- Department of Neurology, Neuroscience Center, The First Hospital of Jilin University, Changchun, China
Background: In recent years, a growing number of researches indicate that S100B may act in migraine, but the relationship between S100B and migraine remains controversial. Therefore, the current study aimed to perform a meta-analysis to quantitatively summarize S100B levels in migraine patients.
Methods: We used Stata 12.0 software to summarize eligible studies from PubMed, EMBASE, Web of Science, Cochrane Library, China National Knowledge Infrastructure (CNKI), and Wanfang databases. We applied standardized mean differences (SMDs) with 95% confidence intervals (95%CIs) to appraise the association between S100B and migraine.
Results: The combined results of nine case-control studies indicated that compared with healthy controls, overall migraine patients had significantly increased S100B levels in peripheral blood (SMD = 0.688, 95%CI: 0.341–1.036, P < 0.001). The S100B levels in migraineurs during ictal periods (SMD =1.123, 95%CI: 0.409–1.836, P = 0.002) and interictal periods (SMD = 0.487, 95%CI: 0313–0.661, P < 0.001), aura (SMD = 0.999, 95%CI: 0.598–1.400, P < 0.001) and without aura (SMD = 0.534, 95%CI: 0.286–0.783, P < 0.001) were significantly higher than those in the controls. The subgroup analyses by age, country, migraine assessment, and assay method of S100B also illustrated a statistically obvious association between S100B levels and migraine, indicating that age may be the most important source of heterogeneity. Sensitivity analysis showed that no individual study has a significant influence on the overall association between S100B and migraine.
Conclusion: This meta-analysis demonstrates that the level of S100B in peripheral blood of patients with migraine was significantly increased. Migraine may be associated with pathological reactions involving S100B, which is instrumental for the clinical diagnosis of migraine and therapy that considers S100B as a potential target.
Introduction
Migraine has been known as a leading cause of disability worldwide through the ongoing Global Burden of Diseases, Injuries, and Risk Factors Study (1, 2), with an estimated prevalence between 2.6 and 21.7% in different regions and populations (3). The prevalence of migraine in women is higher than that in men, and the ratio of male to female prevalence changes with age (4). The severe attack and chronic course of migraine can affect the patient's academic performance, occupation, psychological health, quality of life, social interaction, and even financial stability of individual family members (5–7) which is an important component increasing the burden of public health. Migraine is very prevalent, but its pathogenesis remains unclear. Cortical spreading depression (8), trigeminal system (9), vasodilation (10), and hypothalamus (11, 12) may be involved in the triggering and occurrence of migraine. Increased synaptic connectivity and excitability, and expansion of receptive fields, that is, central sensitization, are relevant to the development of chronic migraine (13).
The S100 protein family comprises 24 calcium-binding proteins that exert intracellular or extracellular regulatory effects. They act as calcium-activated switches and regulate the activity of various targets in different tissues. S100B is mainly serried in astrocytes (14) and other glial cell types, such as oligodendrocytes and Schwann cells, and is not limited to the nervous system (15). S100B is involved in cell proliferation, survival, and differentiation (16). Originally, S100B was considered a marker of neural injury because it can leak from damaged cells. Currently, S100B was also found to be initiatively released by different cell types, specifically under stress status. It is directly related to the progress of a disease, including congenital/perinatal disorders, neurodegenerative diseases, acute brain injury, psychiatric disorders, and inflammatory bowel disease (16). S100B at high concentrations induced toxic/proinflammatory effects (17, 18).
The mechanism of migraine is not fully understood, and the release of many inflammatory factors involves the entire process and its chronicity. Calcitonin gene-related peptide (CGRP) levels increase during ictal periods of migraine, and experiments have shown that it originates from stimulating trigeminal nerve fibers (13), leading to the production and release of inflammatory cytokines and induction to sensitization of trigeminal neurons. Studies have found that chemical stimulation to the trigeminal nerves increases the expression of the inflammatory protein S100B in both neurons and glia. Increased S100B is thought to participate in peripheral sensitization of trigeminal neurons and takes a part in the formation and continuance of inflammatory pain (19). Tetrandrine has been reported to suppress the overexpression of S100B while significantly alleviating trigeminal pain sensitization (20). S100B may play an important pathological role in the occurrence of migraine and may be a potential target for the treatment of migraine. However, there are few studies on the relationship between S100B and migraine, and this relationship is unclear. Some studies believe that S100B rises during ictal or interictal, and some studies have shown that it will increase in the chronic phase. Therefore, this study performed a meta-analysis on the controversy of the relationship between the two in previous studies and explored the relationship between S100B and migraine.
Materials and Methods
This meta-analysis was conducted in terms of the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (21).
Search Strategies
A comprehensive search was executed in the PubMed, Web of Science, EMBASE, Cochrane Library, China National Knowledge Infrastructure (CNKI), and Wanfang databases by two independent investigators (CC and RZ) from inception to October 2021, with no restrictions on population. Languages are limited to English and Chinese. We used the following combination of subject headings and free terms: (S100 calcium binding protein beta subunit OR S100β OR S100B OR S100beta OR S100 calcium-binding protein B) and (migraine disorders OR migraine OR hemicrania OR headache). Detailed search term strategies are presented in the Supplementary Materials. We search the Chinese database for the following phrases: (S100B OR钙结合蛋白B OR 钙结合蛋白β) and (偏头痛) (“钙结合蛋白” means “calcium binding protein,” “偏头痛” means “migraine”).
Study Selection
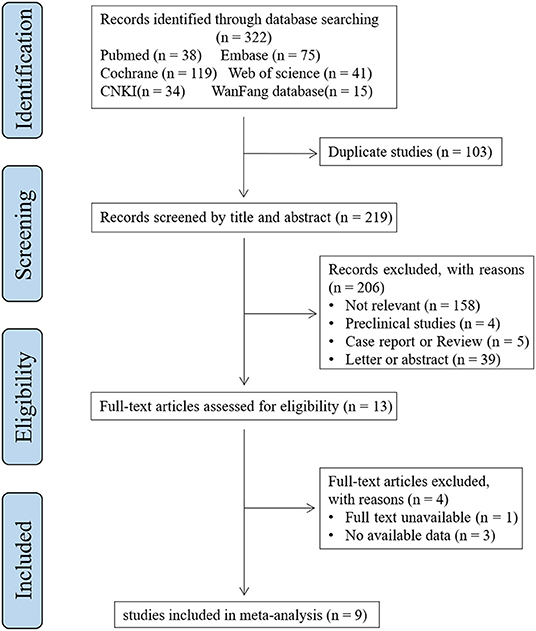
All potential studies were reviewed step-by-step by two researchers (CC and RZ) independently (Figure 1). Irrelevant studies on account of titles or abstracts were excluded first. Provisionally eligible studies were needed to be evaluated for eligibility by inspecting the full text. Any disagreements between the two reviewers were resolved by a third reviewer (MC). The studies included in this meta-analysis met the following inclusion criteria: a) Population: individuals diagnosed with migraine (according to international guidelines); b) Intervention (i.e. assessment): measurement of S100B reported in mean ± SD or can be converted to mean ± SD; c) Comparator: individuals without migraine; d) Outcome: the level of S100B; and e) Study designs: cross-sectional or case-control study. The exclusion criteria were as follows: a) conference abstracts, letters, case reports, reviews, and meta-analyze; b) no full text. Duplicated studies were removed. We included the latest or most complete study, for studies with reduplicated populations.
Data Extraction
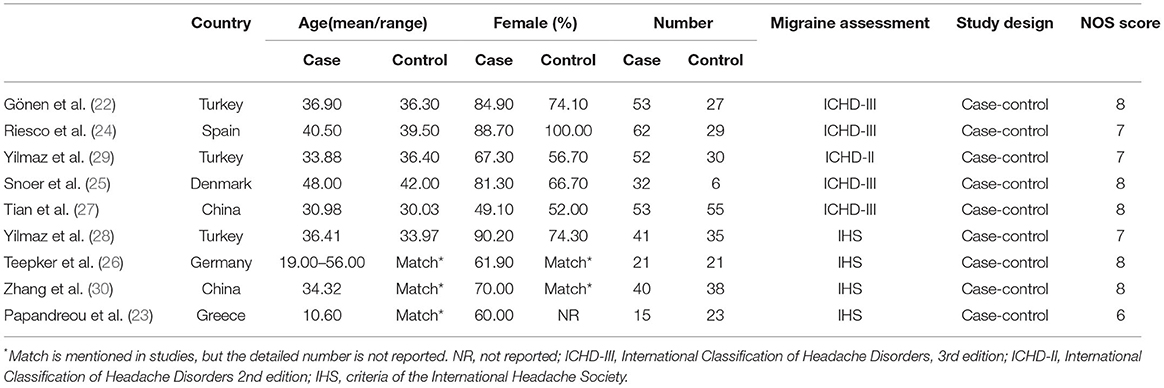
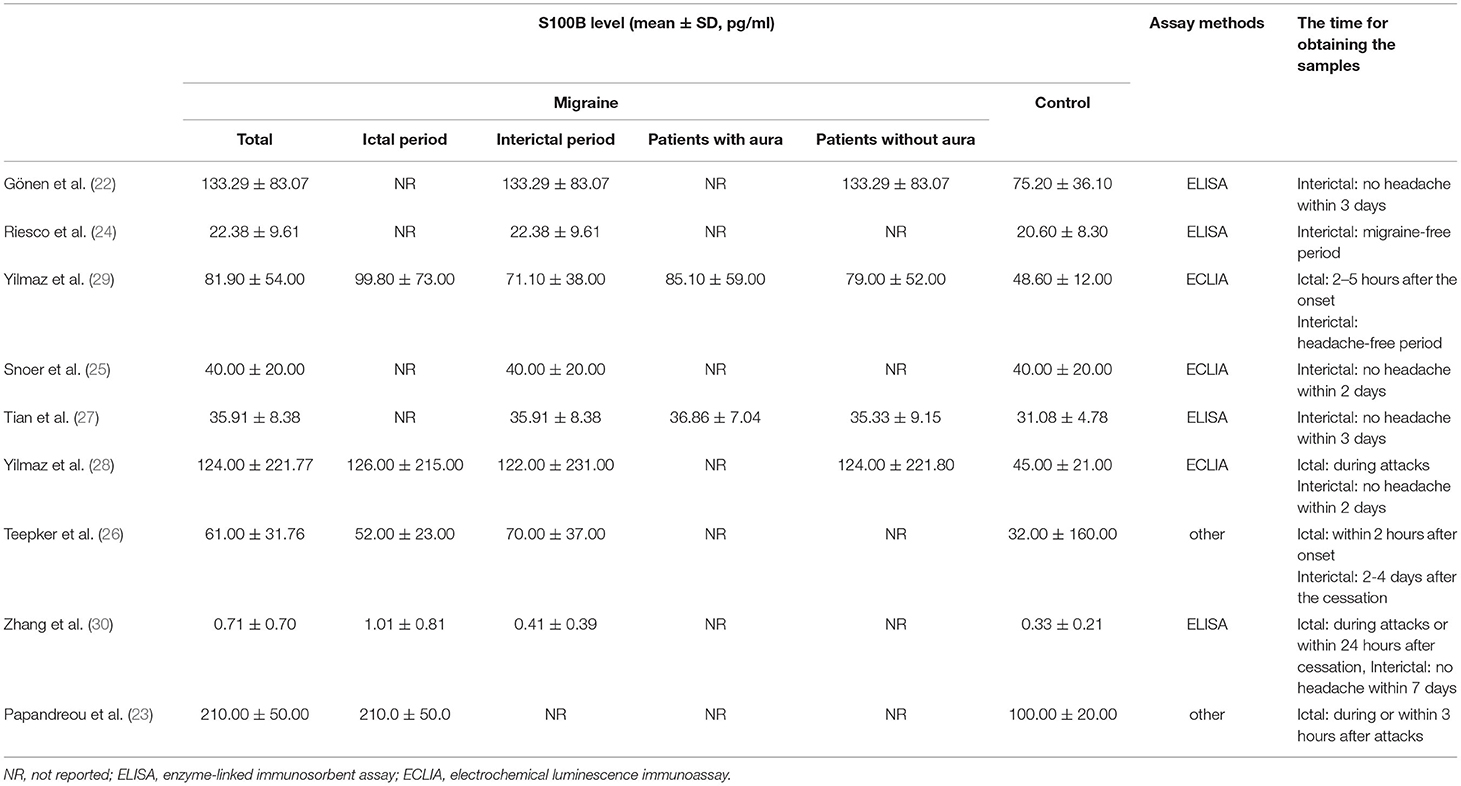
Information was extracted on the first author, publication year, country, age, gender distribution (female %), sample size, diagnosis, and study design (Table 1). When the original study only mentioned the migraine diagnostic criteria proposed by the International Headache Society (IHS), and did not specify which version of the criteria, was expressed by IHS. Data on mean S100B concentrations (mean ± SD) were extracted as primary outcomes and used for combining data by two independent researchers (Table 2).
Quality Assessment
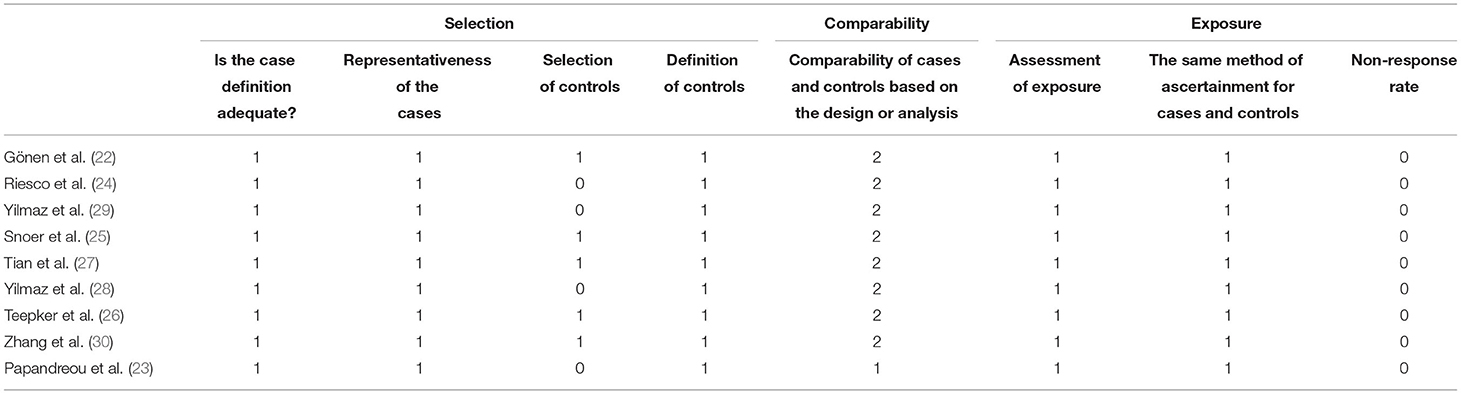
The quality of the included study was estimated by using the Newcastle-Ottawa Scale. This score classifies individual studies across three domains: selection, comparability, and exposure. Two researchers independently estimated the quality of the studies and resolved any disagreements through discussion.
Statistical Analyses
Associations between S100B levels and migraine was assessed by using SMDs with 95%CIs describing continuous data, and the results are visualized as forest plots, which included the contribution of each study (weight) to the overall effect. In the present meta-analysis, results with two-tailed P-values < 0.05, were regarded as statistically significant. Substantial heterogeneity of the included studies was confirmed by >50% and P-value of Q test < 0.10). A random-effects model or a fixed-effects model was selected by I2-value. Sensitivity analyses were performed to examine the potential source of heterogeneity using leave-one-out cross-validation. Begg's rank correlation test and Egger's regression asymmetry test were employed to evaluate publication bias if the number of included studies was >8 in the meta-analysis. Stata (version 12.0, Stata Corp LP, College Station, Texas, USA) was used for the statistical analysis.
Results
Literature Search
The systematic literature search produced 322 references. In total, 103 duplicate references were removed first. We identified 13 studies that were potentially eligible for inclusion after scanning the titles and abstracts. After the full-text assessment, nine studies were evaluated for eligibility and included in the quantitative analysis (22–30). A flowchart of the process used to select the studies is shown in Figure 1.
Characteristics and Quality Assessments of Included Studies
Nine included studies comprising 633 individuals (369 migraine cases and 264 controls) were published between 2005 and 2021. Two of the studies were conducted in China and seven in Europe.
The Newcastle-Ottawa Scale scores of the studies varied from 6 to 8 points (mean score: 6.81), implying the good quality of the included studies. Specific quality scores of the included studies are listed in Table 3.
Results of Meta-Analysis
The Association of S100B Levels With Overall Migraine
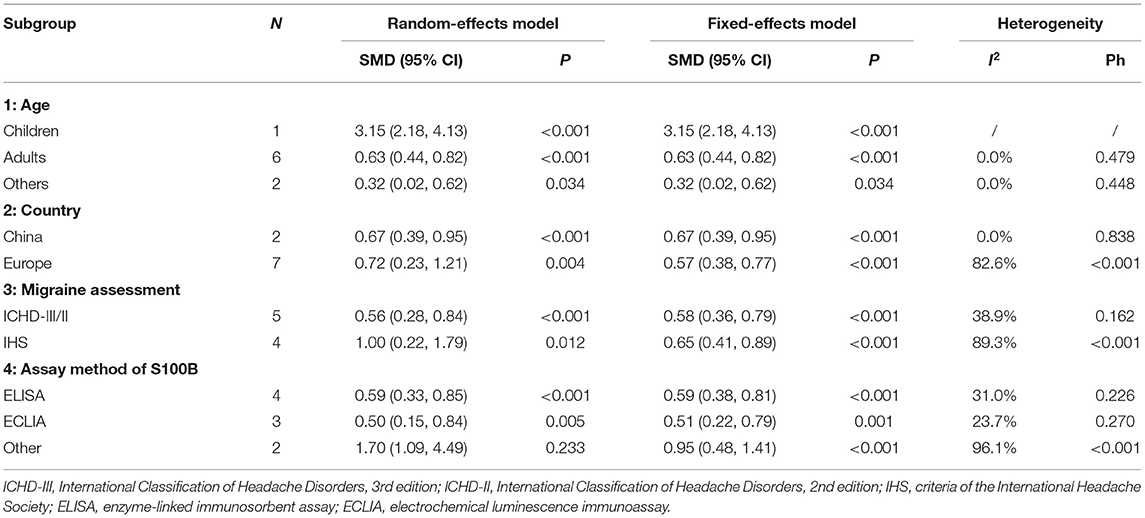
A meta-analysis was carried out using a random-effects model due to the high heterogeneity (I2 = 77.0%, P < 0.001). Synthetic results illustrated that overall migraine patients (with or without aura, ictal or interictal) had significantly elevated S100B levels in peripheral blood compared to healthy controls (SMD = 0.688, 95%CI: 0.341–1.036, P < 0.001) (Figure 2).
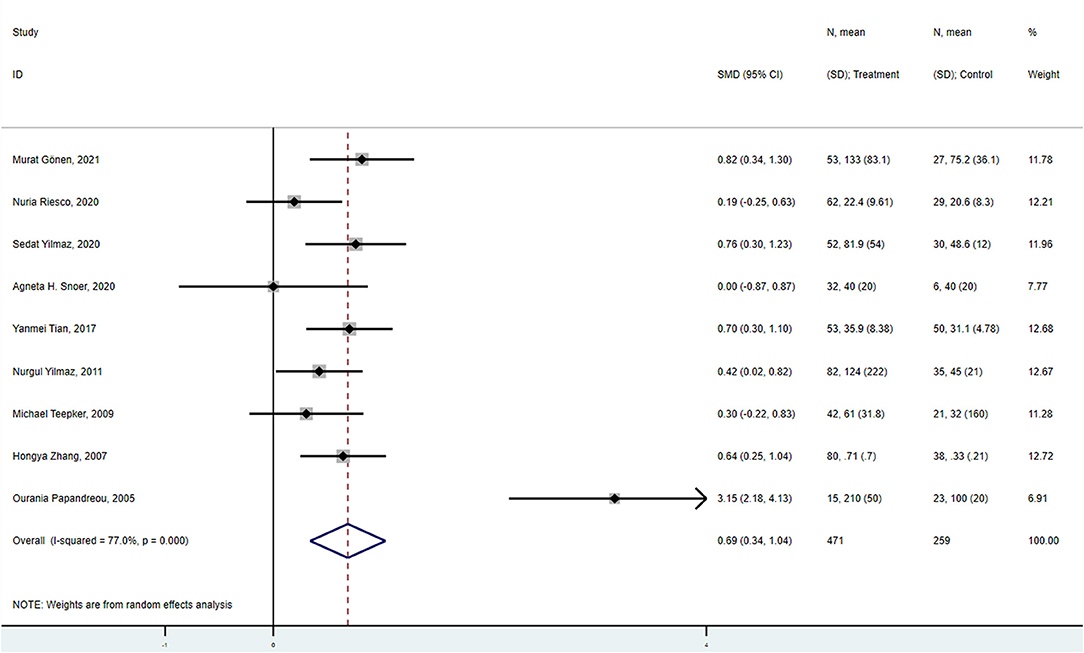
Figure 2. Forest plot for the association between S100B levels and overall migraine. SMD, standardized mean difference; CI, confidence interval.
S100B Levels of Migraineurs in Interictal and Ictal Periods
Of the nine studies included in the meta-analysis, eight studies contained S100B levels of migraineurs in the interictal period and five studies in the ictal period.
The combined results of the meta-analysis (fixed-effects model, I2 = 18.0%, P = 0.288) indicated that S100B levels were significantly higher in interictal patients than in controls (SMD = 0.487, 95%CI: 0313–0.661, P < 0.001) (Figure 3A). Similarly, the combined results of the meta-analysis (random-effects model) comparing the S100B levels of migraine patients in the ictal period to that of controls suggested that SMD was 1.123 (95%CI: 0.409–1.836, P = 0.002), the corresponding I2-statistic was 86.6% (P < 0.001) (Figure 3B). The S100B levels of patients were not significantly difference in interictal period than ictal period (SMD = 0.240, 95%CI: −0.378 to 0.857, P = 0.447, I2 = 82.9%, Ph = 0.001).
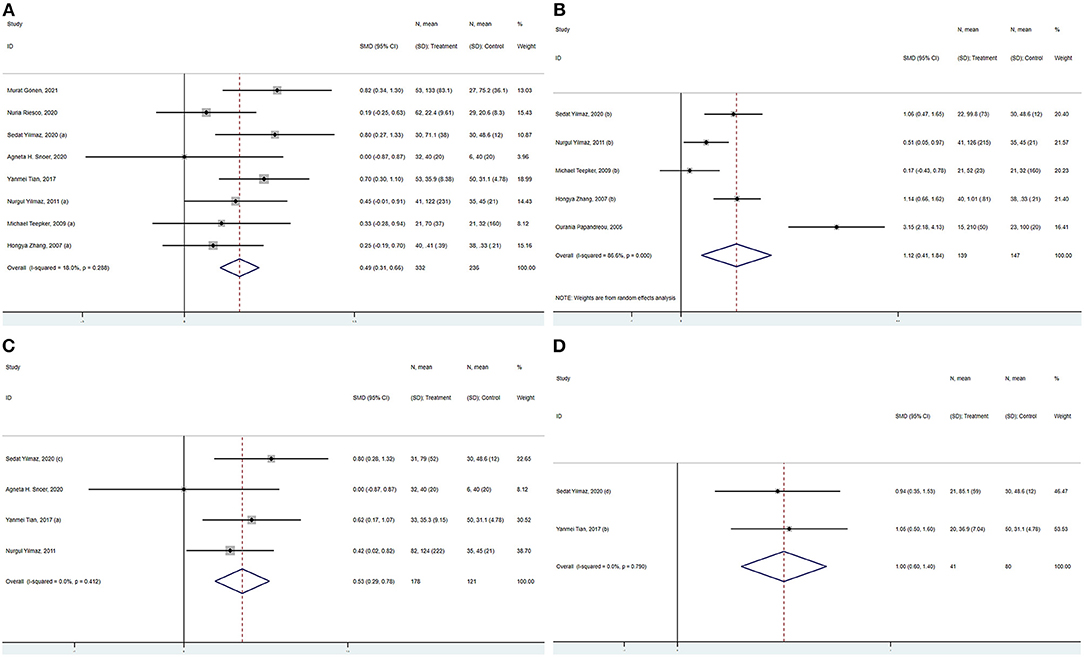
Figure 3. Forest plot for the association between S100B levels and migraine (A) in the interictal period, (B) in the ictal period, (C) without aura, and (D) with aura. SMD, standardized mean difference; CI, confidence interval.
S100B Levels of Migraineurs Without and With Aura
S100B levels were significantly higher in migraineurs without aura than in controls in the meta-analysis pooling four studies (SMD = 0.534, 95%CI: 0.286–0.783, P < 0.001), the corresponding I2-statistic was 0.0% (P = 0.412) (Figure 3C). S100B levels were significantly higher in migraine patients with aura than in controls (SMD = 0.999, 95%CI: 0.598–1.400, P < 0.001, I2 = 0.0%, P = 0.790) (Figure 3D). Migraine patients without aura in the interictal periods also had significantly increased S100B levels in peripheral blood compared with healthy controls (SMD = 0.474, 95%CI: 0.173–0.774, P < 0.001, I2 = 0.0%, P = 0.460). The S100B levels were not significantly difference in patients without aura than with aura (SMD = 0.146, 95%CI: −0.247 to 0.539, P = 0.466, I2 = 0.0%, Ph = 0.860).
S100B Levels of Patients With Chronic Migraine and Episodic Migraine
S100B levels were not significantly difference in patients with chronic migraine than in controls in the meta-analysis pooling two studies (SMD = 0.563, 95%CI: −0.086 to 1.212, P = 0.089), the corresponding I2-statistic was 68.1% (P = 0.076). S100B levels were also not significantly difference in patients with episodic migraine than in controls (SMD = 0.527, 95%CI: −0.369 to 1.423, P = 0.249, I2 = 79.4%, P = 0.028).
Results of Subgroup Analysis
The overall estimation of S100B in migraine showed significant heterogeneity. We performed subgroup analyses to further elucidate the association between S100B and migraine, and to explore the source of heterogeneity. Subgroup analyses by age group were also performed. Pooled data demonstrated that more significant differences in blood S100B levels between migraine patients and healthy controls were found in children (SMD = 3.15, 95% CI: 2.18–4.13) than in adults (SMD = 0.63, 95% CI: 0.44–0.82) and other age groups (including both minors and adults, and unknown age group) (SMD = 0.32, 95% CI: 0.02–0.62). The heterogeneity decreased notably after the age subgroup analysis, which indicated that age or only one study on children may be an important source of heterogeneity. In addition, the results of stratification by country, diagnosis of migraine, and assay method of S100B indicated a correlation between elevated S100B and migraine (Table 4). The I2 was reduced to below 50% in China, International Classification of Headache Disorders, 3/2nd edition (ICHD-III/II), enzyme-linked immunosorbent assay (ELISA), and electrochemical luminescence immunoassay (ECLIA) subgroups, which shows that heterogeneity may be related to the assessment of migraine and assay method of S100B.
Sensitivity Analysis
Sensitivity analysis (including a minimum of three studies) was performed to assess and evaluate the stability and reliability of the overall SMD by leave-one-out cross-validation, which repeated the analysis after the sequential exclusion of each study. The results were not substantially altered after the serial exclusion of individual studies (Figure 4 and Supplementary Figure). Thus, these strategies revealed that the results were stable.
Figure 4. Sensitivity analysis for the association between S100B levels and overall migraine. SMD, standardized mean difference; CI, confidence interval.
Publication Bias
Since the number of studies we included was <10, the results of the Begg rank correlation test (P > |z| = 0.754) and Egger linear regression (P = 0.220) were only used to roughly evaluate publication bias. Positive results may be more likely to be published, which may lead to publication bias in this study.
Discussion
In recent years, some studies have explored the S100B level in migraine patients, but the sample size of participants was small, and the results were not consistent. Our study aimed to review existing studies to synthesize data and uncover further evidence to confirm the correlation between S100B levels and migraine. To the best of our knowledge, this is the first meta-analysis to investigate the correlation between migraine and S100B. The findings of the current meta-analysis including nine studies showed that S100B levels were indeed significantly increased in migraineurs than in the healthy control group, although the results of previous clinical studies on this topic were not consistent.
In 2005, Papandreou et al. first reported significantly elevated S100B levels in pediatric patients with migraine during the ictal period (23). This may indicate that brain cell damage caused by local inflammation leads to the elevation of S100B levels. Subsequently, several studies found that the S100B level in migraine during the interictal period also increased (22, 24, 26–30). These findings suggest that migraine may be an ongoing or progressive disorder with a subclinical course, rather than a paroxysmal disorder characterized by intermittent headaches. During this interval, there are persistent pathological changes and processes in the nervous system. Evidence is increasing for other functional and structural brain changes that occur over time in individuals with migraine (31–33). However, some studies hold different opinions, making the correlation between S100B and migraine controversial. Therefore, a meta-analysis is needed to further verify these results. Our study included nine studies, of which eight contained S100B data of migraineurs during the interictal phase, five comprised ictal data, four had S100B data of migraineurs without aura, two had S100B data of migraineurs with aura, and two had S100B data of patients with episodic migraine and chronic migraine. S100B levels increased in many types of migraine, except chronic migraine and episodic migraine. This may imply that S100B is involved in the pathological process of the occurrence and development of migraine. In these two studies including data of patients with episodic migraine and chronic migraine, the S100B levels of both chronic and episodic migraine in the Riesco et al. study were not significant increases from those in the control group (24), unlike the findings of Gönen et al. (22). This may be related to the fact that preventive medication was not withdrawn before blood samples were collected in the Riesco et al. study. Preventive medication may ameliorate the pathological process of migraine and reduce the expression of S100B at the same time. Due to the small number of included articles, this finding also affected the results of the meta-analysis of the S100B levels of patients with chronic migraine and episodic migraine.
Subgroup analyses were also conducted, and we found that S100B increased more significantly in the children group after stratification by age group. Within the different age subgroups, I2 was 0.0%. This implies that age may be an important source of heterogeneity. Some previous studies have shown that migraineurs experience changes in structure (34) and metabolism (35) in their brains as they age. Some studies have suggested that the expression of S100B increases in the cortex and hippocampus by activating astrocytes with advancing age. These results indicate that S100B may play an important role in brain development and the establishment of proper brain functions. Upregulation of S100B in aging may be relevant to the age-related pathological state of the brain (36). Age could be considered an important factor influencing both S100B expression and migraine progression. However, since there was only one study on children in the subgroup analysis, and the quality evaluation of this study was low, the above results may not be reliable. Therefore, in the future, age stratification is needed to explore the elevated S100B levels in patients with migraine.
S100B is an acidic protein that binds to zinc (Zn2+) and calcium (Ca2+) and exists in the nucleus and cytoplasm of a broad range of cells (37–39). Intracellular S100B modulates a series of biological activities, such as cell proliferation, survival, differentiation, regulation of calcium homeostasis, enzyme regulation, and interaction with the cytoskeleton (40). Extracellular S100B binds to the receptor for advanced glycation end products (RAGE), which is a cell surface receptor of immunoglobulin present on various cell types (41, 42), initiates a complex intracellular signaling cascade (43), up-regulates nitric oxide synthase (NOS), inducing the release of NO and the production of reactive oxygen species (ROS) and NO-dependent neuronal and glial death (44, 45), facilitating glutamate-mediated death of neurons and upregulation of COX-II expression in microglia (46). Elevated S100B has been observed in patients with many neurological diseases, such as brain injury (47), Parkinson's disease (48), Alzheimer's disease (49), epilepsy (50), and multiple sclerosis (MS) (51). Elevated S100B was also observed to participate in the subsequent signaling pathway and inflammation in many animal models of neurological diseases.
Previous research has shown that extracellular signal-regulated kinases (ERK) are modulated by extracellular S100B protein in astrocyte cultures, resulting in increased activation of phosphorylation products (52). ERK and p38 activated by inflammatory factors participate in the generation and maintenance of inflammatory nociceptive hypersensitivity (53). There is little experimental research on the association between S100B levels and migraine. Chemical stimulation has been found to increase the expression of S100B and p38 in the trigeminal nerves in rat models of capsaicin-induced migraine (19, 20). A significant increase in p-ERK protein and S100B expression was also observed in the trigeminal ganglia of rat models of nitroglycerin (NTG) -induced migraine (20). Therefore, glial S100B expression and subsequent upregulation of the p-ERK and p38 pathways may be involved in trigeminal nociceptive hypersensitivity and the pathological process of migraine.
In some neurological diseases, some measures also play a role in ameliorating diseases and reducing the expression of S100B at the same time. S100B knockout mice showed reduced Aβ plaque formation in the cortical region in an experimental Alzheimer's disease model (54). In addition, arundic acid inhibits astrocytic S100B synthesis and induced the protection of dopaminergic neurons against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) toxicity in a mouse model of Parkinson's disease (55). Similarly, arundic acidinhibits both astrocytic over-expression of S100B and the subsequent activation of signaling pathways in the peri-infarct area, which may be shared by ischemic stroke or even multiple sclerosis (56). In a rat model of epilepsy, resveratrol, metformin, dexamethasone, as anti-inflammatory and neuroprotective agents, also significantly reduced the levels of S100B participating in neuroinflammatory processes accompanying epilepsy (57–59). The above studies show that S100B is indeed involved in the process of many neurological diseases. In animal experiments on migraine, tetrandrine can suppress NTG-induced overexpression of S100B and p-ERK in satellite glial cells of the trigeminal ganglia and significantly alleviate trigeminal pain sensitization. This result suggests that S100B may play an important pathological role in the occurrence and maintenance of migraine. The study of S100B level in migraine may provide more directions for the study of migraine pathological mechanisms.
Strengths and Limitation
This study has strengths and limitations. Its strengths lie in this is the first meta-analysis on this topic, and we investigated the relationship between various types of migraine and the level of S100B. These may contribute to the study of the pathological mechanism of migraine.
There are also some limitations in the present study that cannot be neglected. The present research may be biased due to the inability to obtain unpublished literature and the inclusion of only Chinese and English literature. Although this is the first meta-analysis on this topic, the quality of the research results may be affected by the scarcity of related studies and the small sample size of the included studies. Large-scale studies are needed to provide more deterministic evidence. Although we have found an important source of the overall heterogeneity through age subgroups, there was only one article in the children subgroup, and more studies in this age group are needed in the future to confirm the relationship between S100B and child migraine. Due to the lack of more detailed characteristics of migraine, such as the disease duration and frequency of attacks in the included studies, our research was not been able to further explore the changes of S100B in migraines of different severity, which requires further research.
Conclusion
In summary, this study provides evidence that S100B levels were significantly increased in migraine patients compared to controls, and helps to understand the pathological mechanism of migraine and the possible implementation of S100B-targeted therapies for migraine.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
WL guided the design of the study and revised the manuscript. CC designed the study, screened the literature, extracted data, performed the statistical analysis, and wrote the manuscript. RZ and MC supported the screening of literatures and extracted data. NL supported the visualization of the results. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We sincerely thank the originator of Newcastle-Ottawa Scale.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.914051/full#supplementary-material
References
1. Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet. (2017) 390:1211–59. doi: 10.1016/S0140-6736(17)32154-2
2. Disease GBD, Injury I, Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2018) 392:1789–858. doi: 10.1016/S0140-6736(18)32279-7
3. Yeh WZ, Blizzard L, Taylor BV. What is the actual prevalence of migraine? Brain Behav. (2018) 8:e00950. doi: 10.1002/brb3.950
4. Buse DC, Loder EW, Gorman JA, Stewart WF, Reed ML, Fanning KM. Sex differences in the prevalence, symptoms, and associated features of migraine, probable migraine and other severe headache: results of the American migraine prevalence and prevention (AMPP) Study. Headache. (2013) 53:1278–99. doi: 10.1111/head.12150
5. Blumenfeld AM, Varon SF, Wilcox TK, Buse DC, Kawata AK, Manack A. Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the international burden of migraine study (IBMS). Cephalalgia. (2011) 31:301–15. doi: 10.1177/0333102410381145
6. Buse DC, Scher AI, Dodick DW, Reed ML, Fanning KM, Manack Adams A. Impact of migraine on the family: perspectives of people with migraine and their spouse/domestic partner in the cameo study mayo. Clin Proc. (2016) 16:13 doi: 10.101016/jmayocp02013
7. Leonardi M, Steiner TJ, Scher AT, Lipton RB. The global burden of migraine: measuring disability in headache disorders with WHO's classification of functioning, disability and health (ICF). J Headache Pain. (2005) 6:429–40. doi: 10.1007/s10194-005-0252-4
8. Arngrim N, Hougaard A, Ahmadi K, Vestergaard MB, Schytz HW, Amin FM. Heterogenous migraine aura symptoms correlate with visual cortex functional magnetic resonance imaging responses. Ann Neurol. (2017) 82:925–39. doi: 10.1002/ana.25096
9. Goadsby PJ, Edvinsson L, Ekman R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann Neurol. (1988) 23:193–6. doi: 10.1002/ana.410230214
10. Friberg L, Olesen J, Iversen HK, Sperling B. Migraine pain associated with middle cerebral artery dilatation: reversal by sumatriptan. Lancet. (1991) 338:13–7. doi: 10.1016/0140-6736(91)90005-a
11. Denuelle M, Fabre N, Payoux P, Chollet F, Geraud G. Hypothalamic activation in spontaneous migraine attacks. Headache. (2007) 47:1418–26. doi: 10.1111/j.1526-4610.2007.00776.x
12. Schulte LH, May A. The migraine generator revisited: continuous scanning of the migraine cycle over 30 days and three spontaneous attacks. Brain. (2016) 139:1987–93. doi: 10.1093/brain/aww097
13. Andreou AP, Edvinsson L. Mechanisms of migraine as a chronic evolutive condition. J Headache Pain. (2019) 20:117. doi: 10.1186/s10194-019-1066-0
14. Ludwin SK, Kosek JC, Eng LF. The topographical distribution of S-100 and GFA proteins in the adult rat brain: an immunohistochemical study using horseradish peroxidase-labelled antibodies. J Comp Neurol. (1976) 165:197–207. doi: 10.1002/cne.901650206
15. Tubaro C, Arcuri C, Giambanco I, Donato R. S100B protein in myoblasts modulates myogenic differentiation via NF-kappaB-dependent inhibition of MyoD expression. J. Cell. Physiol. (2010) 223:270–82. doi: 10.1002/jcp.22035
16. Michetti F, D'Ambrosi N, Toesca A, Puglisi MA, Serrano A, Marchese E. The S100B story: from biomarker to active factor in neural injury. J Neurochem. (2019) 148:168–87. doi: 10.1111/jnc.14574
17. Niven J, Hoare J, McGowan D, Devarajan G, Itohara S, et al. S100B up-regulates macrophage production of IL1beta and CCL22 and influences severity of retinal inflammation. PLoS ONE. (2015) 10:e0132688. doi: 10.1371/journal.pone.0132688
18. Piazza O, Leggiero E, Benedictis De, Pastore G, Salvatore L, Tufano FR, et al. S100B. induces the release of pro-inflammatory cytokines in alveolar type I-like cells. Int. J. Immunopathol Pharmacol. (2013) 26:383–91. doi: 10.1177/039463201302600211
19. Thalakoti S, Patil VV, Damodaram S, Vause CV. Langford LE, Freeman SE. Neuron-glia signaling in trigeminal ganglion: implications for migraine pathology. Headache. (2007) 47:1008–23. discussion 1024-1005. doi: 10.1111/j.1526-4610.2007.00854.x
20. Qin G, Gui B, Xie J, Chen L, Cui Z. Tetrandrine alleviates nociception in a rat model of migraine via suppressing S100B and p-erk activation in satellite glial cells of the trigeminal Ganglia. J Mol Neurosci. (2018) 64:29–38. doi: 10.1007/s12031-017-0999-5
21. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
22. Gonen M, Ozdogan S, Balgetir F, Demir CF, Aytac E, Mungen B. S100B and neuron-specific enolase levels in episodic and chronic migraine. Acta Neurol Scand. (2021). 143, 298–302. doi: 10.1111/ane.13365
23. Papandreou O, Soldatou A, Tsitsika A, Kariyannis C, Papandreou T, Zachariadi A. Serum S100beta protein in children with acute recurrent headache: a potentially useful marker for migraine. Headache. (2005) 45:1313–6. doi: 10.1111/j.1526-4610.2005.00263.x
24. Riesco N, Cernuda-Morollon E, Martinez-Camblor P, Perez-Pereda S, Pascual J. Peripheral, interictal serum S100B levels are not increased in chronic migraine patients. Headache. (2020) 60:1705–11. doi: 10.1111/head.13919
25. Snoer AH, Vollesen ALH, Beske RP, Guo S, Hoffmann J, Jorgensen NR, S100B. and NSE in cluster headache - evidence for glial cell activation? Headache. (2020) 60:1569–80. doi: 10.1111/head.13864
26. Teepker M, Munk K, Mylius V, Haag A, Moller JC, Oertel WH. Serum concentrations of s100b and NSE in migraine. Headache. (2009) 49:245–52. doi: 10.1111/j.1526-4610.2008.01228.x
27. Tian Y. The significance of serum levels of S100B protein and NSE in patients with migraine[master's thesis]. Hebei: Hebei Medical University (2017)
28. Yilmaz N, Karaali K, Ozdem S, Turkay M, Unal A, Dora B. Elevated S100B and neuron specific enolase levels in patients with migraine-without aura: evidence for neurodegeneration? Cell. Mol Neurobiol. (2011) 31:579–85. doi: 10.1007/s10571-011-9651-z
29. Yilmaz S. Serum NO, S100B, NSE concentrations in migraine and their relationship. J Clin Neurosci. (2020) 82:2–5. doi: 10.1016/j.jocn.10.046.
30. Zhang H, Shi T, Wang X. Changes of neuron-specific enolase and S100 protein content in jugular vein blood in patients with migraine. Chinese J Misdiag. (2007) 7:2995–6.
32. Maleki N, Becerra L, Brawn J, Bigal M, Burstein R, Borsook D. Concurrent functional and structural cortical alterations in migraine. Cephalalgia. (2012) 32:607–20. doi: 10.1177/0333102412445622
33. Palm-Meinders IH, Koppen H, Terwindt GM, Launer LJ, Konishi J, Moonen JM. Structural brain changes in migraine. JAMA. (2012) 308:1889–97. doi: 10.1001/jama.2012.14276
34. Chong CD, Dodick DW, Schlaggar BL, Schwedt TJ. Atypical age-related cortical thinning in episodic migraine. Cephalalgia. (2014) 34:1115–24. doi: 10.1177/0333102414531157
35. Lisicki M, D'Ostilio K, Coppola G, Parisi V, Noordhout de, Magis AMD, et al. Age related metabolic modifications in the migraine brain. Cephalalgia. (2019) 39:978–87. doi: 10.1177/0333102419828984
36. Modi PK, Kanungo MS. Age-dependent expression of S100beta in the brain of mice. Cell Mol Neurobiol. (2010) 30:709–16. doi: 10.1007/s10571-009-9495-y
37. Baudier J, Gerard D. Ions binding to S100 proteins: structural changes induced by calcium and zinc on S100a and S100b proteins. Biochemistry. (1983) 22:3360–9. doi: 10.1021/bi00283a009
38. Baudier J, Gerard D. Ions binding to S100 proteins II Conformational studies and calcium-induced conformational changes in S100 alpha alpha protein: the effect of acidic pH and calcium incubation on subunit exchange in S100a (alpha beta) protein. J Biol Chem 261. (1986) 8204–12.
39. Baudier J, Glasser N, Gerard D. Ions binding to S100 proteins. I. Calcium- and zinc-binding properties of bovine brain S100 alpha alpha, S100a (alpha beta), and S100b (beta beta) protein: Zn2+ regulates Ca2+ binding on S100b protein. (1986) J. Biol. Chem. 261, 8192–8203.
40. Donato R, Cannon BR, Sorci G, Riuzzi F, Hsu K, Weber DJ. Functions of S100 proteins. Curr Mol Med. (2013) 13:24–57.
41. Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. (1999) 97:889–901. doi: 10.1016/s0092-8674(00)80801-6
42. Huttunen HJ, Kuja-Panula J, Sorci G, Agneletti AL, Donato R, Rauvala H. Coregulation of neurite outgrowth and cell survival by amphoterin and S100 proteins through receptor for advanced glycation end products (RAGE) activation. J Biol Chem. (2000) 275:40096–105. doi: 10.1074/jbc.M006993200
43. Bongarzone S, Savickas V, Luzi F, Gee AD. Targeting the receptor for advanced glycation endproducts (RAGE): a medicinal chemistry perspective. J Med Chem. (2017) 60:7213–32. doi: 10.1021/acs.jmedchem.7b00058
44. Hu J, Castets F, Guevara JL, Van Eldik LJ, S100. beta stimulates inducible nitric oxide synthase activity and mRNA levels in rat cortical astrocytes. J. Biol. Chem. (1996) 271:2543–7. doi: 10.1074/jbc.271.5.2543
45. Petrova TV, Hu J, Van Eldik LJ. Modulation of glial activation by astrocyte-derived protein S100B: differential responses of astrocyte and microglial cultures. Brain Res. (2000) 853:74–80. doi: 10.1016/s0006-8993(99)02251-9
46. Bianchi R, Giambanco I, Donato R. S100B/RAGE-dependent activation of microglia via NF-kappaB and AP-1 Co-regulation of COX-2 expression by S100B, IL-1beta and TNF-alpha. Neurobiol Aging. (2010) 31:665–77. doi: 10.1016/j.neurobiolaging.05017
47. Blyth BJ, Farhavar A, Gee C, Hawthorn B, He H, Nayak A. Validation of serum markers for blood-brain barrier disruption in traumatic brain injury. J Neurotrauma. (2009) 26:1497–507. doi: 10.1089/neu.2008-0738
48. Sathe K, Maetzler W, Lang JD, Mounsey RB, Fleckenstein C, Martin HL. S100B is increased in Parkinson's disease and ablation protects against MPTP-induced toxicity through the RAGE and TNF-alpha pathway. Brain. (2012) 135:3336–47. doi: 10.1093/brain/aws250
49. Chaves ML, Camozzato AL, Ferreira ED, Piazenski I, Kochhann R., Dall'Igna O, et al. Serum levels of S100B and NSE proteins in Alzheimer's disease patients. J. Neuroinflammation. (2010) 7:6. doi: 10.1186/1742-2094-7-6
50. Lu C, Li J, Sun W, Feng L, Li L, Liu A. Elevated plasma S100B concentration is associated with mesial temporal lobe epilepsy in Han Chinese: a case-control study. Neurosci Lett. (2010) 484:139–42. doi: 10.1016/j.neulet.08.036.
51. Petzold A, Eikelenboom MJ, Gveric D, Keir G, Chapman M, Lazeron RH. Markers for different glial cell responses in multiple sclerosis: clinical and pathological correlations. Brain. (2002) 125:1462–73. doi: 10.1093/brain/awf165
52. Goncalves DS, Lenz G, Karl J, Goncalves CA, Rodnight R. Extracellular S100B protein modulates ERK in astrocyte cultures. Neuroreport. (2000) 11:807–9. doi: 10.1097/00001756-200003200-00030
53. Ji RR. Peripheral and central mechanisms of inflammatory pain, with emphasis on MAP kinases. Curr Drug Targets Inflamm Allergy. (2004) 3:299–303. doi: 10.2174/1568010043343804
54. Roltsch E, Holcomb L, Young KA, Marks A, Zimmer DB. PSAPP mice exhibit regionally selective reductions in gliosis and plaque deposition in response to S100B ablation. J Neuroinflammation. (2010) 7:78. doi: 10.1186/1742-2094-7-78
55. Kato H, Kurosaki R, Oki C, Araki T. Arundic acid, an astrocyte-modulating agent, protects dopaminergic neurons against MPTP neurotoxicity in mice. Brain Res. (2004) 1030:66–73. doi: 10.1016/j.brainres.09046
56. Tateishi N, Mori T, Kagamiishi Y, Satoh S, Katsube N, Morikawa E. Astrocytic activation and delayed infarct expansion after permanent focal ischemia in rats. Part II: suppression of astrocytic activation by a novel agent (R)-(-)-2-propyloctanoic acid (ONO-2506) leads to mitigation of delayed infarct expansion and early improvement of neurologic deficits. J Cereb Blood Flow Metab 22. (2002) 723–34. doi: 10.1097/00004647-200206000-00011
57. Meng XJ, Wang F, Li CK. Resveratrol is neuroprotective and improves cognition in pentylenetetrazole-kindling model of epilepsy in rats. Indian J Pharm Sci. (2014) 76:125–31.
58. Vazifehkhah S, Khanizadeh AM, Mojarad TB, Nikbakht F. The possible role of progranulin on anti-inflammatory effects of metformin in temporal lobe epilepsy. J Chem Neuroanat. (2020) 109:101849. doi: 10.1016/j.jchemneu.2020.101849
Keywords: S100B, blood levels, migraine, meta-analysis, case-control
Citation: Chu C, Zhong R, Cai M, Li N and Lin W (2022) Elevated Blood S100B Levels in Patients With Migraine: A Systematic Review and Meta-Analysis. Front. Neurol. 13:914051. doi: 10.3389/fneur.2022.914051
Received: 20 April 2022; Accepted: 20 June 2022;
Published: 14 July 2022.
Edited by:
Uwe Reuter, Charité Universitätsmedizin Berlin, GermanyReviewed by:
Lucas Overeem, Charité Universitätsmedizin Berlin, GermanySonia Santos Lasaosa, Lozano Blesa University Clinical Hospital, Spain
Copyright © 2022 Chu, Zhong, Cai, Li and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weihong Lin, bGlud2hAamx1LmVkdS5jbg==
 Chaojia Chu
Chaojia Chu Rui Zhong
Rui Zhong Mengtan Cai
Mengtan Cai Nan Li
Nan Li Weihong Lin
Weihong Lin