
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 10 June 2022
Sec. Pediatric Neurology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.920214
This article is part of the Research TopicInsights in Pediatric Neurology: 2021View all 20 articles
 Andrea Santangelo1
Andrea Santangelo1 Emanuele Bartolini2
Emanuele Bartolini2 Giulia Nuzzi1
Giulia Nuzzi1 Thomas Foiadelli3
Thomas Foiadelli3 Alexandre Michev3
Alexandre Michev3 Tommaso Mina4
Tommaso Mina4 Irene Trambusti1
Irene Trambusti1 Valeria Fichera1
Valeria Fichera1 Alice Bonuccelli1
Alice Bonuccelli1 Gabriele Massimetti5
Gabriele Massimetti5 Diego G. Peroni1,5
Diego G. Peroni1,5 Emanuela De Marco6
Emanuela De Marco6 Luca Coccoli6
Luca Coccoli6 Laura Luti6
Laura Luti6 Sayla Bernasconi6
Sayla Bernasconi6 Margherita Nardi6
Margherita Nardi6 Maria Cristina Menconi6
Maria Cristina Menconi6 Gabriella Casazza6
Gabriella Casazza6 Dario Pruna7
Dario Pruna7 Rosamaria Mura8
Rosamaria Mura8 Chiara Marra9
Chiara Marra9 Daniele Zama10
Daniele Zama10 Pasquale Striano10,11
Pasquale Striano10,11 Duccio M. Cordelli12
Duccio M. Cordelli12 Roberta Battini2,5*
Roberta Battini2,5* Alessandro Orsini1
Alessandro Orsini1Introduction: Stroke-like syndrome (SLS) is a rare subacute neurological complication of intrathecal or high-dose (≥500 mg) Methotrexate (MTX) administration. Its clinical features, evoking acute cerebral ischaemia with fluctuating course symptoms and a possible spontaneous resolution, have elicited interest among the scientific community. However, many issues are still open on the underlying pathogenesis, clinical, and therapeutic management and long-term outcome.
Materials and Methods: We retrospectively analyzed clinical, radiological and laboratory records of all patients diagnosed with SLS between 2011 and 2021 at 4 National referral centers for Pediatric Onco-Hematology. Patients with a latency period that was longer than 3 weeks between the last MTX administration of MTX and SLS onset were excluded from the analysis, as were those with unclear etiologies. We assessed symptom severity using a dedicated arbitrary scoring system. Eleven patients were included in the study.
Results: The underlying disease was acute lymphoblastic leukemia type B in 10/11 patients, while fibroblastic osteosarcoma was present in a single subject. The median age at diagnosis was 11 years (range 4–34), and 64% of the patients were women. Symptoms occurred after a mean of 9.45 days (± 0.75) since the last MTX administration and lasted between 1 and 96 h. Clinical features included hemiplegia and/or cranial nerves palsy, paraesthesia, movement or speech disorders, and seizure. All patients underwent neuroimaging studies (CT and/or MRI) and EEG. The scoring system revealed an average of 4.9 points (± 2.3), with a median of 5 points (maximum 20 points). We detected a linear correlation between the severity of the disease and age in male patients.
Conclusions: SLS is a rare, well-characterized complication of MTX administration. Despite the small sample, we have been able to confirm some of the previous findings in literature. We also identified a linear correlation between age and severity of the disease, which could improve the future clinical management.
Methotrexate (MTX) is an antimetabolite agent acting as a competitive inhibitor of the enzyme dihydrofolate reductase (DHFR), hence blocking the synthesis of folate and tetrahydrofolate and inhibiting DNA synthesis during the S-phase of the cell cycle. Folate antagonists were among the first developed antineoplastic agents, and methotrexate is still a mainstay of treatment for leukemia, lymphomas, gastric, breast, and bladder cancer. Neurological complications of anticancer therapy may either result from direct neurotoxicity or from indirect drug-induced metabolic derangements, cerebrovascular disorders or, in the case of checkpoint inhibitors, autoimmune disorders. MTX yields poor drug penetration across the blood–brain barrier due to its ionization and hydrophobicity (1); neurological toxicity mostly results from intrathecal (IT) administration or from high-dose intravenous (IV) treatments leading to intrathecal inflow.MTX neurotoxicity syndrome may cause acute, subacute, or chronic symptoms (2, 3). The overall incidence of MTX neurotoxicity ranges from 3 to 10% and varies according to dose, route, and frequency of administration (4, 5). Factors are high-dose therapy, intrathecal route, young age, and cranial irradiation (6).
While delayed/chronic neurotoxicity may take from months to years to manifest as leukoencephalopathy, acute to subacute neurotoxicity usually occurs within hours to weeks after MTX administration (7). In particular, the weekly or biweekly administration of high-dose MTX (HD-MTX), a prolonged low-dose oral treatment, and IT administration may produce a subacute MTX neurotoxicity called stroke-like syndrome (SLS), possibly characterized hemiplegia, hemisensory deficits, aphasia, dysarthria, dysphagia, diplopia, and occasionally seizures (2). Symptoms develop approximately 2–14 days after drug administration, they last from 15 min to 72 h, and then resolve spontaneously without sequelae. Watanabe et al. observed that the neurological events did not occur immediately after the first IT-MTX administration but started about 1–2 weeks after IT-MTX administration and often fluctuated until they resolved completely (8). Neuroimaging studies are usually normal, although changes have been described on MRI, such as areas of restricted diffusion on diffusion-weighted imaging and non-enhancing T2 hyper-intense lesions in the white matter. Additional investigations such as CSF analysis, and haematologic exams (e.g., thrombophilic profile) are usually normal, whereas electroencephalography (EEG) might show diffuse slowing of the background activity (9, 10). Treatment may consist of observation and supportive care alone. Dextromethorphan, dexamethasone, aminophylline and folic acid have also been successfully employed in MTX neurotoxicity (11–15), such as SLS. However, as most of these cases resolve spontaneously, the value of these medications is not clear. MTX has been eliminated from the therapeutic regimen in most of the cases of subacute neurotoxicity, but cases have also been reported in which further doses of MTX were given without any complication (15).
The incidence of subacute neurotoxicity is still unclear; in 369 children with diagnosed acute lymphoblastic leukemia (B-ALL) treated with both IV-MTX and/or IT-MTX, subacute encephalopathy occurred in 14 patients (3.8%) (16). In other studies, the incidence was higher, ranging from 0.8 to 3.8% (16–18), whereas in a recent paper the incidence of SLS was much lower (0.2%) (19).
We retrospectively recruited 11 patients aged 4–34 years (median 11.02) from the Pediatric Onco-Hematology Centers of Pisa, Bologna, Pavia, and Cagliari who had been diagnosed with leukemia or osteosarcoma and had presented a stroke-like event, defined as the acute or subacute presentation of 1 or more of the following symptoms after MTX administration: hemiplegia, altered consciousness, seizures, hemianopsia, cranial nerve palsy, unilateral sensory disorders or speech disorders. We attributed the neurological event to MTX-induced stroke-like neurotoxicity if the first neurological symptom occurred within 3 weeks after IT or HD MTX administration once other possible causes had been excluded. Patients with neurological symptoms or patients who had presented with evident signs of symptoms on their first visit were excluded, as were those who had clear extracranial problems (i.e. septic shock). Each patient underwent brain imaging using MRI and/or computed tomography (CT). Electronic medical records were reviewed, and data regarding the mode of MTX administration, the temporal relationship to MTX administration, the type, duration and severity of stroke-like symptoms, and the neurological outcome were recorded.
To assess the severity of the disease, we developed an arbitrary ranking scale based on the clinical judgment assigning a score to a total of 13 observed symptoms (Table 1). Each symptom received a score of 0 (no signs/symptoms); 1 (mild symptoms, with a low impact on daily life) or 2 (major symptom, or potentially life-threatening), with a possible maximum sum score of 20.
For the statistical analysis of quantitative variables, we evaluated mean, median, and standard deviations, which were compared through t-tests. To study the relationship between these variables, the Pearson correlation coefficient was calculated. We described the most relevant correlations with a scatter plot and regression line, calculating the regression coefficient, and the coefficient of determination. The Fisher exact test was employed for the analysis of categorical variables which were expressed as percentages,. Finally, we described the overall survival rates through Kaplan –Meier curves (Figure 1). We used IBM SPSS, ver. 26. for the statistical analysis.
Total eleven patients with MTX-induced stroke-like neurotoxicity were included in the study. The main characteristics of our cohort are reported in Table 2. Of 11 patients, 64% were women. Of these, ten subjects had acute lymphoblastic leukemia type B (B-ALL) and 1 had fibroblastic osteosarcoma of the right femur. The median age at the time of diagnosis was 11 years (range, 4 – 34 years). None of the patients with SLS showed evidence of CNS leukemia or CNS metastasis at the original presentation. As regards the treatment of patients with B-ALL, 5 (55%) were treated according to the AIEOP-BFM 2009 protocol, and the others (45%) according to the AIEOP-BFM 2017 protocol. The patient with osteosarcoma was treated according to the ISG/OS-2 PGOP NEG protocol. About 6 patients (55%) received IT administration of 12 mg of MTX, 3 (27%) also underwent HD intravenous therapy with 5 g/m (2), only 1 patient received intravenous therapy exclusively, while 1 was treated with oral and IT MTX.
The mean interval between the most recent MTX exposure and SLS was 9.45 days (± 0.75), with a median of 9 days (range 2–13). Figure 1 shows the survival rate of the analyzed population.
SLS episodes occurred in 1 patient during the induction phase, in 4 during re-induction and in 3 patients during the maintenance phase.
Symptoms lasted 24 h in 5 patients, 72 h in 3 patients, and 96 h in 3 patients, as seen in Table 3. The clinical presentation typically included mild paresis and paraesthesias, disturbances of speech and eventually motor impairment
In detail, 7 patients (63.6%) showed limb hyposthenia, 3 (27.3%) had hemiplegia, and 3 (27.3%) developed paraesthesias. In 4 patients (36.4%) tongue deviation and/or protrusion of the buccal opening was observed. About 8 patients (72.7%) had speech disorders, mainly aphasia and/or dysarthria, whereas an altered mental status occurred in 2 cases (18.2%).
Other observed symptoms were deafness, choreic movements, tremors, drooling, or facial nerve palsy. Of these 1 patient experienced seizures and increased blood pressure.
The analysis of these symptoms revealed a different gender distribution: limb hyposthenia occurred in 5 (71.4%) women and in 2 (50%) men; no men presented paraesthesia while buccal opening and/or tongue deviation manifested in 28% of females and 50% of males. Impaired consciousness was not observed in men, whereas it occurred in 28% of women (Figure 2).
Our analysis displayed a severity score ranging from 1 to 10, with a mean value of 4.9 (± 2.3), and a median of 5.
We did not observe any correlation between the severity of the clinical picture and the age of the female patients, or with the administration modality. On the other hand, a significant correlation was detected in the male subgroup, in which age showed a linear correlation with the severity of the clinical picture (r: 0.98; p-value: 0.017), as shown in Figure 3. A trend toward a linear correlation was also observed between severity and the time elapsed since the last administration of MTX (Figure 4), although no statistical significance has been detected (r: 0.56; p-value: 0.71).
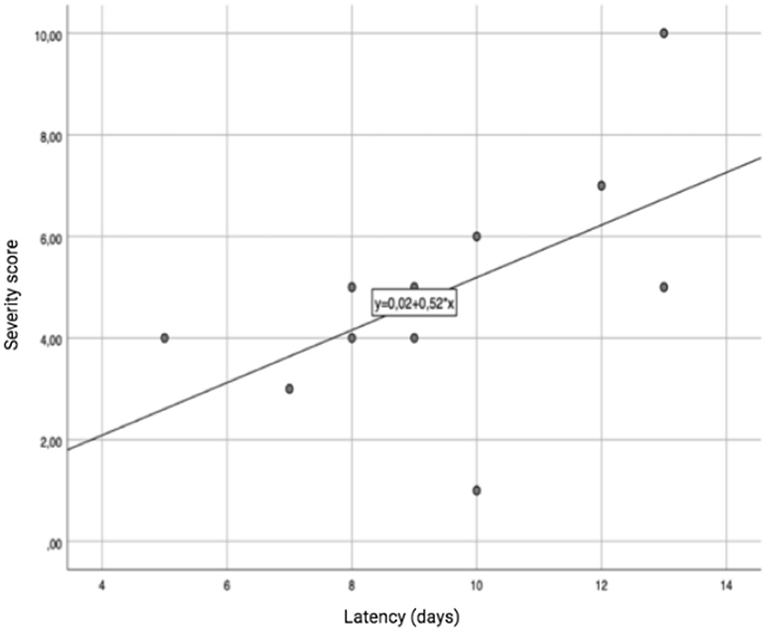
Figure 4. Correlation between the latency time since the last administration of MTX and the severity of the clinical picture.
Total 3 (27%) MRIs were reported as normal, while 8/11 (73%) showed focal or diffuse hyperintensity of the periventricular or subcortical white matter on T2-weighted images; these lesions showed restricted diffusion on ADC map, suggesting cytotoxic oedema (Figure 5). As expected, CT was much less sensitive for white matter changes and was reported as normal in 9/11 cases; only 1/11 patients (Patient 4) presented with mild white matter hypodensity and slight dilation of the liquor spaces which was perhaps due to therapy outcomes. EEG was performed on all patients, and resulted abnormal for mild interhemispheric asymmetry in 2 subjects (Patients 7 and 9). A cerebro-spinal fluid analysis was not performed.
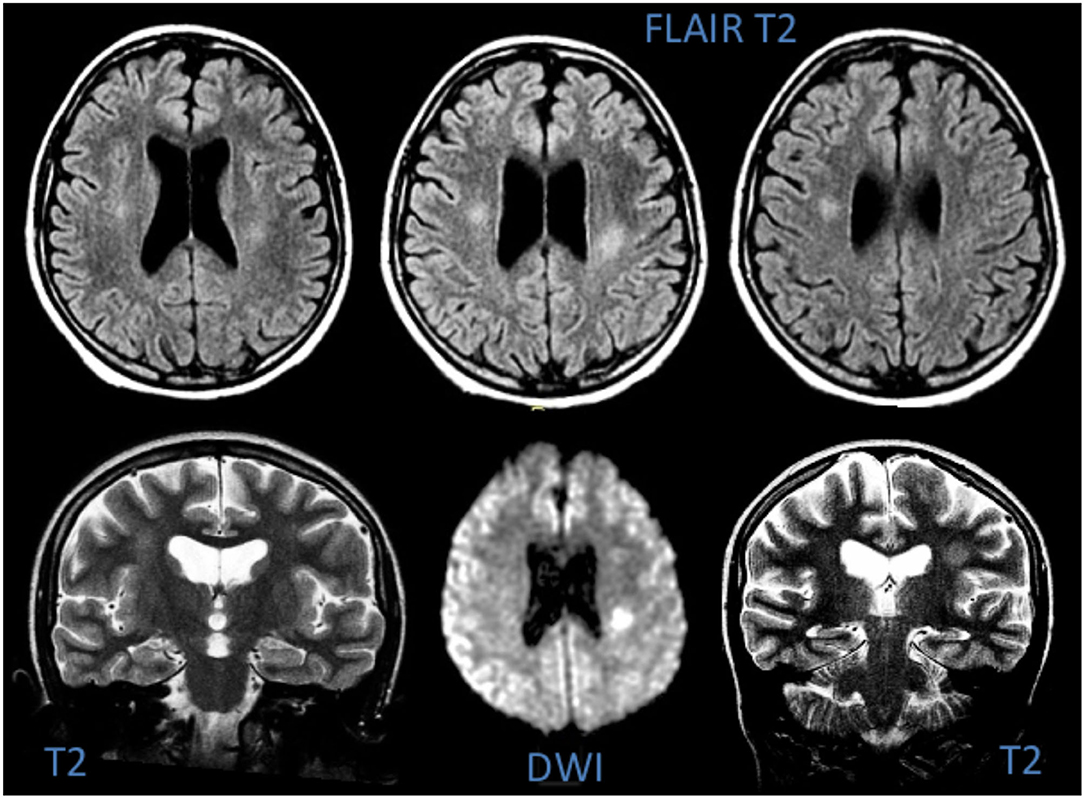
Figure 5. MRI of Patient 6, showing hyperintensity of the deep white matter on T2-weighted images and signs of cytotoxic oedema in DWI.
Treatment decisions were made in each case by the local oncology group in joint sessions. As shown in Table 3, 5/11 patients received no therapy, whereas 6/11 patients received pharmacological treatments: 4/11 antiseizure medications (midazolam, clonazepam, levetiracetam), 2/11 (Patients 2 and 6) anti-hypertensive agents (amlodipine, nebivolol), 2/11 (Patients 1 and 8) supplemental folinic acid, n = 1/11 (Patient 1), empiric anticoagulant therapy with low-molecular weight heparin, dexamethasone, and gabapentin.
In 5 patients IT-MTX was re-established following the initial SLS. Patients 5 and 6, who experienced clinical symptoms during Protocol M, did not receive the fourth dose of MTX. Patients 4 and 8 discontinued reinduction and phase IB with a subsequent therapy modification. Only 1 patient presented a MTX-induced SLS relapse upon re-exposure during the re-induction therapy (20%), with right paraesthesia, right hemiparesis and dysarthria. The brain MRI showed a T2-weighted hyperintense lesion in the left subcortical white matter. The patient was treated with midazolam with a subsequent remission of symptoms. During maintenance, after 12 doses of oral MTX, the patient re-presented with right hemiparaesthesia and re-flaring of the above mentioned hyperintense lesion. The oncologist decided to stop 6 MP and MTX and to recommence therapy by reducing the dose.
Methotrexate-induced stroke-like syndrome is a rare complication of intrathecal or high dose administration of MTX [≥500 mg/m (2)]. Its peculiar and worrisome clinical features, together with its predisposition to a spontaneous resolution, have elicited the interest of the scientific community since its first descriptions (1). A review of the literature showed 11 reports of neurological serious adverse events associated with MTX administration, determined to be consistent with MTX-induced stroke-like neurotoxicity, characterized by focal neurological dysfunction that could occur with disturbances in speech, vision, or altered mental status, sensorial or motor deficits.
Herein, we examined the main features of such toxicity in a pediatric population through the analysis of a multicentric cohort of 11 patients, so as to highlight the main characteristics of this manifestation and eventually achieve a better understanding of its pathogenesis.
Different hypotheses have been proposed on the mechanisms underlying SLS. Direct damage induced by MTX might result in astrocytosis, axonal loss, and demyelination (20, 21). Several MTX-related biochemical changes could also indirectly affect the central nervous system, such as higher levels of homocysteine and a lower production of methionine resulting from the inhibition of DHFR. Decreased levels of S-adenosylmethionine have been associated with demyelination (22), whereas homocysteine appears to have a direct toxic effect on vascular endothelium (23), elicit oxidative stress (24) and alter coagulation (25), although abnormalities in hemostasis have never been observed in patients with MTX-related neurotoxicity (8, 15).
Moreover, homocysteine-derived metabolites, namely sulfur-containing amino acids, are known to be excitatory agonists of the N-methyl-D-aspartate (NMDA) receptor (26), whose intensive stimulation could lead to seizures and excitotoxicity, eventually leading to neuronal damage and degeneration (27). Furthermore, MTX can decrease the levels of adenosine, biopterins, homovanillic acid, and 5-hydroxyndoleacetic acid (22, 28), which also seem to be involved in the development of neurological alterations (7).
According to the literature, the main risk factors for MTX-induced neurotoxicity include high-dose or intrathecal therapy, association with cranial radiation and age >10 years old (9). Some authors proposed a high MTX/leucovorin ratio as an additional SLS trigger (29).
Our findings support these parameters as risk factors; all of our patients were on IT or HD MTX, and the median age was 11 years (mean: 12 years ± 8.4). However, it is not infrequent to observe SLS in younger patients (8, 30), as we observed in Patient 9. Interestingly, we diagnosed SLS also in a 34-year old patient (Patient 2). The onset of SLS in this patient could be partially explained by the higher doses of MTX used in ISG/OS-2 PGOP NEG protocol and the tendency toward a reduced clearance of MTX in older patients (8). Consistently with this hypothesis, we detected a linear correlation between the age and the severity of the clinical picture in our male patients, which had not been described in previous works. However, no studies have linked SLS with pharmacokinetic parameters of methotrexate.
In our series, the onset of SLS could be observed in induction, re-induction and maintenance treatments. All these phases included the co-administration of Ara-C and Cyclophosphamide. These drugs may promote neurological complications in high-dose regimens, and different authors have hypothesized their role in facilitating MTX-induced neurotoxicity. In particular, we could presume that cyclophosphamide plays a contributing role, since it is not employed in other IT /HD MTX-based schedules which are not characterized by SLS (e.g. trials for acute myeloid leukemia).
Furthermore, SLS was not observed upon the first administration of IT or HD-MTX. This could lead us to presume that there is a possible sensitization or progressive accumulation of this drug as a predisposing factor for its neurotoxicity.
In all our cases, the onset of SLS occurred within 1 or 2 weeks after the last administration of MTX (median 9 days), all patients presented fluctuating symptoms which eventually resolved spontaneously and involved mostly 1 haemisoma. These results were in agreement with those in previous literature. Moreover, 2 patients presented transitory increased blood pressure, 1 diffuse tremor and another patient presented seizures.
The severity of the clinical picture was assessed through an arbitrary scale with a total score ideally ranging from 0 to 20. All patients presented a total score between 1 and 10. According to our observations, the main symptoms presented by our patients determine a significant impairment in their quality of life, such as speech disorders, limb hyposthenia, deviation of the oral opening and hemiplegia.
Interestingly, Patient 6 showed choreic movements. Choreoathetosis had been previously reported in SLS patients and could lead us to presume a possible involvement of basal ganglia.
We failed to identify a correlation between the severity of the clinical picture and the time which had elapsed since the last administration of MTX, although this could be due to the small group of patients.
All patients underwent a CT scan, which was normal in 9/11 cases. Brain MRI, performed between 1 and 3 days after the onset of symptoms, showed a hyperintensity in centrum semiovale with restricted diffusion in the same area on T2-weighted sequence. Such findings have been observed in other studies also (8, 31–33), and are similar to those seen in the early phases of cerebral infarction (8). This might suggest that the pathogenesis of SLS could be an ischemic lesion of deep white matter.
Interestingly, 3 of our patients showed a normal MRI. Such findings could be related to inter-subject variability, or to the waxing/waning phase of the disease in which the exam was performed.
MTX administration is associated with higher concentrations of different molecules, even in CSF, such as adenosine and homocysteine, which could lead to damage of the vascular endothelium. It has been therefore hypothesized that the deep white matter, which is less vascularised, may be more prone to developing ischaemic lesions (7).
Moreover, other authors have suggested that the peculiar fluctuating manifestations of SLS might be the sign of a progressive depolarization of neuronal and axonal membranes (1, 9), similarly to migraine-associated cortical spreading depression (CSD). In this case, a pivotal role could be played by the MTX-associated astrocytosis (20, 25). It has been observed that astrocytes may show intracellular calcium waves that spread over long distances (34) and can modulate neuronal and vascular activity.
As regards the treatment of SLS, different drugs could reverse the biochemical effects of MTX (7). Two of our patients received additional folic acid, which may have an effect on the above-mentioned ratio with MTX concentrations, preventing relapses or fluctuations and possibly improving recovery. In both cases, the treatment was followed by remission of the symptoms. Notably, rescue with leucovorin has been successfully employed by some authors in patients rechallenged with HD or intrathecal MTX to prevent relapses of SLS (16). Aminophylline, due to its action as a competitive antagonist of adenosine, has also been widely administered as secondary prophylaxis in patients with MTX-induced stroke-like syndrome (9, 14, 16, 30–32). Moreover, other authors (31, 35), have reported the use of dextromethorphan in some patients. The efficacy of this treatment could be related to its antagonizing action on the NMDA receptor, which might inhibit the excitatory effects of homocysteine metabolites. Total 2 patients required anti-hypertensive therapy. Of these, 1 patient, (Patient. 6), who also presented choreic movements, required the administration of midazolam, the latter being also effectively employed in our patients with tremor and seizures. To our knowledge, no specific treatment has been proposed for SLS besides supporting therapy. Given the predisposition to a spontaneous resolution of symptoms, a gold standard of management is still to be defined.
Although SLS is a well-known complication of MTX administration, its clinical features and correct treatment are still largely debated. The worrisome clinical picture, which occurs in other complications of chemotherapy, such as Posterior Reversible Encephalopathy Syndrome (36), represents a major concern for the clinician. Better comprehension of the syndrome is therefore needed. Through the analysis of 1 of the largest cohorts of patients in literature, we have been able to achieve a better understanding of the clinical features and severity of MTX-induced SLS. Our results, despite the limited sample, have confirmed some of the main assessments observed in literature thus far. Nonetheless, we have also detected a linear correlation between age and severity of the disease, which could lead to changes in the management of SLS.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
AS, AO, GN, IT, and VF contributed to conception and design of the study. AS, GN, IT, and VF organized the database. GM performed the statistical analysis. AS wrote the first draft of the manuscript. AS, GN, IT, EB, AO, and RB wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
This work has been partially supported by grant-RC 1.22 and the 5 x 1,000 voluntary contributions, Italian Ministry of Health (RB and EB).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We acknowledge Dr. Ailish Lynam for her precious and careful work in the reviewing process of this manuscript.
1. Newton HB. Neurological Complications of Chemotherapy to the Central Nervous System. Handbook of Clinical Neurology. Elsevier. (2012) p. 903–16.
2. Walker RW, Allen JC, Rosen G, Caparros B. Transient cerebral dysfunction secondary to high-dose methotrexate. J Clin Oncol. (1986) 4:1845–50. doi: 10.1200/JCO.1986.4.12.1845
3. Yim YS, Mahoney Jr DH, Oshman DG. Hemiparesis and ischemic changes of the white matter after intrathecal therapy for children with acute lymphocytic leukemia. Cancer. (1991) 67:2058–61. doi: 10.1002/1097-0142(19910415)67:8<2058::AID-CNCR2820670808>3.0.CO;2-G
4. Rubnitz JE, Relling MV, Harrison PL, Sandlund JT, Ribeiro RC, Rivera GK, et al. Transient encephalopathy following high-dose methotrexate treatment in childhood acute lymphoblastic leukemia. Leukemia. (1998) 12:1176–81. doi: 10.1038/sj.leu.2401098
5. Rollins N, Winick N, Bash R, Booth T. Acute methotrexate neurotoxicity: findings on diffusion-weighted imaging and correlation with clinical outcome. Am J Neuroradiol. (2004) 25:1688–95.
6. Fisher MJ, Khademian ZP, Simon EM, Zimmerman RA, Bilaniuk LT. Diffusion-weighted MR imaging of early methotrexate-related neurotoxicity in children. Am J Neuroradiol. (2005) 26:1686–9.
7. Vezmar S, Becker A, Bode U, Jaehde U. Biochemical and clinical aspects of methotrexate neurotoxicity. Chemotherapy. (2003) 49:92–104. doi: 10.1159/000069773
8. Watanabe K, Arakawa Y, Oguma E, et al. Characteristics of methotrexate-induced stroke-like neurotoxicity. Int J Hematol. (2018) 108:630–6. doi: 10.1007/s12185-018-2525-0
9. Inaba H, Khan R, Laningham F, Crews K, Pui C-H, Daw N. Clinical and radiological characteristics of methotrexate-induced acute encephalopathy in pediatric patients with cancer. Annals oncol. (2008) 19:178–84. doi: 10.1093/annonc/mdm466
10. Borgna-Pignatti C, Battisti L, Marradi P, Balter R, Caudana R. Transient neurologic disturbances in a child treated with moderate-dose methotrexate. Br J Haematol. (1992) 81:3. doi: 10.1111/j.1365-2141.1992.tb08256.x
11. Drachtman RA, Cole PD, Golden CB, et al. Dextromethorphan is effective in the treatment of subacute methotrexate neurotoxicity. Pediatr Hematol Oncol. (2002) 19:319–27. doi: 10.1080/08880010290057336
12. Jaksic W, Veljkovic D, Pozza C, Lewis I. Methotrexate-induced leukoencephalopathy reversed by aminophylline and high-dose folinic acid. Acta Haematol. (2004) 111:230–2. doi: 10.1159/000077573
13. Shuper A, Stark B, Kornreich L, Cohen IJ, Aviner S, Steinmetz A, et al. Methotrexate treatment protocols and the central nervous system: significant cure with significant neurotoxicity. J Child Neurol. (2000) 15:573–80. doi: 10.1177/088307380001500902
14. Bernini JC, Fort DW, Griener JC, Kane BJ, Chappell WB, Kamen BA. Aminophylline for methotrexate-induced neurotoxicity. Lancet. (1995) 345:544–7. doi: 10.1016/S0140-6736(95)90464-6
15. Packer RJ, Grossman RI, Belasco JB. High dose systemic methotrexate-associated acute neurologic dysfunction. Med Pediatr Oncol. (1983) 11:159–61. doi: 10.1002/mpo.2950110304
16. Bhojwani D, Sabin ND, Pei D, Yang JJ, Khan RB, Panetta JC, et al. Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J clin oncol. (2014) 32:949. doi: 10.1200/JCO.2013.53.0808
17. Baytan B, Evim MS, Güler S, Güneş AM, Okan M. Acute central nervous system complications in pediatric acute lymphoblastic leukemia. Pediatr Neurol. (2015) 53:312–8. doi: 10.1016/j.pediatrneurol.2015.03.006
18. Parasole R, Petruzziello F, Menna G, et al. Central nervous system complications during treatment of acute lymphoblastic leukemia in a single pediatric institution. Leuk Lymphoma. (2010) 51:1063–71. doi: 10.3109/10428191003754608
19. Banerjee J, Niinimäki R, Lähteenmäki P, et al. The spectrum of acute central nervous system symptoms during the treatment of childhood acute lymphoblastic leukaemia. Pediatr Blood Cancer. (2020) 67:e27999. doi: 10.1002/pbc.27999
20. Gregorios JB, Gregorios AB, Mora J, Marcillo A, Fojaco RM, Green B. Morphologic alterations in rat brain following systemic and intraventricular methotrexate injection: light and electron microscopic studies. J Neuropathol Experiment Neurol. (1989) 48:33–47. doi: 10.1097/00005072-198901000-00004
21. Gilbert MR, Harding BL, Grossman SA. Methotrexate neurotoxicity: in vitro studies using cerebellar explants from rats. Cancer Res. (1989) 49:2502−5.
22. Hyland K, Smith I, Bottiglieri T, et al. Demyelination and decreased S-adenosylmethionine in 510-methylenetetrahydrofolate reductase deficiency. Neurology. (1988) 38:459–459. doi: 10.1212/WNL.38.3.459
23. Chambers JC, McGregor A. Acute hyperhomocysteinaemia and endothelial dysfunction. Lancet. (1998) 351:36–7. doi: 10.1016/S0140-6736(05)78090-9
24. Preibisch G, Kiiffner C, Elstner EF. Biochemical model reactions on the prooxidative activity of homocysteine. Zeitschrift für Naturforschung C. (1993) 48:58–62. doi: 10.1515/znc-1993-1-211
25. Ling Q, Hajjar KA. Inhibition of endothelial cell thromboresistance by homocysteine. J nutrition. (2000) 130:373S−6S. doi: 10.1093/jn/130.2.373S
26. Curras MC, Dingledine R. Selectivity of amino acid transmitters acting at N-methyl-D-aspartate and amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors. Mol Pharmacol. (1992) 41:520–6.
27. Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. New England J Med. (1994) 330:613–22. doi: 10.1056/NEJM199403033300907
28. Quinn CT, Griener JC, Bottiglieri T, Arning E, Winick NJ. Effects of intraventricular methotrexate on folate, adenosine, and homocysteine metabolism in cerebrospinal fluid. J Pediatr Hematol Oncol. (2004) 26:386–8. doi: 10.1097/00043426-200406000-00011
29. Mahoney DH, Shuster JJ, Nitschke R. Acute neurotoxicity in children with B-precursor acute lymphoid leukemia: an association with intermediate-dose intravenous methotrexate and intrathecal triple therapy–a pediatric oncology group study. J Clin Oncol. (1998) 16:1712–22. doi: 10.1200/JCO.1998.16.5.1712
30. Bond J, Hough R, Moppett J, Vora A, Mitchell C, Goulden N. “Stroke-like syndrome” caused by intrathecal methotrexate in patients treated during the UKALL 2003 trial. Leukemia. (2013) 4:954–6. doi: 10.1038/leu.2012.328
31. Cruz-Carreras MT, Chaftari P, Shamsnia A, Guha-Thakurta N, Gonzalez C. Methotrexate-induced leukoencephalopathy presenting as stroke in the emergency department. Clin Case Rep. (2017) 10:1644–8. doi: 10.1002/ccr3.1110
32. Agarwal A, Vijay K, Thamburaj K, Ouyang T. Transient leukoencephalopathy after intrathecal methotrexate mimicking stroke. Emerg Radiol. (2011) 18:345–7. doi: 10.1007/s10140-010-0931-6
33. Sandoval C, Kutscher M, Jayabose S, Tenner M. Neurotoxicity of intrathecal methotrexate: MR imaging findings. AJNR Am J Neuroradiol. (2003) 24:1887–90.
34. Charles A. Advances in the basic and clinical science of migraine. Ann Neurol. (2009) 65:491–8. doi: 10.1002/ana.21691
35. Rogers P, Pan WJ, Drachtman RA, Haines C. A stroke mimic: methotrexate-induced neurotoxicity in the emergency department. J Emerg Med. (2017) 52:559–61. doi: 10.1016/j.jemermed.2016.11.016
Keywords: stroke-like syndrome, methotrexate, pseudo-stroke, neurotoxicity, subacute toxicity
Citation: Santangelo A, Bartolini E, Nuzzi G, Foiadelli T, Michev A, Mina T, Trambusti I, Fichera V, Bonuccelli A, Massimetti G, Peroni DG, De Marco E, Coccoli L, Luti L, Bernasconi S, Nardi M, Menconi MC, Casazza G, Pruna D, Mura R, Marra C, Zama D, Striano P, Cordelli DM, Battini R and Orsini A (2022) The Clinical Impact of Methotrexate-Induced Stroke-Like Neurotoxicity in Paediatric Departments: An Italian Multi-Centre Case-Series. Front. Neurol. 13:920214. doi: 10.3389/fneur.2022.920214
Received: 14 April 2022; Accepted: 10 May 2022;
Published: 10 June 2022.
Edited by:
Kette D. Valente, University of São Paulo, BrazilReviewed by:
Piali Mandal, Lady Hardinge Medical College and All India Institute of Medical Sciences, IndiaCopyright © 2022 Santangelo, Bartolini, Nuzzi, Foiadelli, Michev, Mina, Trambusti, Fichera, Bonuccelli, Massimetti, Peroni, De Marco, Coccoli, Luti, Bernasconi, Nardi, Menconi, Casazza, Pruna, Mura, Marra, Zama, Striano, Cordelli, Battini and Orsini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roberta Battini, cm9iZXJ0YS5iYXR0aW5pQGZzbS51bmlwaS5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.