
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Neurol., 20 June 2022
Sec. Stroke
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.922677
 Hong-yu Lin1†
Hong-yu Lin1† Qing-qing Wei1†
Qing-qing Wei1† Jian-yi Huang1
Jian-yi Huang1 Xing-hua Pan1
Xing-hua Pan1 Ning-chao Liang1
Ning-chao Liang1 Cai-xia Huang1*
Cai-xia Huang1* Teng Long1
Teng Long1 Wen Gao2
Wen Gao2 Sheng-liang Shi3
Sheng-liang Shi3Background: The relationship between mortality and seizures after intracerebral hemorrhage (ICH) has not yet been understood until now. A meta-analysis was performed to assess the effect of post-ICH seizures on mortality among patients with ICH.
Methods: PubMed and Embase were searched from the establishment of the databases to December 2021 to identify literature that evaluated the relationship between post-ICH seizures and mortality in ICH. Crude odds ratios and adjusted odds ratios with a 95% confidence interval (CI) were pooled using a random-effects model.
Results: Thirteen studies involving 245,908 participants were eventually included for analysis. The pooled estimate suggested that post-ICH seizures were not associated with significantly increased mortality in patients with ICH (crude odds ratios 1.35, 95% CI: 0.91–2; adjusted adds ratios 1.22, 95% CI: 0.78–1.88). However, the relationship was not consistent in subgroup analysis or robust in a sensitivity analysis.
Conclusions: This meta-analysis proved that post-ICH seizures were not associated with significantly increased mortality in patients with ICH. However, this result could be influenced by confounding factors, so more high-quality research is needed.
Intracerebral hemorrhage (ICH) is the second most common cause of stroke, the incidence of which is 24.6 per 100,000 person-years, with mortality that has maintained a rate of 35.2–45.5% for several decades, which is at a much higher level compared with that of ischemic stroke (1, 2). ICH complications, such as hematoma expansion, perihematomal edema, the intraventricular extension of the hemorrhage with hydrocephalus, seizures, venous thromboembolic events, hyperglycemia, increased blood pressure, fever, and infections, can in turn increase mortality and adverse outcome after ICH onset. Of these complications, seizures are a frequent complication with an overall 30-day risk of about 8%, but their impact on ICH clinical outcomes and mortality remains to be elucidated (3).
Most previous research focused on exploring the relationship between mortality and adverse outcome and seizures after stroke, including ischemic and hemorrhagic stroke; however, the results were inconclusive on account of numerous confounding factors (4–6). As there is a higher incidence of seizures following hemorrhagic stroke compared with ischemic stroke (5–9), we performed a systematic review and meta-analysis to assess current and relevant literature to evaluate the relationship between mortality and post-ICH seizures diminutively aimed at avoiding the impact of other stroke subtypes.
We conducted this systematic review and meta-analysis in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols guidelines (10).
We performed systematic searches of PubMed and Embase from the establishment of the databases to December 2021 to identify literature that evaluated mortality and seizures in patients with ICH. MeSH terms, explored EMTREE headings, and keywords were applied, and the search terms included “mortality,” “seizure,” “epileptic,” and “intracerebral hemorrhage” and their variants. No language restrictions were set. We also searched references in included literature to identify additional studies. A detailed search strategy is shown in Supplementary Materials 1, 2.
Our main outcome was the relationship between mortality and post-ICH seizures at the longest available follow-up. On account of the different types of post-ICH seizures, such as early seizures (ESs), late seizures (LSs), status epilepticus (SE), and any seizures (Ass), having a potential effect on mortality, subgroup analysis was used to evaluate the relationship between mortality and different types of seizures. A crude odds ratio (OR) and adjusted odds ratio (aOR), derived from a multivariable model adjusted for measured confounders, were respectively used to confirm this relationship.
Two researchers (HY-L and QQ-W) independently screened and evaluated the article titles and abstracts for inclusion. A third researcher (JY-H) was employed in the case of disagreements. Articles that were not relevant to the mortality of seizure onset after patients with ICH, or that merely reported the mortality of seizure onset after other stroke subtypes, such as subarachnoid hemorrhage, cerebral venous sinus thrombosis, and cerebral infarction, were excluded during the screening process. Articles that reported the relationship between mortality and seizures after a whole stroke were excluded, and articles where relevant data of ICH subtypes could not be extracted were also excluded. We also excluded duplicate records, case reports, reviews, and unavailable full text.
Diagnosis of seizures was defined as convulsive seizures or non-convulsive seizures by using an electroencephalogram (EEG) or not. According to definitions of the International League Against Epilepsy (11), seizures are generally divided into ESs and LSs, in which the former occurs within 7 days and the latter occurs after 7 days (12). ESs and LSs, as well as SE and ASs that were not able to be classified, were all included in this study. Considering that previous studies used a non-standard cut-off point for ES and LS and were not able to acquire initial data, we used an established cut-off point of each study for analysis.
Data extracted from the articles included study design, research time, sample size, the definition of seizures, effect size of the association between post-ICH seizures and mortality, and confounding factors were included in the multivariate analysis.
Two researchers (XH-P and CX-H) independently utilized the Newcastle-Ottawa Scale (NOS) to assess each study. This scale awards a maximum of nine stars including four stars for selection of participants and measurement of exposure, two stars for comparability, and three stars for assessment of outcomes and adequacy of follow-up (13). Scores of 0 to 3, 4 to 6, and 7 to 9 were defined as low, moderate, and high-quality studies, respectively.
We acquired the crude ORs and aORs and their 95% confidence interval (CI) from the included studies. If the crude OR and 95% CI were not mentioned in the article, we calculated their quantitative value by the number of deaths and survivals in each of the included studies. These calculated crude ORs and aORs, and their associated 95% Cis, were then used in the meta-analysis.
The heterogeneity between the studies was quantitatively determined by the I2 value, which was divided into three levels: low (I2 = 25–49%), moderate (I2 = 50–74%), and high (I2 ≥ 25–49%). We performed subgroup analysis using categorical data to identify potential sources of heterogeneity according to the study sample size, including no previous seizure/epileptic episodes, previous seizure/epileptic episodes, and seizure type. Furthermore, we performed sensitivity analyses to explore potential sources of heterogeneity and resulting robustness by omitting one study at a time.
Publication bias was analyzed and is represented as a funnel plot. Funnel plot symmetry was assessed with Egger's test. A random-effects model, using the DerSimonian and Laird method for variance estimation, was performed in the meta-analysis (14). Statistical significance was set at a P-value > 0.05 (two-tailed). Stata software version 12.0 (Stata corp LP, College Station, TX, USA) was used to perform the data analysis in this meta-analysis.
There were 9,298 studies (5,377 in Embase, 3,921 in PubMed) in the primary searches for initial review; 7,695 remained after 1,603 duplicates were removed and 13 studies (15–27) were eventually included (Figure 1), in which three were retrospective and 10 prospective. A total of 245,908 patients was included and the sample size of each study differed widely with a range between 228 and 245,908. Study populations were single hospital-based, multi hospital-based, and population-based, and were from Asia, America, and Europe. Definitions of seizures included ESs, LSs, SE, and in-hospital seizures that could not be classified. ESs were defined as seizures occurring within 7, 14, or 30 days after ICH onset.
Three included studies (17, 24, 26) provided crude ORs and 95% CIs directly, two others (16, 25) provided no related data on crude ORs, and the remaining eight studies (15, 18–23, 27) provided the numbers of deaths and survivals of seizure and non-seizure groups, which were then used to calculate the crude ORs and 95% CIs as described in the methods. Among them, Claessens et al. (22) provided the number of deaths and survivals associated with ESs and LSs separately; we, therefore, analyzed the two as a whole seizure in the main outcome and analyzed each one respectively in the subgroup analysis. Only six included studies (16, 17, 21, 23–26) provided aORs and 95% CIs. Detailed information is presented in Table 1.
Two studies received nine stars after NOS quality assessment, seven received eight stars, three received seven stars, and one received six stars, which, taken together, indicated moderate to high-quality studies (Table 2).
Post-ICH seizures were not associated with significantly increased mortality in patients with ICH (crude OR 1.35, 95% CI 0.91–2; Figure 2). The p-value for the statistic was <0.001, which suggested that the true effect sizes did not vary among the studies included in the meta-analysis. The I2 statistic was 83.9%, which demonstrated a high heterogeneity, suggesting that 83.9% of the variance in the observed effects was due to differences among the true effect sizes and not sampling errors. Publication bias analysis did not highlight any differences between observed and estimated values (Figure 3). Egger's test was not statistically significant (p = 0.95).
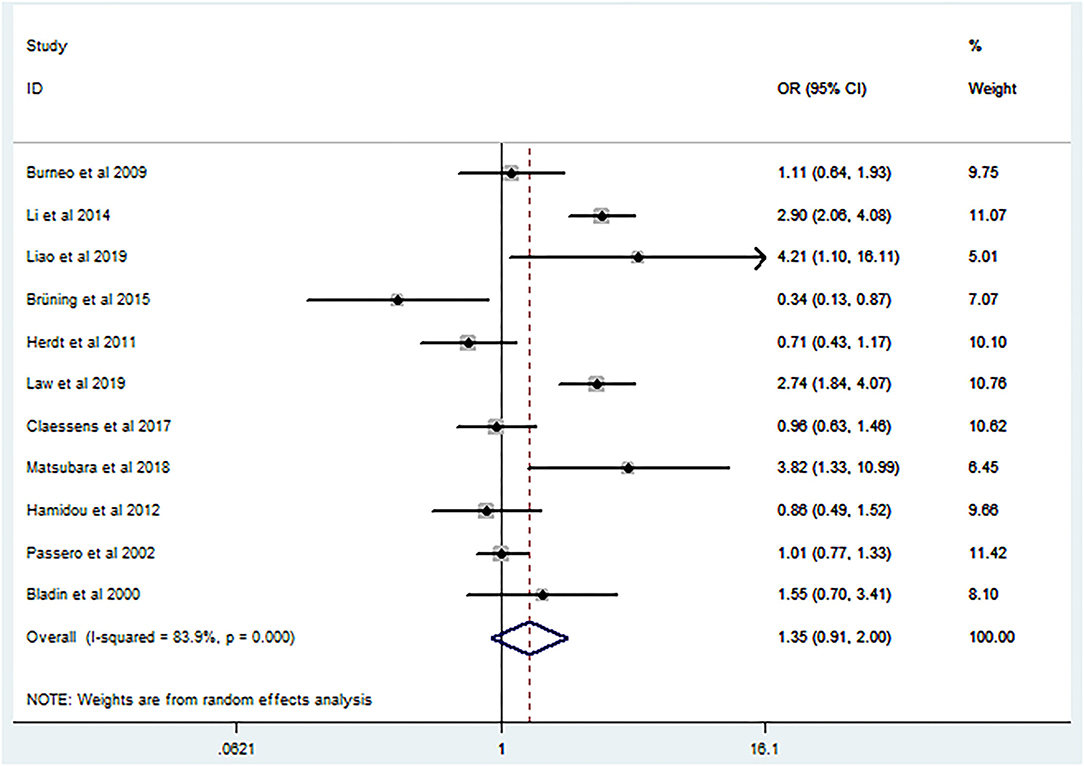
Figure 2. Forest plot of crude odds ratio on the relationship between mortality and seizures after intracerebral hemorrhage.

Figure 3. Funnel plot for publication bias of the relationship between mortality and seizures after intracerebral hemorrhage.
In agreement with the above results, aOR (aOR 1.22, 95% CI 0.78–1.88) also showed a high heterogeneity (I2 = 91%, p = 0.00; Figure 4). Because only six studies were included in the aOR meta-analysis, funnel plot asymmetry was not conducted given the limited specificity and power of these tests when fewer than ten studies are included.
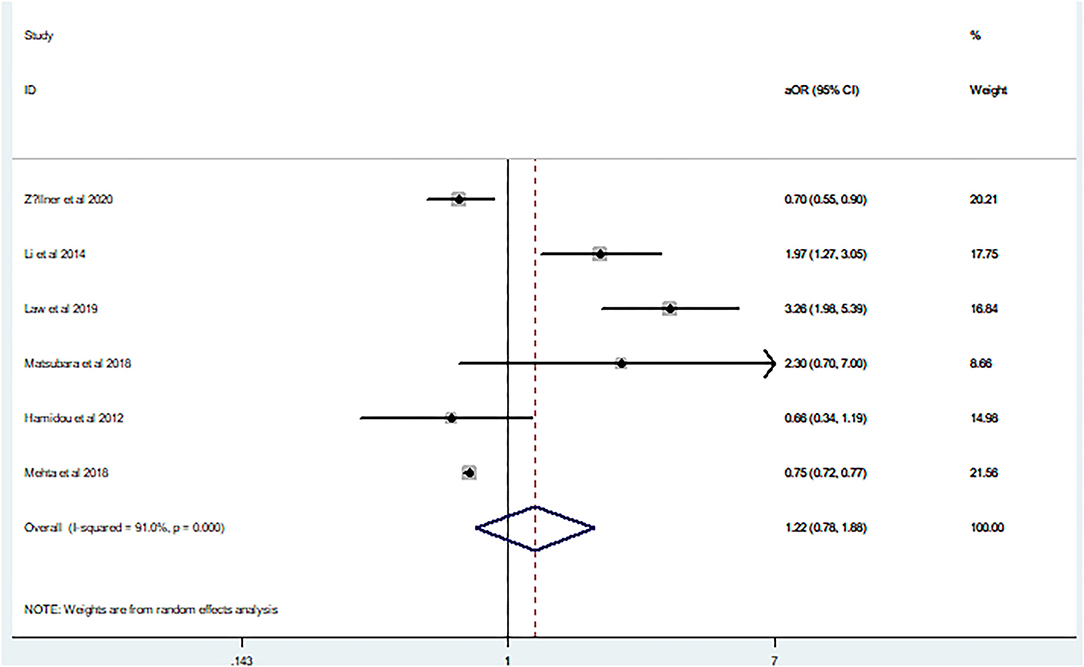
Figure 4. Forest plot of the adjusted odds ratio on the relationship between mortality and seizures after intracerebral hemorrhage.
We performed subgroup analysis on the study sample size, including no previous seizures/epileptic episodes, previous seizures/epileptic episodes, and seizure type. The results showed that the crude OR in the subgroup of the sample size was ≥ 1,000, with previous seizures/epileptic episodes and SE showing a positive relationship, whereas aOR in the subgroup of no previous seizures/epileptic episodes showed a positive relationship (Table 3).
When the study of Brüning et al. was omitted for meta-analysis, there was a slight positive relationship between post-ICH seizures and mortality (OR 1.49, 95% CI 1.01–2.20). When other studies were omitted in turn, this relationship became non-significant (Table 4).
In the sensitivity analysis of aOR, there was no relationship between post-ICH seizures and mortality when each study was omitted (Table 5). Hence, our results were relatively robust in the sensitivity analysis.
This meta-analysis explored the relationship between seizures and mortality in patients with ICH, the results of which indicated that no relationship was evident. That is, post-ICH seizures were not associated with significantly increased mortality in patients with ICH.
However, there were interactions between seizures and ICH. ESs could be caused by mechanical effects of the expanding hemorrhage, the disruption of cortical networks by hematoma via its structural damaging properties, and/or irritation of the cortex due to products of blood metabolism (28). LSs are thought to be related to gliosis and chemical-cellular repair processes creating an epileptogenic focus (7, 8, 29). Seizures could in turn increase the severity and mortality since they occur after ICH. Early epileptiform activity has a negative impact on perihematomal areas, possibly by increasing the cerebral blood flow and glucose metabolic demand in hypoxic tissue and may also be related to the molecular pathophysiology associated with the activation of cytotoxic, oxidative, and inflammatory pathways that result in surrounding cells death (30–32). Additionally, symptomatic seizures due to stroke or other diseases resulted in increased early mortality rates (33).
To our surprise, only four included studies corresponded with the above theory (17, 18, 21, 23). among which Matsubara et al. (23) supported a positive result only in the crude analysis, but not in the adjusted analysis. Six included studies (15, 20, 22, 24, 26, 27), found no relationship between seizures and ICH, while the remaining three (16, 19, 25) found that post-ICH seizures were associated with reduced odds of mortality. The cause of the reduced odds of mortality reduction was related to the fact that seizure patients had less severe neurologic injuries, and more severely injured patients may be more likely to suffer non-convulsive seizures that would be underdiagnosed without EEG, and seizures patients tended to receive intensive care once seizures occurred (16, 19, 25).
Our results inferred those seizures were not associated with significantly increased mortality in patients with ICH, which was consistent with the analysis of crude OR (1.35, 95% CI 0.91–2) and aOR (1.22, 95% CI 0.78–1.88). This result was relatively robust in the sensitivity analysis but could be influenced by some potential risk factors. Furthermore, the sample sizes of the included studies may have influenced the robustness of the results. Overall, as the findings indicated that the occurrence of seizures in patients with preexisting epilepsy tended to be higher than in those without preexisting epilepsy (19), mortality rates may be different between patients with preexisting epilepsy and those without. In addition, the mechanism of different types of post-ICH seizures was not dissimilar and their impact on ICH may have led to discrepancies in the data. Because of these three potential sources of heterogeneity, we conducted a subgroup analysis. We found that a positive relationship tended to appear as the sample sizes increased. Inclusion of no previous seizures/epileptic episodes and previous seizures/epileptic episodes may have led to inconsistent results and which are considered potential confounding factors. Type of seizure had no effect on increased mortality in patients with ICH, except SE. However, only one study provided data on SE.
There is controversy surrounding whether patients should receive antiepileptic drugs (AEDs) as primary pharmacological prevention of seizures after spontaneous intracerebral hemorrhage (34). The use of AEDs depends on a balance of the effect of seizures on prognosis of patients with ICH, the effect of AEDs on reducing incidence of post-ICH seizures and mortality of patients with ICH after the occurrence of seizures, and the toxicity and side effects of AEDs. A recent meta-analysis inferred that the use of AEDs as primary prevention among adult patients with spontaneous intracerebral hemorrhage was not associated with improved neurological function during long-term follow-up (34). Moreover, according to European Stroke Organization guidelines for the management of post-stroke seizures and epilepsy, little evidence exists for the recommendation of primary seizure prophylaxis for ICH (35). Hence, our results that no association was found between post-ICH seizures and mortality support these recommendations.
Our study had several limitations. First, included studies were all observational studies and many potential confounding factors likely remained. Second, the cut-off points of ES and LS in included studies were inconsistent, most of which was defined as 7 days; however, other was extended to 14 days or even 30 days from stroke onset. The incidence rate of ES and LS could be influenced by the inconsistent cut-off point and could eventually influence mortality. Third, most included studies did not have a standard method of seizure monitoring and relied on clinical detection of seizures, which has resulted in under-representation of non-convulsive seizures that may have impacted clinical outcomes. Fourth, follow-up times of the included studies varied as the mortality rates may increase with increasing time. Fifth, most studies did not provide data on AEDs usage, which may affect the occurrence of post-ICH seizures and further weaken its effect on the outcome of ICH. Finally, our results were not consistent in all subgroup analyses, which indicated that the results could be influenced by many factors.
Conversely, our study had several strengths. It is the first meta-analysis published to date on the relationship of mortality and seizures after ICH. Furthermore, we explored this relationship by using crude ORs and aORs, respectively, and the results were found to be consistent. Finally, we conducted subgroup and sensitivity analyses and the results were found to be relatively robust relatively in the sensitivity analysis.
In conclusion, the available evidence revealed that post-ICH seizures were not associated with a significant increased mortality in patients with ICH. However, the results of the current study may be influenced by confounding factors and, therefore, more high-quality research is needed.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
H-yL and Q-qW: conceptualization, literature search, and original draft. J-yH and WG: methodology. X-hP and C-xH: data collection. TL and N-cL: data analysis. S-lS: review, editing, and supervision. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.922677/full#supplementary-material
ICH: intracerebral hemorrhage; ES: early seizures; LS: late seizures; SE: status epilepticus; AS: any seizures; OR: odds ratio; aOR: adjusted odds ratio; EEG: electroencephalogram; NOS: Newcastle-Ottawa Scale; CI: confidence interval; AEDs: antiepileptic drugs; GCS: Glasgow Coma Scale; NCSE: nonconvulsive status epilepticus; ICD-9-CM: International Classification of Diseases-Ninth Revision-Clinical Modification; CT: computed tomography; NR: not reported.
1. Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. (2009) 8:355–69. doi: 10.1016/S1474-4422(09)70025-0
2. Van Asch CJ, Luitse MJ, Rinkel GJ, van der Tweel I, Algra A, Klijn CJ. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. (2010) 9:167–76. doi: 10.1016/S1474-4422(09)70340-0
3. Balami JS, Buchan AM. Complications of intracerebral haemorrhage. Lancet Neurol. (2012) 11:101–18. doi: 10.1016/S1474-4422(11)70264-2
4. Kilpatrick CJ, Davis SM, Tress BM, Rossiter SC, Hopper JL, Vandendriesen ML. Epileptic seizures in acute stroke. Arch Neurol. (1990) 47:157–60. doi: 10.1001/archneur.1990.00530020053014
5. Szaflarski JP, Rackley AY, Kleindorfer DO, Khoury J, Woo D, Miller R, et al. Incidence of seizures in the acute phase of stroke: a population-based study. Epilepsia. (2008) 49:974–81. doi: 10.1111/j.1528-1167.2007.01513.x
6. Lekoubou A, Bishu KG, Ovbiagele B. Mortality and trends in stroke patients with seizures: A contemporary nationwide analysis. Epilepsy Res. (2019) 156:106166. doi: 10.1016/j.eplepsyres.2019.106166
7. Doria JW, Forgacs PB. Incidence, Implications, and management of seizures following ischemic and hemorrhagic stroke. Curr Neurol Neurosci Rep. (2019) 19:37. doi: 10.1007/s11910-019-0957-4
8. Derex L, Rheims S, Derex LP. Seizures and epilepsy after intracerebral hemorrhage: an update. J Neurol. (2021) 268:2605–15. doi: 10.1007/s00415-021-10439-3
9. Merlino G, Gigli GL, Bax F, Serafini A, Corazza E, Valente M. Seizures do not affect disability and mortality outcomes of stroke: a population-based study. J Clin Med. (2019) 8:2006. doi: 10.3390/jcm8112006
10. Moher D, Shamseer L, Ghersi D, Liberati A, Petticrew M, Shekelle P, et al. PRISMA for systematic review protocols (PRISMA-P). Syst Rev. (2015) 4:1–9. doi: 10.1186/2046-4053-4-1
11. Guidelines for epidemiologic studies on epilepsy Commission Commission on epidemiology and prognosis. International league against epilepsy. Epilepsia. (1993) 34:592–596. doi: 10.1111/j.1528-1157.1993.tb00433.x
12. Fisher RS, Cross JH, French JA, Higurashi N, Hirsch E, Jansen FE, et al. Operational classification of seizure types by the international league against epilepsy: position paper of the ILAE commission for classification and terminology. Epilepsia. (2018) 58:522–530. doi: 10.1111/epi.13670
13. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. The Ottawa Hospital Research Institute. (2000). Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed August 2, 2019).
14. Kontopantelis E, Reeves D. Performance of statistical methods for meta analysis when true study effects are non-normally distributed: A comparison between DerSimonian-Laird and restricted maximum likelihood. Stat Methods Med Res. (2012) 21:657–9. doi: 10.1177/0962280211413451
15. Burneo JG, Fang J, Saposnik G. Impact of seizures on morbidity and mortality after stroke: A Canadian multi-centre cohort study. Eur J Neurol. (2010) 17:52–8. doi: 10.1111/j.1468-1331.2009.02739.x
16. Zöllner JP, Konczalla J, Stein M, Roth C, Krakow K, Kaps M, et al. Acute symptomatic seizures in intracerebral and subarachnoid hemorrhage: A population study of 19,331 patients. Epilepsy Res. (2020) 161:106286. doi: 10.1016/j.eplepsyres.2020.106286
17. Li Z, Zhao X, Wang Y, Wang C, Liu L, Shao X, et al. Association between seizures and outcomes among intracerebral hemorrhage patients: the China national stroke registry. J Stroke Cerebrovasc Dis. (2015) 24:455–64. doi: 10.1016/j.jstrokecerebrovasdis.2014.09.021
18. Liao HC, Chen SH, Yang CD, Chen YW. Clinical profile and outcomes of early seizures in Asian patients with acute intracerebral hemorrhage. J Acute Med. (2019) 9:172–7.
19. Brüning T, Awwad S, Al-Khaled M. Do early seizures indicate survival of patients with nontraumatic intracerebral hemorrhage? Cerebrovasc Dis. (2016) 41:68–73. doi: 10.1159/000442297
20. De Herdt V, Dumont F, Henon H, Derambure P, Vonck K, Leys D, et al. Early seizures in intracerebral hemorrhage: Incidence, associated factors, and outcome. Neurology. (2011) 77:1794–800. doi: 10.1212/WNL.0b013e31823648a6
21. Law ZK, England TJ, Mistri AK, Woodhouse LJ, Cala L, Dineen R, et al. Incidence and predictors of early seizures in intracerebral haemorrhage and the effect of tranexamic acid. Eur Stroke J. (2020) 5:123–9. doi: 10.1177/2396987320901391
22. Claessens D, Bekelaar K, Schreuder FH, de Greef BT, Vlooswijk MC, Staals J, et al. Mortality after primary intracerebral hemorrhage in relation to post-stroke seizures. J Neurol. (2017) 264:1885–91. doi: 10.1007/s00415-017-8573-1
23. Matsubara S, Sato S, Kodama T, Egawa S, Nakamoto H, Toyoda K, et al. Nonconvulsive status epilepticus in acute intracerebral hemorrhage. Stroke. (2018) 49:1759–61. doi: 10.1161/STROKEAHA.118.021414
24. Hamidou B, Aboa-Eboulé C, Durier J, Jacquin A, Lemesle-Martin M, Giroud M, et al. Prognostic value of early epileptic seizures on mortality and functional disability in acute stroke: the Dijon Stroke Registry (1985-2010). J Neurol. (2013) 260:1043–51. doi: 10.1007/s00415-012-6756-3
25. Mehta A, Zusman BE, Choxi R, Shutter LA, Yassin A, Antony A, et al. Seizures after intracerebral hemorrhage: Incidence, risk factors, and impact on mortality and morbidity. World Neurosurg. (2018) 112:e385–92. doi: 10.1016/j.wneu.2018.01.052
26. Passero S, Rocchi R, Rossi S, Ulivelli M, Vatti G. Seizures after spontaneous supratentorial intracerebral hemorrhage. Epilepsia. (2002) 43:1175–80. doi: 10.1046/j.1528-1157.2002.00302.x
27. Bladin CF, Alexandrov AV, Bellavance A, Bornstein N, Chambers B, Coté R, et al. Seizures after stroke: A prospective multicenter study. Arch Neurol. (2000) 57:1617–22. doi: 10.1001/archneur.57.11.1617
28. Serafini A, Gigli GL, Gregoraci G, Janes F, Cancelli I, Novello S, et al. Are early seizures predictive of epilepsy after a stroke? Results of a population-based study. Neuroepidemiology. (2015) 45:50–8. doi: 10.1159/000382078
29. Klein P, Dingledine R, Aronica E, Bernard C, Blümcke I, Boison D, et al. Commonalities in epileptogenic processes from different acute brain insults: do they translate? Epilepsia. (2018) 59:37–66. doi: 10.1111/epi.13965
30. La Fougère C, Rominger A, Förster S, Geisler J, Bartenstein P. PET and SPECT in epilepsy: a critical review. Epilepsy Behav. (2009) 15:50–5. doi: 10.1016/j.yebeh.2009.02.025
31. Goffin K, Dedeurwaerdere S, Van Laere K, Van Paesschen W. Neuronuclear assessment of patients with epilepsy. Semin Nucl Med. (2008) 38:227–39. doi: 10.1053/j.semnuclmed.2008.02.004
32. Newton MR, Berkovic SF, Austin MC, Rowe CC, McKay WJ, Bladin PF. Postictal switch in blood flow distribution and temporal lobe seizures. J Neurol Neurosurg Psychiatry. (1992) 55:891–4. doi: 10.1136/jnnp.55.10.891
33. Hesdorffer DC, Benn EK, Cascino GD, Hauser WA. Is a first acute symptomatic seizure epilepsy? Mortality and risk for recurrent seizure. Epilepsia. (2009) 50:1102–8. doi: 10.1111/j.1528-1167.2008.01945.x
34. Angriman F, Tirupakuzhi Vijayaraghavan BK, Dragoi L, Lopez Soto C, Chapman M, Scales DC. Antiepileptic drugs to prevent seizures after spontaneous intracerebral hemorrhage. Stroke. (2019) 50:1095–9. doi: 10.1161/STROKEAHA.118.024380
Keywords: intracerebral hemorrhage, outcome, mortality, seizures, systematic review, meta-analysis
Citation: Lin H-y, Wei Q-q, Huang J-y, Pan X-h, Liang N-c, Huang C-x, Long T, Gao W and Shi S-l (2022) Relationship Between Mortality and Seizures After Intracerebral Hemorrhage: A Systematic Review and Meta-Analysis. Front. Neurol. 13:922677. doi: 10.3389/fneur.2022.922677
Received: 18 April 2022; Accepted: 16 May 2022;
Published: 20 June 2022.
Edited by:
Gian Luigi Gigli, University of Udine, ItalyReviewed by:
Giovanni Merlino, Udine University Hospital, ItalyCopyright © 2022 Lin, Wei, Huang, Pan, Liang, Huang, Long, Gao and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cai-xia Huang, aHVhbmdjYWl4aWEwODExQG91dGxvb2suY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.