS in drinkers under multiple genetic models, however, rs243849 in MMP2 in the literature evidently reduced the risk of IS in Hainan population. Apart from drinking, the reasons for this difference may be related to environmental, climatic and dietary factors in the northern and southern Chinese populations. Furthermore, this study was the first to confirm that rs14070 in MMP2 apparently reduced the risk of IS in Chinese Shaanxi population under multiple genetic models. In brief, the polymorphism differences of MMP2 were significantly associated with the risk of IS in Chinese Shaanxi populations, and the key role of MMP2 may depend on polymorphism differences.
Genetic susceptibility to IS may be associated with race, age, smoking, and drinking. A study found that long-term smoking, body mass index (BMI) ≥30, inactivity or unhealthy diet will increase the risk of IS by 66% (23). Rs1800795 in IL-6 in Asian populations was significantly associated with stroke occurrence, whereas rs1800795 on IL-6 in Oceania populations was not associated with IS occurrence (1). Another study demonstrated that SNPs in PITX2 significantly reduced the risk of IS in Chinese Han males (2). In addition, SNPs in HTRA1 were significantly associated with the risk of IS among Chinese Han smokers (5). In our study, analysis results illustrated that rs243849 in MMP2 was associated with evidently increased IS risk in Chinese drinking population under allelic, co-dominant, dominant and additive genetic models. However, rs14070 in MMP2 can still significantly reduce IS susceptibility in Chinese Shaanxi population older than 55 years, females, smokers and drinkers. These study suggested that genetic susceptibility to IS is closely related to race, sex, advanced age, smoking and drinking.
The function of MMP2 depends on endogenous inhibitors (TIMP), angiogenesis factors (VEGF, TGF-β) and inflammatory factors (28). The regulation of ECM degradation mainly depends on the balance between MMPs-TIMP (29). Imbalance of MMP-TIMP can lead to neurological diseases (stroke, Alzheimer's disease), atherosclerosis and cardiovascular diseases (30). Notably, the secretion and regulation of MMP2 lead to BBB damage in the early stage of stroke (31). As a major inhibitor of MMP2 (19), TIMP2 likewise has a dual role (29). VEGF interacting with MMP2 regulated neovascular remodeling and neuroprotection after stroke injury (32). Similarly, TGF-β can promote the remodeling of ECM and inhibit the disruption of BBB (32). Furthermore, MMP-2 and TGF-β can be bidirectionally regulated, which is beneficial to angiogenesis and reconstruction (30). In this study, we demonstrated that MMP2 mainly regulated TIMPs and angiogenesis factors (VEGF, TGF-β). Subsequently, they participated in the Relaxin signaling pathway to regulate angiogenesis, inflammation and vascular endothelial function. Collectively, MMP2 can regulate inflammation, angiogenesis, and ECM degradation, which play a crucial role in IS occurrence and recovery.
Interesting findings were revealed in this study, which provided a reliable basis to future research on MMP2 regulation of IS. However, potential limitations of our study deserve consideration. In this study, only 3 SNPs in MMP2 were explored, and we will continue to explore the association of other SNPs in MMP2, MMPs and gene interactions with IS susceptibility in the future (16). Additionally, the current study was limited to a single ethnic group, other ethnic groups should be validated in the future (13, 17). Finally, in order to further determine the potential impact of MMP2 genetic variation on the risk of IS, cell and animal models will be required to verify its regulatory mechanism in different stages of IS (2).
Conclusion
In general, this study explored the association of rs1053605, rs243849 and rs14070 in MMP2 with the risk of IS in Chinese Shaanxi population. Stratified analysis indicated that rs243849 in MMP2 obviously increased the risk of IS among drinking population, while rs14070 in MMP2 evidently reduced IS susceptibility in females, participants with older than 55, smokers, and drinkers. This study illustrated that genetic variation of MMP2 played an essential role in the occurrence and recovery of IS, which provided support for the early diagnosis and treatment of IS.
Data availability statement
The datasets generated and/or analyzed during the current study are available in the zenodo repository (https://zenodo.org/record/6826121#.Ys6AkfkaWUk).
Ethics statement
The studies involving human participants were reviewed and approved by this study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Affiliated Hospital of Northwest University (Xi'an No.3 Hospital). The patients/participants provided their written informed consent to participate in this study.
Author contributions
SL and SY performed the manuscript. XiaobZ and YZ took part in genotyping. JZ, XiaoZ, and WL participated in the statistical analysis of the data. XN and GZ modified the manuscript. WS, MC, and YT designed the study, co-supervised the work, and finalized the manuscript. All authors have read and approved the final manuscript.
Funding
This study was supported by Natural Science Foundation of China (No. 82104155), Key Research and Development Program of Shaanxi (2020ZDLSF04-03 and 2021SF-096), and Xi'an Science and Technology Planning Project (21YXYY0038 and 21YXYJ0004).
Acknowledgments
The authors thank all the participants in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.931437/full#supplementary-material
References
1. Chai J, Cao XL, Lu F. Association of Interleukin-6-174g/C polymorphism with ischemic stroke: an updated meta-analysis. Front Neurol. (2021) 12:799022. doi: 10.3389/fneur.2021.799022
2. Zhao W, Hu X, Hao J, Guo L, Zhang W, Liu J, et al. Effect of Pitx2 Genetic Variants on the Susceptibility to Stroke in the Chinese Han Population. Infect Genet Evol. (2022) 98:105201. doi: 10.1016/j.meegid.2021.105201
3. Ching SC, Wen LJ, Ismail NIM, Looi I, Kooi CW, Peng LS, et al. Slc17a3 Rs9379800 and ischemic stroke susceptibility at the northern region of Malaysia. J Stroke Cerebrovasc Dis. (2021) 30:105908. doi: 10.1016/j.jstrokecerebrovasdis.2021.105908
4. Zhang Z, Mei Y, Xiong M, Lu F, Zhao X, Zhu J, et al. Genetic variation of inflammatory genes to ischemic stroke risk in a Chinese Han Population. Pharmgenomics Pers Med. (2021) 14:977–86. doi: 10.2147/PGPM.S320483
5. Tian Y, Tang W, Yang S, Zhao Y, Chen Y, Zhao X, et al. Htra1 variants and the interaction with smoking confer the genetic susceptibility to ischemic stroke. Int J Med Sci. (2021) 18:1840–7. doi: 10.7150/ijms.45856
6. Balkaya M, Cho S. Genetics of stroke recovery: Bdnf Val66met polymorphism in stroke recovery and its interaction with aging. Neurobiol Dis. (2019) 126:36–46. doi: 10.1016/j.nbd.2018.08.009
7. Li Q, Wu H, Yue W, Dai Q, Liang H, Bian H, et al. Prevalence of stroke and vascular risk factors in China: a nationwide community-based study. Sci Rep. (2017) 7:6402. doi: 10.1038/s41598-017-06691-1
8. Markidan J, Cole JW, Cronin CA, Merino JG, Phipps MS, Wozniak MA, et al. Smoking and risk of ischemic stroke in young men. Stroke. (2018) 49:1276–8. doi: 10.1161/STROKEAHA.117.018859
9. Niu F, Wei B, Yan M, Li J, Ouyang Y, Jin T. Matrix Metalloproteinase-2 gene polymorphisms are associated with ischemic stroke in a hainan population. Medicine. (2018) 97:e12302. doi: 10.1097/MD.0000000000012302
10. Misra S, Talwar P, Kumar A, Kumar P, Sagar R, Vibha D, et al. Association between matrix metalloproteinase family gene polymorphisms and risk of ischemic stroke: a systematic review and meta-analysis of 29 studies. Gene. (2018) 672:180–94. doi: 10.1016/j.gene.2018.06.027
11. Wang B, Wang Y, Zhao L. Mmp-9 Gene Rs3918242 polymorphism increases risk of stroke: a meta-analysis. J Cell Biochem. (2018) 119:9801–8. doi: 10.1002/jcb.27299
12. Kelly PJ, Lemmens R, Tsivgoulis G. Inflammation and stroke risk: a new target for prevention. Stroke. (2021) 52:2697–706. doi: 10.1161/STROKEAHA.121.034388
13. Li Y, Ouyang QR, Li J, Chen XR, Li LL, Xu L, et al. Correlation between matrix metalloproteinase-2 polymorphisms and first and recurrent atherosclerotic ischemic stroke events: a case-control study. J Int Med Res. (2021) 49:3000605211022967. doi: 10.1177/03000605211022967
14. Wu G, Cai H, Cai H, Chen Z, Tan L, Qin X, et al. Influence of the Cyclooxygenase-2 Gene−765g/C and−1195g/a Polymorphisms on Development of Ischemic Stroke. J Stroke Cerebrovasc Dis. (2016) 25:2126–35. doi: 10.1016/j.jstrokecerebrovasdis.2016.06.001
15. Wang L, Deng L, Yuan R, Liu J, Li Y, Liu M. Association of matrix metalloproteinase 9 and cellular fibronectin and outcome in acute ischemic stroke: a systematic review and meta-analysis. Front Neurol. (2020) 11:523506. doi: 10.3389/fneur.2020.523506
16. Fan D, Zheng C, Wu W, Chen Y, Chen D, Hu X, et al. Mmp9 Snp and Mmp Snp-Snp interactions increase the risk for ischemic stroke in the han hakka population. Brain Behav. (2022) 12:e2473. doi: 10.1002/brb3.2473
17. Shen C, Fan D, Fu H, Zheng C, Chen Y, Hu Z. Single nucleotide polymorphisms in the Angptl4 gene and the Snp-Snp interactions on the risk of atherosclerotic ischaemic stroke. BMC Neurol. (2021) 21:108. doi: 10.1186/s12883-021-02138-3
18. Strbac D, Goricar K, Dolzan V, Kovac V. Matrix metalloproteinases polymorphisms as baseline risk predictors in malignant pleural mesothelioma. Radiol Oncol. (2018) 52:160–6. doi: 10.2478/raon-2018-0005
19. Wang N, Zhou S, Fang XC, Gao P, Qiao Q, Wu T, et al. Mmp-2,−3 and Timp-2,−3 polymorphisms in colorectal cancer in a Chinese Han population: a case-control study. Gene. (2020) 730:144320. doi: 10.1016/j.gene.2019.144320
20. Wu W, Liu D, Jiang S, Zhang K, Zhou H, Lu Q. Polymorphisms in gene Mmp-2 modify the association of cadmium exposure with hypertension risk. Environ Int. (2019) 124:441–7. doi: 10.1016/j.envint.2019.01.041
21. Pourasgari M, Mohamadkhani A. Heritability for stroke: essential for taking family history. Casp J Intern Med. (2020) 11:237–43. doi: 10.22088/cjim.11.3.237
22. Malik R, Chauhan G, Traylor M, Sargurupremraj M, Okada Y, Mishra A, et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat Genet. (2018) 50:524–37. doi: 10.1038/s41588-018-0058-3
23. Rutten-Jacobs LC, Larsson SC, Malik R, Rannikmäe K, Sudlow CL, Dichgans M, et al. Genetic risk, incident stroke, and the benefits of adhering to a healthy lifestyle: cohort study of 306 473 Uk biobank participants. BMJ. (2018) 363:k4168. doi: 10.1136/bmj.k4168
24. Stewart JC, Cramer SC. Genetic variation and neuroplasticity: role in rehabilitation after stroke. J Neurol Phys Ther. (2017) 41 Suppl 3(Suppl 3 IV STEP Spec Iss):S17–s23. doi: 10.1097/NPT.0000000000000180
25. Lin HF, Hsi E, Huang LC, Liao YC, Juo SH, Lin RT. Methylation in the Matrix Metalloproteinase-2 Gene Is Associated with Cerebral Ischemic Stroke. J Investig Med. (2017) 65:794–9. doi: 10.1136/jim-2016-000277
26. Zhang S, An Q, Wang T, Gao S, Zhou G. Autophagy- and Mmp-2/9-Mediated Reduction and Redistribution of Zo-1 Contribute to Hyperglycemia-Increased Blood-Brain Barrier Permeability During Early Reperfusion in Stroke. Neuroscience. (2018) 377:126–37. doi: 10.1016/j.neuroscience.2018.02.035
27. Kim EH, Kim ES, Shin D, Kim D, Choi S, Shin YJ, et al. Carnosine protects against cerebral ischemic injury by inhibiting matrix-metalloproteinases. Int J Mol Sci. (2021) 22:7495. doi: 10.3390/ijms22147495
28. Dusanovic Pjevic M, Jekic B, Beslac Bumbasirevic L, Vojvodic L, Damnjanovic T, Grk M, et al. Tt Genotype of the Mmp-9-1562c/T polymorphism may be a risk factor for thrombolytic therapy-induced hemorrhagic complications after acute ischemic stroke. Pharmacotherapy. (2021) 41:562–71. doi: 10.1002/phar.2532
29. Zhang Y, Fan F, Zeng G, Zhou L, Zhang Y, Zhang J, et al. Temporal analysis of blood-brain barrier disruption and cerebrospinal fluid matrix metalloproteinases in rhesus monkeys subjected to transient ischemic stroke. J Cereb Blood Flow Metab. (2017) 37:2963–74. doi: 10.1177/0271678X16680221
30. Zeng XL, Sun L, Zheng HQ, Wang GL, Du YH, Lv XF, et al. Smooth Muscle-Specific Tmem16a expression protects against angiotensin ii-induced cerebrovascular remodeling via suppressing extracellular matrix deposition. J Mol Cell Cardiol. (2019) 134:131–43. doi: 10.1016/j.yjmcc.2019.07.002
31. Shen Y, Gu J, Liu Z, Xu C, Qian S, Zhang X, et al. Inhibition of Hif-1α reduced blood brain barrier damage by regulating Mmp-2 and Vegf during acute cerebral ischemia. Front Cell Neurosci. (2018) 12:288. doi: 10.3389/fncel.2018.00288
Keywords: MMP2, susceptibility, single nucleotide polymorphism (SNP), rs243849, rs14070, ischemic stroke (IS)
Citation: Li S, Yang S, Zhang X, Zhang Y, Zhang J, Zhang X, Li W, Niu X, Shi W, Zhang G, Chang M and Tian Y (2022) Impact of MMP2 rs243849 and rs14070 genetic polymorphisms on the ischemic stroke susceptibility in Chinese Shaanxi population. Front. Neurol. 13:931437. doi: 10.3389/fneur.2022.931437
Received: 29 April 2022; Accepted: 30 June 2022;
Published: 25 July 2022.
Edited by:
Dennis John Cordato, University of New South Wales, AustraliaReviewed by:
Zhenyu Li, Chongqing University, ChinaAlejandro Ferraz Prado, Federal University of Pará, Brazil
Copyright © 2022 Li, Yang, Zhang, Zhang, Zhang, Zhang, Li, Niu, Shi, Zhang, Chang and Tian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ye Tian, dGlhbnllMjAyMjAyQDE2My5jb20=; Mingze Chang, Y2hhbmdtaW5nemUxOTFAMTYzLmNvbQ==
 Shilin Li
Shilin Li Shiyao Yang2
Shiyao Yang2 Xiaobo Zhang
Xiaobo Zhang Ye Tian
Ye Tian