
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 01 November 2022
Sec. Movement Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.929569
 Ruxin Gu1†
Ruxin Gu1† Jun Zhu1†
Jun Zhu1† Min Zhong1
Min Zhong1 Yinyin Jiang1
Yinyin Jiang1 Sha Zhu1
Sha Zhu1 Yaxi Wang1
Yaxi Wang1 Xu Jiang1
Xu Jiang1 Bo Shen1
Bo Shen1 Jun Yan1
Jun Yan1 Li Zhang1,2*†
Li Zhang1,2*† Yang Pan1*†
Yang Pan1*†Hallucination is a common non-motor symptom in patients with Parkinson's disease (PD). Additionally, sleep disorders are associated with an increased risk of hallucinations in PD patients. This study aimed to examine the association between hallucination and objective sleep parameters in PD patients. We retrospectively recruited 278 PD patients who underwent polysomnography and clinical assessments and classified them into non-hallucination and hallucination groups. Hallucinations were observed in 77 older PD patients who had more severe motor symptoms and higher scores on the Non-Motor Symptoms Questionnaire (NMSQ), Hamilton Depression Scale (HAMD) and Hamilton Anxiety Scale (HAMA) but lower scores on the Montreal Cognitive Assessment (MOCA) and PD Sleep Scale (PDSS) than PD patients without hallucinations. Analysis of the polysomnographic variables in patients with hallucinations showed that they exhibited a decrease in total sleep time, sleep efficiency (SE), rapid eye movement (REM) sleep time and slow wave sleep (SWS, N3) time and percentage but a significant increase in wake time after sleep onset (WASO), periodic limb movement index (PLMI) scores, and stage 2 NREM (N2)percentage. Logistic regression analysis revealed that higher NMSQ scores, lower MOCA scores, lower SE, and a lower percentage of N3 sleep were associated with hallucinations in PD patients. Our results suggested that PD patients with hallucinations had worse sleep quality and differences in sleep architecture (measured by polysomnography).
Except typical motor deficits, Parkinson's disease (PD) patients suffer from a range of non-motor symptoms (NMS), such as Parkinson's disease psychosis (PDP), sleep disturbances, cognitive deficits, and autonomic dysfunction (1). With disease progression, NMS predominate, reducing quality of life; this is especially true for PDP and sleep disturbances, which are associated with higher mortality and greater rates of nursing home placement (2, 3).
Approximately 2/3 of patients with PD experience hallucinations during their disease course (4). Most patients with PDP present with minor hallucinations (MH) and well-structured visual hallucinations (VH), which may precede motor symptoms (5).
Sleep disturbances are common in PD, can present in prodromal stages, such as REM sleep behavior disorder (RBD) (6). Moreover, RBD may increase the risk of hallucinations (7).
Several studies (8) have suggested that PD patients who developed hallucinations had worse sleep quality than those who did not, with reduced sleep duration, reduced efficiency and increased daytime sleepiness. This is consistent with evidence reporting that poor sleep quality played a causal role in the development of psychotic experiences, such as auditory hallucinations and VH (9). In addition, imaging data from PD patients with hallucinations or sleep disturbances showed dysfunction of the cholinergic system in the brain; both groups of patients exhibited similar neurobiological processes of cholinergic dysfunction (10). These evidences indicate that sleep disorders associated with hallucinations, but few studies have accurately quantified sleep architecture to determine the association between hallucinations and sleep disorders.
Therefore, we aimed to examine and compare clinical characteristics and sleep parameters in a cohort of PD patients with or without hallucinations.
From January 2020 to March 2022, PD patients were recruited from the Affiliated Brain Hospital of Nanjing Medical University. This cross-sectional study evaluated their sleep disturbances. All participants met the Movement Disorder Society PD Criteria (11). Data from 18 patients who did not cooperate with sleep monitoring were excluded, resulting in the inclusion of 278 PD patients (Supplementary Figure S1). This study was approved by the Ethics Committee of the Affiliated Brain Hospital of Nanjing Medical University and written informed consent was obtained from all participants. We excluded patients with secondary Parkinsonism, focal brain injury (MRI evidence), severe ocular disorders and other psychiatric disorders. The patients did not take antipsychotics and hypnotics. Patients were also excluded if they had cognitive impairment, as demonstrated by a Montreal Cognitive Assessment (MoCA) score < 20 (12).
Structured interviews were conducted using a standard questionnaire to obtain demographic characteristics and specific disease-related data, including sex, age, body mass index (BMI), duration of disease, and medication use. The questionnaire was administered by trained experts during face-to-face interviews. Levodopa equivalent doses (LEDs) were calculated according to previously published recommendations (13).
Motor symptoms were assessed using the Unified Parkinson's Disease Rating Scale motor subscale (UPDRS-III) and the Hoehn and Yahr scale (HY). Non-motor symptoms were assessed using the Non-Motor Symptom Questionnaire (NMSQ) (14), excluding the score on the hallucinations/delusions domain. Cognitive function was assessed according to the MOCA, which is more sensitive than the Mini-Mental State Examination (MMSE) for detecting mild cognitive impairment (15). The PD Sleep Scale (PDSS), Hamilton Anxiety Scale (HAMA) and Hamilton Depression Scale (HAMD) were used to assess subjective sleep quality and the severity of anxiety and depression, respectively (16–18).
The presence of hallucinations was assessed based on the “Hallucinations and Psychosis” item of the Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): 0: Normal = No hallucinations or psychotic behavior. 1: Slight = Illusions or non-formed hallucinations, but the patient recognizes them without loss of insight. 2: Mild = Formed hallucinations independent of environmental stimuli. No loss of insight. 3: Moderate = Formed hallucinations with loss of insight. 4: Severe = Patients have delusions or paranoid. A total of 201 patients were categorized into the non-hallucinations group. A questionnaire comprising 11 items on the nature and properties of patient hallucinations, including temporal factors, content and external factors, was used to further assessed hallucinations in patients with MDS-UPDRS hallucination scores ≥ 1 (19).
Standard PSG assessment was performed at both sites using a video-enabled Compumedics Grael-HD 64 (Compumedics Grael-HD, Australia) polygraph throughout the night. The time of the PSG recording was fixed (between 23:00 and 07:00). We collected the following channels for sleep staging: 6 electroencephalogram channels (F3/M2, F4/M1, C3/M2, C4/M1, O1/M2, O2/M1); 2 electrooculogram channels (E1, E2), an electromyogram channel, a nasal pressure channel, a respiratory thermistor channel, an oximetry channel, a snoring sensor channel, an electrocardiography channel, a thoracic and abdominal respiratory effort sensor, a body position channel and 2 leg movement sensors.
Movements, sleep stages and cardiopulmonary events were assessed manually according to the American Academy of Sleep Medicine (AASM) guidelines (20), including periodic limb movement index (PLMI), five sleep stages (wake, stage N1, stage N2, stage N3, and REM), micro-arousal index (MAI) and apnea hypopnea index (AHI). Total sleep time (TST), wake time after sleep onset (WASO), sleep efficiency (SE), sleep latency (SL), and REM sleep latency (RSL) were recorded. Sleep disorders were determined for WASO ≥ 60 min, SL ≥ 30 min, and SE < 85% on the PSG as well as PDSS < 105 (21).
Descriptive data are expressed as the mean ± standard deviation (SD) and number (n) or percentage (%). The two groups were compared, using Student's t test for continuous variables and the chi-square test for categorical variables. Clinical scores (HAMA, HAMD, MOCA, NMSQ, PDSS and PSG data) were compared by one-way analyses of covariance (ANCOVA) with adjustments for confounding factors, including age, UPDRS-III score and HY stage. A binary logistic regression model was used to identify possible factors associated with hallucinations. Receiver operating characteristic (ROC) curves were plotted to evaluate the abilities of the NMSQ, MoCA and 2 sleep variables to predict PD with hallucinations. Statistical analyses were performed using SPSS 27.0 (IBM Corporation, New York, USA), and a p < 0.05 value was considered statistically significant.
The final analysis included data from of 278 patients consisted of 112 females (40.3%) and 166 males (59.7%), with a mean age of 66.80 ± 8.64 years. Among these patients, 77 (25.8%) were diagnosed with hallucinations. Almost all of the patients who had hallucinations experienced minor hallucinations and/or well-structured visual hallucinations, sometimes with difficulty in distinguishing between the two. Some patients also reported other forms of hallucinations: 3 had tactile hallucinations, 4 had olfactory hallucinations, and 9 had auditory hallucinations (Table 1). Patients reported that hallucinations could be more likely to occur at night or in dim lighting (75.3%). The visual hallucinations tended to consist of mundane content, including unfamiliar or deceased people, animals, and objects. The following were significantly associated with hallucinations in the univariate analyses (Table 2): older age (P = 0.014, P < 0.05), higher UPDRS-III scores (P = 0.002, P < 0.05), and greater HY stage (P < 0.001). There were no differences in sex, BMI or drug types between the patients with hallucinations and those without. Although patients with hallucinations had a longer duration of disease and higher LEDs, these differences were not significant.
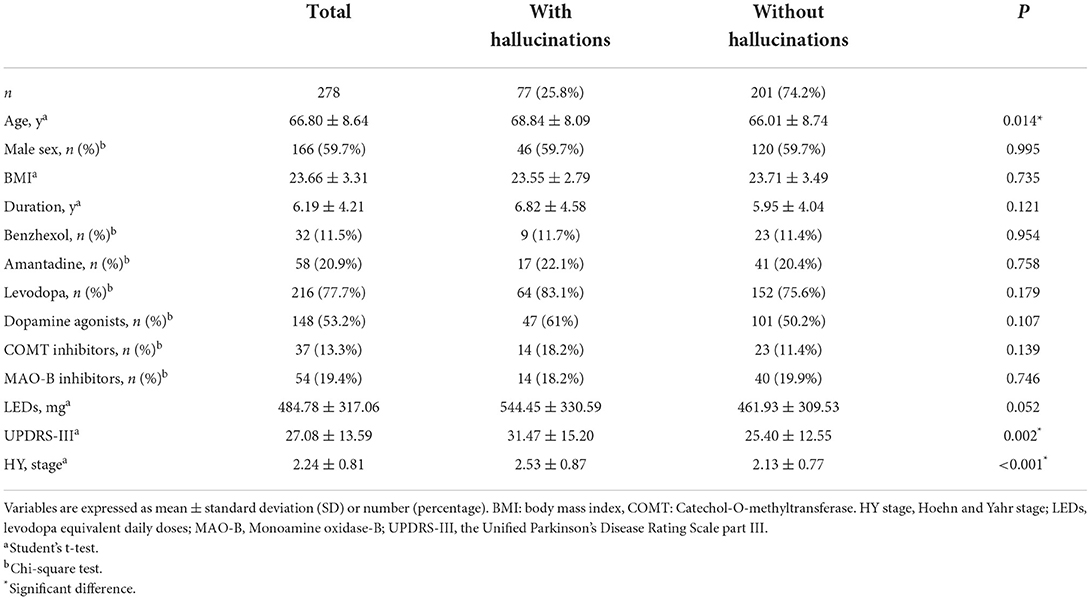
Table 2. Demographic and clinical characteristics of Parkinson's disease patients based on the presence/absence of hallucinations.
The differences in non-motor symptoms between patients with and without hallucinations are shown in Table 3. After controlling for some variables, the patients with hallucinations were associated with significantly higher NMSQ (P < 0.001), HAMD (P = 0.005, P < 0.001), and HAMA (P = 0.002, P < 0.001) scores than the patients without hallucinations. In addition, hallucinations were associated with lower PDSS (P < 0.001) and MOCA scores (P < 0.001). These patients had worse cognitive performance and more severe sleep disturbances, depression, and anxiety.
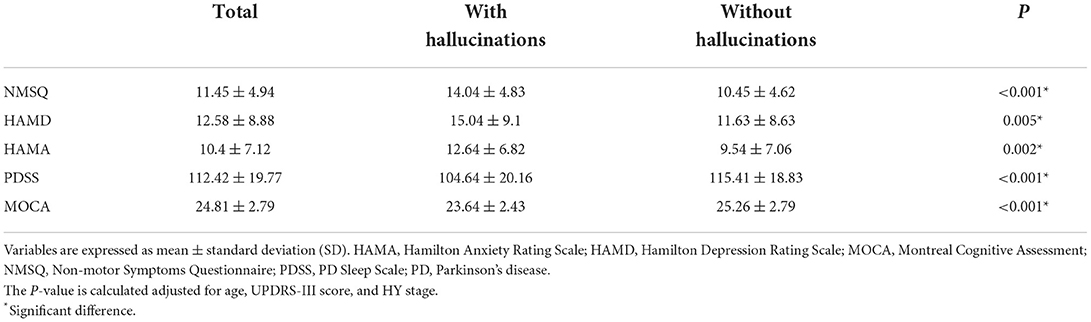
Table 3. Non-motor characteristics of Parkinson's disease patients based on the presence/absence of hallucinations.
Approximately half of the patients (50.7%) in the cohort were diagnosed with sleep disorders (Table 4). Among the patients with sleep disorders, 53 (43.8%) reported hallucinations and 88 (68.8%) did not. In addition, we found a significant decrease in the TST, SE, REM sleep time, slow wave sleep (SWS, N3) time, and percentage and a corresponding increase in WASO and percentage of N2 sleep in PD patients with hallucinations. Moreover, the PLMI was higher in the hallucination group. However, light sleep (N1) time and percentage, MAI and AHI did not differ significantly between the two groups. Among patients with hallucinations (Supplementary Table S1), 45 (58.4%) experienced clear-cut hallucinations and 22 (28.6%) experienced hypnagogic hallucinations. Patients with clear-cut hallucinations had longer stage N1 sleep time (p = 0.007) and a higher percentage of N1 sleep (p = 0.003) than patients with hypnagogic hallucinations.
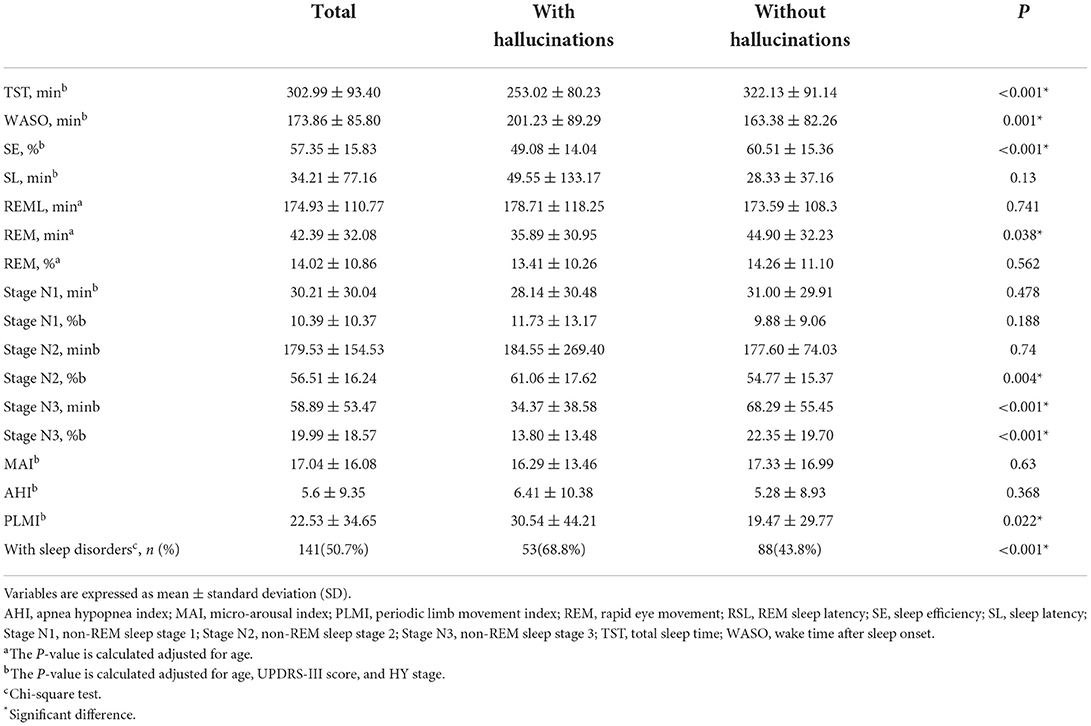
Table 4. Polysomnographic variables according to the presence/absence of hallucinations in Parkinson's disease patients.
Multivariate logistic regression analysis (Table 5) revealed that hallucinations were significantly related to the NMSQ score [OR: 1.17, 95% CI: (1.09–1.25)], MOCA score [OR: 0.82, 95% CI: (0.73–0.92)] and PSG variables such as sleep efficiency [OR: 0.95, 95% CI: (0.93–0.97)] and percentage of SWS out of the TST [OR: 0.96, 95% CI: (0.94–0.98)]. Hallucinators had more complex non-motor symptoms and more severe cognitive impairment. Improving sleep quality by elevating sleep efficiency and increasing deep sleep percentage may protect against hallucinations.
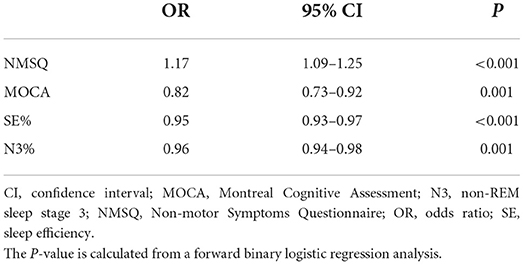
Table 5. Logistic regression analyses for independent predictors of hallucinations in Parkinson's disease patients.
A receiver operating characteristic (ROC) curve was used to assess the predictive value of the factors influencing hallucination occurrence (Figure 1). The NMSQ score, MOCA score, SE%, and N3% (Figure 1A) were used as independent indicators to plot the ROC curves, with the NMSQ score (AUC = 0.706, 95% CI: 0.638–0.774) and SE% (AUC = 0.717, 95% CI: 0.654–0.781) exhibiting good predictive value and the MOCA score (AUC = 0.671, 95% CI: 0.604–0.738) and N3% (AUC = 0.651, 95% CI: 0.579–0.723) exhibiting relatively less accurate predictive value. The ROC curve for the combined four indicators (Figure 1B) had a high sensitivity (77.9%) and specificity (78.1%) (AUC = 0.833: 95% CI: 0.779–0.887).
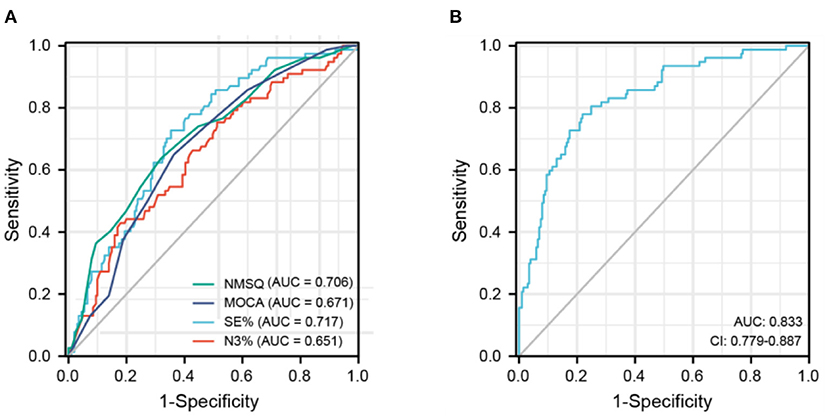
Figure 1. Receiver operating characteristic curve of predicting hallucinations in Parkinson's disease patients. (A) NMSQ score, MOCA score, SE%, N3% for independently predicting hallucinations occurrence in PD patients. (B) A combination of NMSQ score, MOCA score, SE%, N3% for predicting hallucinations occurrence in PD patients. AUC, area under the curve; MOCA, Montreal Cognitive Assessment; N3, non-REM sleep stage 3; NMSQ, Non-motor Symptoms Questionnaire; SE, sleep eciency.
In previous cross-sectional studies, the prevalence of hallucinations in PD patients ranged from 21.5% to 50% (22–28). Our study reported that a quarter (25.8%) of PD patients experienced hallucinations. All patients with hallucinations experienced MHs or VHs, which were the most common modality of hallucination. As previously reported, PDP may occur in de-novo patients (5), MH can be observed in the earliest stages of the disease, followed by well-structured hallucinations, with gradual loss of insight and eventually progresses to delusions (29). Although the most hallucinatory content is not frightening, its development worried some PD patients. Hallucinations can be perceived as threatening when patients lose their insight (30).
Among our PD patients, the presence of hallucinations was significantly associated with older age, more severe motor and non-motor symptoms, such as HY stage, UPDRS-III score, depression, anxiety, impaired cognition and sleep disturbances. Multivariate analysis identified two clinical variables, the NMSQ score and MOCA score, as independent risk factors for hallucinations, supported by the results of similar studies (4, 27). Overall, as PD progressed, the motor symptoms became more severe and non-motor symptoms, including hallucinations and sleep disturbances, became more complex.
To the best of our knowledge, this is the first large sample study to explore the sleep architecture of Chinese PDP patients with PSG. The hallucinations group (68.8%) had a higher rate of sleep disorders than the non-hallucination group (43.8%). In addition, we found that patients with hallucinations exhibited less balanced and more fragmented sleep structure, with a corresponding reduction in the proportion of REM sleep and slow wave sleep. These findings support the hypothesis that REM sleep may intrude in the wakeful state and lead to visual hallucinations (31). Univariate analysis showed that the PDSS score was associated with hallucinations, but multivariate analysis revealed that it was not an independent predictor. We can determine that although questionnaire is convenient for assessing subjective sleep quality, it is less accurate than PSG measures.
Previous studies have reported a link between sleep disturbances and the occurrence of hallucinations. Healthy volunteers reported significant increases in paranoia and hallucinations after sleep loss (32). vConversely, treating volunteers with insomnia resulted in a reduction in paranoia and hallucinations (33). In the present study with PD patients, logistics regression analysis revealed that the SE% and N3% variables were independent predictors of hallucinations in PD patients. That is, reductions in sleep efficiency and deep sleep, which were found to lead to poor sleep quality, lethargy or fatigue the next day (34), may directly or indirectly exacerbate psychiatric problems in PD patients. First, sleep problems have functional impacts on perception and memory in PD patients (35, 36). The integrated theoretical hallucination model emphasizes dysregulation of gating and filtering exogenous perception and endogenous image creation; thus, aberrant release of stored percepts may be a way of compensating for reduced visual input, thus causing hallucinations (31, 37). Second, sleep is associated with neuroplasticity (38) and is essential for removing pathological brain proteins such as α-syn, amyloid-β, and tau proteins (39–41). In mice, sleep deprivation increased levels of tau protein in the interstitial fluid (ISF) almost 2-fold. Similar changes could be seen in the CSF of humans, as sleep deprivation significantly increased CSF α-syn, amyloid-β, and tau protein levels by 36.4, 30, and 51.5%, respectively. Chronic sleep disorders can interfere with the normal clearance process, leading to secondary pathological changes that may induce hallucinations in patients as the disease progresses. Finally, the development of hallucination (e.g., visual form) may occur in parallel to sleep problems throughout the course of PD (42), because the impaired neural structures affected by the disease are involved in sleep-wake control as well as visual perception (43). Although accumulating evidence strongly supports the interaction between sleep disturbances and hallucinations in PD patients, the underlying mechanisms of this bidirectional relationship remain unclear.
Although research on PD-related sleep problems has focused on the REM phase, NREM sleep, such as SWS, also plays a role in the pathogenesis of hallucinations (44). SWS is not only a major contributor to neurophysiological processes such as memory and attention, but it is also crucial for brain metabolite clearance (39, 45). Moreover, deeper sleep relates to slower motor progression and better cognitive performance in PD (46, 47).
Given the heterogeneity and complexity of PD and that combined indicators were more accurate than single indicators in predicting hallucinations in PD patients, we believe that various variables should be integrated to evaluate the condition of PD patients and predict the occurrence of hallucinations.
This study also has some limitations. For example, we monitored participant sleep for only one night, and there may have been a first-night effect. Moreover, hallucination symptoms and sleep conditions fluctuate; thus, measuring them at arbitrary time points does not provide a complete picture. Finally, our findings differed from previous findings due to a lack of specific hallucinatory content assessments and patient feedback. Future longitudinal cohort studies can assess the temporal association between psychosis and sleep disorders to determine whether sleep hygiene practices can alleviate sleep problems and hallucinations in PD patients.
In conclusion, approximately 1/4 of PD patients in our study experienced hallucinations. Over time, PD patients with sleep disturbances have an increased risk of psychotic hallucinations. Based on the PSG characteristics of PD patients with hallucinations in the present study, we propose that adjusting sleep behavior may control hallucinatory symptoms and improve the quality of life of individuals with PD.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of the Affiliated Brain Hospital of Nanjing Medical University. The patients/participants provided their written informed consent to participate in this study.
LZ, JZ, and RG conceived and designed the study. LZ and JY obtained the funding. RG, JZ, MZ, SZ, YJ, YW, XJ, and BS collected the data. RG, JZ, and YP conducted the data analysis. JZ and RG drafted the manuscript. All authors contributed to the article and approved the submitted version.
This work was funded by National Natural Science Foundation of China (Grant No. 82171249), Special Funds of the Jiangsu Provincial Key Research and Development Projects (Grant Nos. BE2018610 and BE2019612), Nanjing Rehabilitation Medicine Center Project, Jiangsu Provincial Cadre Health Projects (BJ20005), Jiangsu Provincial Elderly Health Research Project (LD2021013 and LR2021018), Nanjing Medical Science and Technology Development Foundation (QRX17026), and Nanjing Industrial and Information Development Special Fund Project.
We thank the patients and their families for their participation in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.929569/full#supplementary-material
1. Armstrong MJ, Okun MS. Diagnosis and Treatment of Parkinson Disease: A Review. Jama. (2020) 323:548–60. doi: 10.1001/jama.2019.22360
2. Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci. (2017) 18:435–50. doi: 10.1038/nrn.2017.62
3. Weerkamp NJ, Tissingh G, Poels PJ, Zuidema SU, Munneke M, Koopmans RT, et al. Non-motor symptoms in nursing home residents with Parkinson's disease: prevalence and effect on quality of life. J Am Geriatr Soc. (2013) 61:1714–21. doi: 10.1111/jgs.12458
4. Zhu K, van Hilten JJ, Putter H, Marinus J. Risk factors for hallucinations in Parkinson's disease: results from a large prospective cohort study. Mov Disord. (2013) 28:755–62. doi: 10.1002/mds.25389
5. Lenka A, Pagonabarraga J, Pal PK, Bejr-Kasem H, Kulisvesky J. Minor hallucinations in Parkinson disease: a subtle symptom with major clinical implications. Neurology. (2019) 93:259–66. doi: 10.1212/WNL.0000000000007913
6. Mahlknecht P, Seppi K, Poewe W. The concept of prodromal Parkinson's disease. J Parkinsons Dis. (2015) 5:681–97. doi: 10.3233/JPD-150685
7. Kim YE, Jeon BS. Clinical implication of REM sleep behavior disorder in Parkinson's disease. J Parkinsons Dis. (2014) 4:237–44. doi: 10.3233/JPD-130293
8. Barnes J, Connelly V, Wiggs L, Boubert L, Maravic K. Sleep patterns in Parkinson's disease patients with visual hallucinations. Int J Neurosci. (2010) 120:564–9. doi: 10.3109/00207454.2010.494790
9. Yu M, Du Y, Liu K, Liang X, Huang C, He R, et al. Sleep duration and auditory hallucinations: Genetic correlation and two-sample Mendelian randomization study. J Affect Disord. (2021) 291:409–14. doi: 10.1016/j.jad.2021.04.038
10. Lenka A, Hegde S, Jhunjhunwala KR, Pal PK. Interactions of visual hallucinations, rapid eye movement sleep behavior disorder and cognitive impairment in Parkinson's disease: a review. Parkinsonism Relat Disord. (2016) 22:1–8. doi: 10.1016/j.parkreldis.2015.11.018
11. Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, et al. MDS clinical diagnostic criteria for Parkinson's disease. Mov Disord. (2015) 30:1591–601. doi: 10.1002/mds.26424
12. Gill DJ, Freshman A, Blender JA, Ravina B. The Montreal cognitive assessment as a screening tool for cognitive impairment in Parkinson's disease. Mov Disord. (2008) 23:1043–6. doi: 10.1002/mds.22017
13. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. (2010) 25:2649–53. doi: 10.1002/mds.23429
14. Chaudhuri KR, Martinez-Martin P, Schapira AH, Stocchi F, Sethi K, Odin P, et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson's disease: the NMSQuest study. Mov Disord. (2006) 21:916–23. doi: 10.1002/mds.20844
15. Ciesielska N, Sokołowski R, Mazur E, Podhorecka M, Polak-Szabela A, Kedziora-Kornatowska K. Is the Montreal Cognitive Assessment (MoCA) test better suited than the Mini-Mental State Examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis. Psychiatr Pol. (2016) 50:1039–52. doi: 10.12740/PP/45368
16. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. (1959) 32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x
17. Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. (1988) 45:742–7. doi: 10.1001/archpsyc.1988.01800320058007
18. Chaudhuri KR, Pal S, DiMarco A, Whately-Smith C, Bridgman K, Mathew R, et al. The Parkinson's disease sleep scale: a new instrument for assessing sleep and nocturnal disability in Parkinson's disease. J Neurol Neurosurg Psychiatry. (2002) 73:629–35. doi: 10.1136/jnnp.73.6.629
19. Zhu J, Shen B, Lu L, Lan W, Pan Y, Zhang L, et al. Prevalence and risk factors for visual hallucinations in Chinese patients with Parkinson's disease. J Neurol Sci. (2017) 372:471–6. doi: 10.1016/j.jns.2016.10.043
20. Berry RB, Budhiraja R, Gottlieb DJ, Gozal D, Iber C, Kapur VK, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM manual for the scoring of sleep and associated events. Deliberations of the sleep apnea definitions task force of the American academy of sleep medicine. J Clin Sleep Med. (2012) 8:597–619. doi: 10.5664/jcsm.2172
21. Zhu J, Zhong M, Yan J, Jiang X, Wu Z, Pan Y, et al. Non-motor symptoms affect sleep quality in early-stage parkinson's disease patients with or without cognitive dysfunction. Front Neurol. (2020) 11:292. doi: 10.3389/fneur.2020.00292
22. Lee AH, Weintraub D. Psychosis in Parkinson's disease without dementia: common and comorbid with other non-motor symptoms. Mov Disord. (2012) 27:858–63. doi: 10.1002/mds.25003
23. Barrett MJ, Smolkin ME, Flanigan JL, Shah BB, Harrison MB, Sperling SA. Characteristics, correlates, and assessment of psychosis in Parkinson disease without dementia. Parkinsonism Relat Disord. (2017) 43:56–60. doi: 10.1016/j.parkreldis.2017.07.011
24. Ffytche DH, Pereira JB, Ballard C, Chaudhuri KR, Weintraub D, Aarsland D. Risk factors for early psychosis in PD: insights from the Parkinson's progression markers initiative. J Neurol Neurosurg Psychiatry. (2017) 88:325–31. doi: 10.1136/jnnp-2016-314832
25. Mack J, Rabins P, Anderson K, Goldstein S, Grill S, Hirsch ES, et al. Prevalence of psychotic symptoms in a community-based Parkinson disease sample. Am J Geriatr Psychiatry. (2012) 20:123–32. doi: 10.1097/JGP.0b013e31821f1b41
26. Gibson G, Mottram PG, Burn DJ, Hindle JV, Landau S, Samuel M, et al. Frequency, prevalence, incidence and risk factors associated with visual hallucinations in a sample of patients with Parkinson's disease: a longitudinal 4-year study. Int J Geriatr Psychiatry. (2013) 28:626–31. doi: 10.1002/gps.3869
27. Zhang Y, Zhang GY, Zhu XB, Zhang ZE, Gan J, Liu ZG. Clinical characteristics of minor hallucinations in Chinese Parkinson's disease patients. Front Aging Neurosci. (2021) 13:723405. doi: 10.3389/fnagi.2021.723405
28. Omoto S, Murakami H, Shiraishi T, Bono K, Umehara T, Iguchi Y. Risk factors for minor hallucinations in Parkinson's disease. Acta Neurol Scand. (2021) 143:538–44. doi: 10.1111/ane.13380
29. Ffytche DH, Creese B, Politis M, Chaudhuri KR, Weintraub D, Ballard C, et al. The psychosis spectrum in Parkinson disease. Nat Rev Neurol. (2017) 13:81–95. doi: 10.1038/nrneurol.2016.200
30. Marinus J, Zhu K, Marras C, Aarsland D, van Hilten JJ. Risk factors for non-motor symptoms in Parkinson's disease. Lancet Neurol. (2018) 17:559–68. doi: 10.1016/S1474-4422(18)30127-3
31. Muller AJ, Shine JM, Halliday GM, Lewis SJ. Visual hallucinations in Parkinson's disease: theoretical models. Mov Disord. (2014) 29:1591–8. doi: 10.1002/mds.26004
32. Reeve S, Emsley R, Sheaves B, Freeman D. Disrupting sleep: the effects of sleep loss on psychotic experiences tested in an experimental study with mediation analysis. Schizophr Bull. (2018) 44:662–71. doi: 10.1093/schbul/sbx103
33. Freeman D, Sheaves B, Goodwin GM Yu LM, Nickless A, Harrison PJ, et al. The effects of improving sleep on mental health (OASIS): a randomised controlled trial with mediation analysis. Lancet Psychiatry. (2017) 4:749–58. doi: 10.1016/S2215-0366(17)30328-0
34. Yin Z, Bai Y, Guan B, Jiang Y, Wang Z, Meng F, et al. A quantitative analysis of the effect of bilateral subthalamic nucleus-deep brain stimulation on subjective and objective sleep parameters in Parkinson's disease. Sleep Med. (2021) 79:195–204. doi: 10.1016/j.sleep.2020.10.021
35. Kirszenblat L, Ertekin D, Goodsell J, Zhou Y, Shaw PJ, van Swinderen B. Sleep regulates visual selective attention in Drosophila. J Exp Biol. (2018) 221:jeb191429. doi: 10.1242/jeb.191429
36. Klinzing JG, Niethard N, Born J. Mechanisms of systems memory consolidation during sleep. Nat Neurosci. (2019) 22:1598–610. doi: 10.1038/s41593-019-0467-3
37. Diederich NJ, Goetz CG, Stebbins GT. Repeated visual hallucinations in Parkinson's disease as disturbed external/internal perceptions: focused review and a new integrative model. Mov Disord. (2005) 20:130–40. doi: 10.1002/mds.20308
38. Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration. Neuron. (2014) 81:12–34. doi: 10.1016/j.neuron.2013.12.025
39. Ju YS, Ooms SJ, Sutphen C, Macauley SL, Zangrilli MA, Jerome G, et al. Slow wave sleep disruption increases cerebrospinal fluid amyloid-β levels. Brain. (2017) 140:2104–11. doi: 10.1093/brain/awx148
40. Wang C, Holtzman DM. Bidirectional relationship between sleep and Alzheimer's disease: role of amyloid, tau, and other factors. Neuropsychopharmacology. (2020) 45:104–20. doi: 10.1038/s41386-019-0478-5
41. Holth JK, Fritschi SK, Wang C, Pedersen NP, Cirrito JR, Mahan TE, et al. The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science. (2019) 363:880–4. doi: 10.1126/science.aav2546
42. Zahed H, Zuzuarregui JRP, Gilron R, Denison T, Starr PA, Little S. The neurophysiology of sleep in Parkinson's disease. Mov Disord. (2021) 36:1526–42. doi: 10.1002/mds.28562
43. Damulewicz M, Ispizua JI, Ceriani MF, Pyza EM. Communication among photoreceptors and the central clock affects sleep profile. Front Physiol. (2020) 11:993. doi: 10.3389/fphys.2020.00993
44. Manni R. Rapid eye movement sleep, non-rapid eye movement sleep, dreams, and hallucinations. Curr Psychiatry Rep. (2005) 7:196–200. doi: 10.1007/s11920-005-0053-0
45. Léger D, Debellemaniere E, Rabat A, Bayon V, Benchenane K, Chennaoui M. Slow-wave sleep: from the cell to the clinic. Sleep Med Rev. (2018) 41:113–32. doi: 10.1016/j.smrv.2018.01.008
46. Schreiner SJ, Imbach LL, Werth E, Poryazova R, Baumann-Vogel H, Valko PO, et al. Slow-wave sleep and motor progression in Parkinson disease. Ann Neurol. (2019) 85:765–70. doi: 10.1002/ana.25459
Keywords: Parkinson's disease, hallucination, sleep disorders, slow wave sleep, sleep quality
Citation: Gu R, Zhu J, Zhong M, Jiang Y, Zhu S, Wang Y, Jiang X, Shen B, Yan J, Zhang L and Pan Y (2022) Characteristics of sleep structure in Parkinson's disease patients with hallucinations based on polysomnography. Front. Neurol. 13:929569. doi: 10.3389/fneur.2022.929569
Received: 27 April 2022; Accepted: 10 October 2022;
Published: 01 November 2022.
Edited by:
Xiangdong Tang, Sichuan University, ChinaReviewed by:
Iva Stankovic, University of Belgrade, SerbiaCopyright © 2022 Gu, Zhu, Zhong, Jiang, Zhu, Wang, Jiang, Shen, Yan, Zhang and Pan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Zhang, bmV1cm9femhhbmdsaUAxNjMuY29t; Yang Pan, bmV1cm9fcGFueWFuZ0AxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.