
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 30 May 2022
Sec. Endovascular and Interventional Neurology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.913653
This article is part of the Research TopicNew Insights into the Treatment of Aneurysms with Flow Diverters: Novel Indications and Therapeutic AdvancesView all 18 articles
Purpose: To investigate the effect and safety of flow diverters in the management of small (<10 mm in diameter) unruptured intracranial aneurysms.
Materials and Methods: One hundred and ten patients with 145 small intracranial aneurysms treated with flow diverters were retrospectively enrolled. The clinical, endovascular, and follow-up data were analyzed.
Results: One hundred twenty-one flow diverters were deployed for the treatment of 145 small intracranial aneurysms in 110 patients, and the stenting success rate was 99.1%. In 133 (91.7%) aneurysms, only flow-diverting devices were deployed, and in the rest 12 (8.3%) of aneurysms, coils were used to loosely pack the aneurysm after deployment of a flow-diverting device. Five patients (4.5%) experienced ischemic complications, but no hemorrhagic complications were occurred. All patients had clinical follow-up 6–18 (median 12) after the procedure, with the modified Rankin scale score (mRS) 0 in 101 patients, 1 in four patients, 2 in three patients, 4 in one patient, and 5 in one patient. Digital subtraction angiography was performed at follow-up in 90 (81.8%) patients with 118 (81.4%) aneurysms 6–18 months (median 12) after the procedure, with the Raymond grade I in 90 (76.2%) aneurysms and Raymond grade III in 28 (23.7%). Eighteen patients with 22 partially occluded aneurysms at the first angiographic follow-up experienced the second digital subtraction angiography 12–36 months (median 26) after the procedure, and 21 (95.5%) aneurysms were completely occluded. Two patients had asymptomatic in-stent stenosis.
Conclusion: Treatment of small unruptured intracranial aneurysms with flow diverters can be performed safely and effectively with satisfactory outcomes.
Ever since the approval of flow diversion by the Food and Drug Administration for the treatment of intracranial aneurysms, flow-diverting devices have been increasingly used in the treatment of intracranial aneurysms, especially large and giant aneurysms, which are associated with worse outcomes than small ones (1–8). The indications of treatment for the flow-diverting devices are aneurysms with a maximal diameter of over 10 mm that include large and giant aneurysms and a wide neck with a width of over 4 mm (1, 9) and for aneurysms <10 mm with a narrow neck, traditional stent-assisted coiling embolization is comparatively better. Nonetheless, the management of small intracranial aneurysms remains controversial, with difficulties frequently reported in the literature in both endovascular embolization and surgical clipping, as well as a high crossover rate (up to 18%) from endovascular embolization to surgical clipping (10–12). Favorable clinical and angiographic outcomes of endovascular embolization of small ruptured aneurysms have been reported recently in a study with a long-term follow-up of 5 years (13), which may indicate that experience accumulation may lead to good outcomes. With the accumulation of clinical experience in using the flow-diverting devices for intracranial aneurysms, the indication of flow diversion has been greatly expanded from large and giant unruptured aneurysms to ruptured, blister, and dissecting aneurysms as off-label use (14). This is because flow-diverting devices are different from conventional regular arterial stents in that they have a higher metal coverage surface to divert blood flow away from the aneurysm, promote flow stasis and thrombosis within the aneurysm cavity, and remodel the parent artery for aneurysm regression (14, 15). These advantages can be used to treat complex, large, and giant intracranial aneurysms, which are hard for traditional endovascular or surgical approaches, resulting in a low complication rate and a low recurrence rate (14, 16–21). For small aneurysms <10 mm in diameter (22, 23), the flow-diverting devices also have some specific advantages, such as simple operation and low intraprocedural aneurysm rupture rates, and thus can be used in the treatment of small aneurysms. It was hypothesized that flow-diverting devices could be safely and effectively used in the treatment of small intracranial aneurysms. This study was consequently performed to investigate the effect and safety of flow-diverting devices in the treatment of small intracranial aneurysms.
This retrospective study was approved by the ethics committee of our hospital, and all patients or their family members had given the signed informed consent to participate. From March 2014 to April 2019, patients with small unruptured intracranial aneurysms treated with flow-diverting devices in our hospital were enrolled. The inclusion criteria were consecutive patients with small (<10 mm) unruptured intracranial aneurysms, which were treated with flow-diverting devices (Pipeline Embolization Device, Medtronic, Irvine, CA, USA, and Tubridge, MicroPort Medical Company, Shanghai, China) that include saccular, dissecting, or fusiform aneurysms. The exclusion criteria were patients with larger (>10 mm) aneurysms, ruptured aneurysms, and aneurysms, which had been treated previously using surgical clipping or endovascular embolization.
Three to 5 days before the endovascular procedure, dual antiplatelet therapy was administered for all patients with clopidogrel (75 mg/d) and aspirin (100 mg/d) (24). The endovascular procedure was conducted under general anesthesia and heparinization. Percutaneous access was obtained using femoral artery puncture, and a microcatheter was navigated through the guiding catheter to the aneurysm. An appropriate flow-diverting device was selected and sent to the right location for deployment. In aneurysms with an aneurysm neck > 7 mm, an irregular dome, or a daughter sac, coils were used to embolize the aneurysm. For patients with the device being opened poorly, long operation time in the procedure, and suspected thrombosis at the aneurysm neck, Tirofiban was administered intravenously after stent deployment (25), with the beginning injection dose of 5 μg/kg injected within 3 min followed by instillation in the dose of 0.05 μg/kg−1/min−1, which was 1/2 of the conventional dose. After the endovascular procedure, Tirofiban was continually administered for 24–36 h, and aspirin (100 mg/d) and clopidogrel (75 mg/d) were continued in all patients for 3 months followed by long-term use of aspirin (100 mg/d) alone.
Clinical and angiographic follow-up was scheduled in all patients, and the treatment effect of the flow-diverting device was evaluated 6 months after the procedure using the Raymond grading system (26), with the Raymond grade I as complete obliteration of aneurysm, grade II as a residual neck, and grade III as any opacification of the aneurysm sac or residual aneurysm. The clinical prognosis was assessed with the modified Rankin scale (mRS) scores.
Statistical analysis was performed with the SPSS software version 19.0 (IBM, Chicago, IL, USA). Continuous data in normal distribution were presented as mean ± standard deviation (SD). Enumeration data were presented as frequency and percentages.
One hundred and ten patients with 145 aneurysms who met the inclusion criteria were enrolled that include 77 (70%) male and 33 (30%) female patients with an age range of 35–78 years (mean 53.7 ± 18.3; Table 1). Clinical symptoms included headache or dizziness in 56 (50.9%) patients and ischemic cerebral diseases in 19 (17.3%) patients. The rest 35 (31.8%) patients were incidentally found. Aneurysm location involved the internal carotid artery (ICA) C4–C7 segments in 131 (90.3%) aneurysms, ICA C2 segment in two (1.4%), V4 segment of the vertebral artery in nine (6.2%), middle cerebral artery M1 segment in one (0.7%), and M2 segment in two (1.4%). Among 110 patients, 77 (70%) patients had one aneurysm each, 22 (20%) had two aneurysms each, and eight (5.5%) had three aneurysms each.
In the endovascular procedure, 121 flow-diverting devices were deployed to treat 145 aneurysms (Figure 1) that include 20 (16.53%) Tubridge and 101 (83.47%) Pipeline devices. In 133 (91.7%) aneurysms, only flow-diverting devices were implanted, and in the rest of 12 (8.3%) aneurysms, coils were inserted into the aneurysm sac for loose packing after placement of the flow-diverting device. In one patient with a 4-mm aneurysm at the tortuous paraclinoid segment of ICA, the distal end of the Tubridge device (4.5 × 30 mm) was herniated into the aneurysm cavity when the micro-guidewire was withdrawn, and repeated attempts did not succeed in correct deployment of the Tubridge stent, resulting in failure of stenting. All the other patients had successful stent deployment, with a success rate of stenting of 99.1% (109/110). In 17 devices, good opening and wall adherence were obtained with balloon expansion or micro-guidewire “massage” after stent deployment.
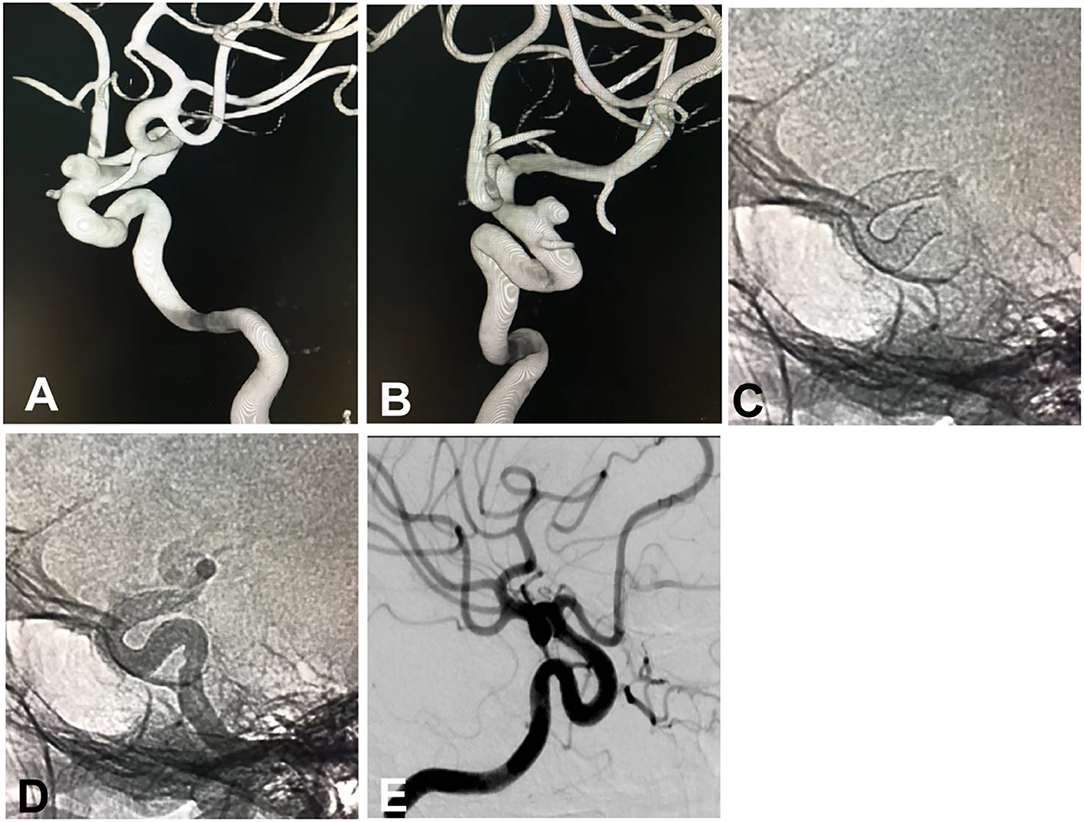
Figure 1. A small intracranial aneurysm in a 53-year-old woman with dizziness was treated with the deployment of a Pipeline embolization device. (A,B) The small aneurysm was located at the sixth (ophthalmic) segment of the left internal carotid artery, measuring 5.1 × 3.0 mm in the sac with a 4-mm neck. (A) Lateral position, (B) Oblique position. (C) Immediately after deployment of a Pipeline device of 4.25 × 20 mm, the stent was shown to have a good opening on angiography. (D) Angiography immediately following the deployment of the stent, the parent artery was shown to be patent with good wall adherence of the stent. (E) Follow-up angiography 8 months later revealed patent parent artery and complete occlusion of the aneurysm.
Five peri-procedural ischemic complications occurred, i.e., one patient who was treated with a Pipeline device combined with coiling and four treated with the deployment of a Pipeline device only, resulting in a complication rate of 4.5%. No hemorrhagic complications took place in this cohort. In the case with the distal stent end herniating in the aneurysm cavity, the parent artery was occluded, and the anterior cerebral artery had good compensation, resulting in an mRS of 2 at a 3-month follow-up. In one case with poor stent adherence to the arterial wall, cerebral infarction had occurred in the area supplied by the choroidal artery and anterior cerebral artery covered by the stent, resulting in an mRS score of 4 at follow-up evaluation. In one case with in-stent thrombosis leading to occlusion of the middle cerebral artery, the mRS was 5 at follow-up. In one case with an atherosclerotic plaque at the parent artery near the aneurysm neck leading to slight stenosis of the parent artery, in-stent thrombosis occurred 4 h after the procedure, and acute endovascular thrombectomy was performed, resulting in recanalization and good recovery of the patient. Complete occlusion of the aneurysm and an mRS of 0 were present at the 3-month follow-up. In one case with cerebral ischemic symptoms (hemiplegia), intravenous pumping of Tirofiban resulted in good recovery.
All patients had clinical follow-up 6–18 (median 12) after the procedure, with the mRS 0 in 101 (91.8%) patients, 1 in four (3.6%) patients, 2 in three (2.7%) patients, 4 in one (0.9%) patient, and 5 in one (0.9%) patient. Digital subtraction angiography was performed at follow-up in 90 (81.8%) patients with 118 (81.4%) aneurysms 6–18 months (median 12) after the procedure, with the Raymond grade I in 90 (76.2%) aneurysms and Raymond grade III in 28 (23.7%). Eighteen patients with 22 partially occluded aneurysms at the first angiographic follow-up experienced the second digital subtraction angiography 12–36 months (median 26) after the procedure, and 21 (95.5% or 21/22) aneurysms were completely occluded (Raymond grade I, Figure 1). Two patients had asymptomatic in-stent stenosis.
In this study, the safety and effect of flow diverters in treating small unruptured intracranial aneurysms were investigated, and it was found that treatment of small unruptured intracranial aneurysms with flow diverters could be performed safely and effectively with satisfactory outcomes.
Small intracranial aneurysms refer to a aneurysms with the maximal diameter <10 mm regardless of the aneurysm nature, such as saccular, dissecting, or fusiform, accounting for a large proportion of cerebral aneurysms (22, 23, 27). These small aneurysms may be irregular, with daughter sacs, in the posterior circulation, and should be treated actively to prevent possible rupture even though they are not the conventional indications for use of flow diverters. Traditionally, stent-assisted coiling has achieved good clinical and imaging outcomes in the treatment of small (<10 mm) unruptured intracranial aneurysms (22). However, endovascular treatment of intracranial aneurysms that include small unruptured ones still faces great challenges, such as incomplete occlusion, recurrence of wide-necked aneurysms, difficult access or unstable placement of embolization catheters due to anatomical characteristics of aneurysms or poor remodeling, intraprocedural rupture during the process of dense embolization, and difficulties and complex management of multiple tandem aneurysms. Thus, it is natural to use flow diverters for the treatment of these kinds of aneurysms (28). The advantages of using flow diverters for these aneurysms included simplified operation with no need to use an embolization catheter into the aneurysm sac for coiling, decreased recurrence or retreatment rate in wide-necked and complex aneurysms with increased long-term effects, and loose packing in some aneurysms with no or decreased risk of aneurysm rupture. However, in conventional stent-assisted coiling, the embolization outcome may be affected by stent types, size of the first coil, proper shaping of the microcatheter, packing of the last coil, and dense packing.
Because of the advantages of flow-diverting devices, the embolization operation with flow-diverting devices is not so difficult, and the rate of peri-procedural complications is decreased. No patients experienced hemorrhagic complications, and the ischemic complication rate was only 4.5% in our study. The ischemic complication rate had been reported to be 2.7 (19) and 8.7% (28) in the use of flow-diverting devices for the treatment of intracranial aneurysms and 4.6–11.2% in traditional stent-assisted coiling of intracranial aneurysms (22, 29), similar to ours. In a meta-analysis of 41 studies that involved 2,614 patients with aneurysms <10 mm treated with flow diverters, the complication rate was reported to be 7.8% (95% CI 4.8–11.4%) (30), and another meta-analysis that investigated the safety and efficiency of flow diverters in treating small aneurysms (<10 mm) also reported procedural-related neurological mortality of 0.87% and morbidity of 5.22%. These complication rates in these meta-analyses were similar to the above complication rates of intracranial aneurysms treated with either flow diverters or stent-assisted coiling. Many reasons may contribute to the occurrence of ischemic complications, such as inexperience, poor adherence or insufficient opening of the stent, insufficiency of antiplatelet therapy, and adjunctive coiling. In our study, the five ischemic complications may probably be associated with the early inexperience in using the flow diverters and possible stenosis of the parent artery. To decrease the ischemic complications, the following aspects should be paid attention to. Because poor wall adherence is an independent risk factor for ischemic complications (31), the flow-diverting device should be deployed to have good wall adherence. With experience accumulation in the process of learning, the flow-diverting device can be deployed with good wall adherence, and the technical complication rate related to wall adherence can be significantly decreased. Moreover, adequate antiplatelet therapy should be administered in the peri-procedural period to prevent possible ischemic complications. In our study, a small dose of Tirofiban was used 24–48 h after deployment of the device in patients with good thromboelastogram, which can significantly decrease ischemic complications without increasing the risk of rupture of intracranial aneurysms based on our experience (25, 32). In patients with parent artery stenosis >50%, balloon expansion should be performed in advance to relieve the stenosis before deployment of the flow-diverting device so as to obtain good wall adherence after deployment. The use of a microcatheter for “massaging” the flow diverter or a balloon to expand the flow diverter can effectively increase the rate of good wall adherence.
In our study, the aneurysm complete occlusion rate (Raymond grade I) was 76.2% at the first angiographic follow-up 6–18 months after the procedure, but 95.5% at the second angiographic follow-up 12–36 months (median 26) after the procedure, similar to those reported by other researchers in the treatment of intracranial aneurysms using traditional stent-assisted coiling (33) or flow diverters (34). Complete occlusion of the aneurysms may depend on several factors. Firstly, the long-term outcome of aneurysm occlusion primarily relies on neointima to completely cover the aneurysmal neck, which may require a period of 20–24 wk based on animal experiments (35). A short period of time between 6 and 18 months may not be sufficient for the neointima to cover the aneurysm neck for complete occlusion. Moreover, aneurysm occlusion outcome may also be affected by the stent adherence, metal coverage, and parent artery tortuosity. Aneurysm complete occlusion rate after treatment with flow diverters has an apparent time dependence, with the complete occlusion rate of 73.6% at 6-month follow-up but 95.2% at 5 years after endovascular treatment (34), similar to the outcomes in our study. An adjusted complete occlusion rate of 74.9% (95% CI of 69.6–79.8%) of aneurysms <10 mm has been reported at 12 months after treatment with flow diverters in a meta-analysis (30). A complete occlusion rate of 84.23% (95% CI 80.34–87.76%) has also been reported in a systematic review and meta-analysis investigating the safety and efficiency of flow diverters in the treatment of small aneurysms <10 mm (27). The use of one or multiple flow diverters may also affect aneurysm complete occlusion rate, with multiple diverters being frequently deployed for large and giant aneurysms and one diverter for small aneurysms. It may thus be more appropriate to define the primary end point of the use of flow diverters in the treatment of aneurysms as the cure rate from 12 to 18 months after treatment.
Metal coverage and mesh size of the stent at the aneurysm neck may be factors significantly affecting the complete occlusion rate of intracranial aneurysms (36), and additional coiling in conjunction with the Pipeline embolization device may effectively increase the complete occlusion rate of intracranial aneurysms, especially for large and giant ones (37). Nonetheless, adjunctive coiling after deployment of a flow diverter may increase the risk of ischemic stroke (38, 39). In our practice, additional coiling was usually performed only in irregular aneurysms > 10 mm with daughter sacs. For small unruptured intracranial aneurysms, it is not necessary to use coils in conjunction with flow diverters for complete aneurysm occlusion so as to avoid increased operation difficulty and risk of complications.
This study had some limitations, such as the retrospective and one-center study nature, no control, no randomization, and Chinese patients enrolled only. Future studies with randomization, control, and multiple centers will have to be performed to resolve these issues for better outcomes.
In conclusion, the use of flow diverters in the treatment of small unruptured intracranial aneurysms may result in good outcomes and fewer peri-procedural complications and may become the preferential choice for small unruptured aneurysms.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by Ethics Committee of Henan Provincial People's Hospital. The patients/participants provided their written informed consent to participate in this study.
LL and T-XL: study design. LL, B-LG, Q-JS, G-LZ, Z-LW, and L-FZ: data collection. LL, B-LG, and T-XL: data analysis. Z-LW and L-FZ: study supervision. LL: writing of the original version. B-LG: revision of the original version. All authors agree to be accountable for all aspects of the work and approve the final version of the article.
This study was supported by the 13th Five-year Plan of China for Research and Development (2016YFC1300702), Henan Province Science and Technology Key Project (182102310658), and Scientific and Technological Project of Henan Province (222102310208).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Becske T, Kallmes DF, Saatci I, McDougall CG, Szikora I, Lanzino G, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology. (2013) 267:858–68. doi: 10.1148/radiol.13120099
2. Jia L, Wang J, Zhang L, Zhang Y, You W, Yang X, et al. Evaluating the tubridge flow diverter for large cavernous carotid artery aneurysms. Chin Neurosurg J. (2020) 6:36. doi: 10.1186/s41016-020-00215-z
3. Kan P, Siddiqui AH, Veznedaroglu E, Liebman KM, Binning MJ, Dumont TM, et al. Early postmarket results after treatment of intracranial aneurysms with the pipeline embolization device: A u.S. Multicenter experience. Neurosurgery. (2012) 71:1080–7. doi: 10.1227/NEU.0b013e31827060d9
4. Liu JM, Zhou Y, Li Y, Li T, Leng B, Zhang P, et al. Parent artery reconstruction for large or giant cerebral aneurysms using the tubridge flow diverter: a multicenter, randomized, controlled clinical trial (parat). AJNR Am J Neuroradiol. (2018) 39:807–16. doi: 10.3174/ajnr.A5619
5. Oishi H, Teranishi K, Yatomi K, Fujii T, Yamamoto M, Arai H. Flow diverter therapy using a pipeline embolization device for 100 unruptured large and giant internal carotid artery aneurysms in a single center in a japanese population. Neurol Med Chir. (2018) 58:461–7. doi: 10.2176/nmc.oa.2018-0148
6. Peschillo S, Caporlingua A, Resta MC, Peluso JPP, Burdi N, Sourour N, et al. Endovascular treatment of large and giant carotid aneurysms with flow-diverter stents alone or in combination with coils: a multicenter experience and long-term follow-up. Oper Neurosurg. (2017) 13:492–502. doi: 10.1093/ons/opx032
7. Wang Z, Tian Z, Li W, Wang J, Zhu W, Zhang M, et al. Variation of mass effect after using a flow diverter with adjunctive coil embolization for symptomatic unruptured large and giant intracranial aneurysms. Front Neurol. (2019) 10:1191. doi: 10.3389/fneur.2019.01191
8. Zhou Y, Yang PF, Fang YB, Xu Y, Hong B, Zhao WY, et al. A novel flow-diverting device (tubridge) for the treatment of 28 large or giant intracranial aneurysms: a single-center experience. AJNR Am J Neuroradiol. (2014) 35:2326–33. doi: 10.3174/ajnr.A3925
9. Yakovlev SB AS, Dorokhov PS, Bocharov AV, Bukharin EY, Arkhangel'skaya YN, Aref'eva IA. Endovascular treatment of large and giant intracranial aneurysms using flow-diverting stents. Zh Vopr Neirokhir Im N N Burdenko. (2015) 79:19–27. doi: 10.17116/neiro201579419-27
10. Catapano JS NC, Frisoli FA, Sagar S, Baranoski JF, Cole TS, Labib MA, et al. Small intracranial aneurysms in the barrow ruptured aneurysm trial (brat). Acta Neurochir. (2021) 163:123–9. doi: 10.1007/s00701-020-04602-4
11. Chalouhi N PD, Tjoumakaris S, Jabbour P, Gonzalez LF, Starke RM, Ali MS, et al. Treatment of small ruptured intracranial aneurysms: comparison of surgical and endovascular options. J Am Heart Assoc. (2012) 1:e002865. doi: 10.1161/JAHA.112.002865
12. McDougall CG SR, Zabramski JM, Partovi S, Hills NK, Nakaji P, Albuquerque FC. The barrow ruptured aneurysm trial. J Neurosurg. (2012) 116:135–44. doi: 10.3171/2011.8.JNS101767
13. Peng F FX, Tong X, Zhang B, Wang L, Guo E, Qi P, et al. Endovascular treatment of small ruptured intracranial aneurysms (<5 mm) : long-term clinical and angiographic outcomes and related predictors. Clin Neuroradiol. (2020) 30:817–26. doi: 10.1007/s00062-019-00835-8
14. Kan P, Sweid A, Srivatsan A, Jabbour P. Expanding indications for flow diverters: ruptured aneurysms, blister aneurysms, and dissecting aneurysms. Neurosurgery. (2020) 86:S96–103. doi: 10.1093/neuros/nyz304
15. Fiorella D, Lylyk P, Szikora I, Kelly ME, Albuquerque FC, McDougall CG, et al. Curative cerebrovascular reconstruction with the pipeline embolization device: the emergence of definitive endovascular therapy for intracranial aneurysms. J Neurointerv Surg. (2018) 10:i9–18. doi: 10.1136/jnis.2009.000083.rep
16. Brinjikji W, Murad MH, Lanzino G, Cloft HJ, Kallmes DF. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke. (2013) 44:442–7. doi: 10.1161/STROKEAHA.112.678151
17. Campi A, Ramzi N, Molyneux AJ, Summers PE, Kerr RS, Sneade M, et al. Retreatment of ruptured cerebral aneurysms in patients randomized by coiling or clipping in the international subarachnoid aneurysm trial (isat). Stroke. (2007) 38:1538–44. doi: 10.1161/STROKEAHA.106.466987
18. Goertz L, Dorn F, Kraus B, Borggrefe J, Schlamann M, Forbrig R, et al. Safety and efficacy of the derivo embolization device for the treatment of ruptured intracranial aneurysms. J Neurointerv Surg. (2019) 11:290–5. doi: 10.1136/neurintsurg-2018-014166
19. Kallmes DF, Hanel R, Lopes D, Boccardi E, Bonafe A, Cekirge S, et al. International retrospective study of the pipeline embolization device: a multicenter aneurysm treatment study. AJNR Am J Neuroradiol. (2015) 36:108–15. doi: 10.3174/ajnr.A4111
20. Molyneux AJ. Indications for treatment of cerebral aneurysms from an endovascular perspective: the creation of an evidence base for interventional techniques. Neurosurg Clin N Am. (2005) 16:313–6, ix. doi: 10.1016/j.nec.2004.08.015
21. Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, et al. International subarachnoid aneurysm trial (isat) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. (2005) 366:809–17. doi: 10.1016/S0140-6736(05)67214-5
22. McLaughlin N, McArthur DL, Martin NA. Use of stent-assisted coil embolization for the treatment of wide-necked aneurysms: a systematic review. Surg Neurol Int. (2013) 4:43. doi: 10.4103/2152-7806.109810
23. Merritt WC BH, Ducruet AF, Becker TA. Definitions of intracranial aneurysm size and morphology: a call for standardization. Surg Neurol Int. (2021) 6:506. doi: 10.25259/SNI_576_2021
24. Park KY OT, Kostynskyy A, Kortman H, Hilario A, Nicholson P, Agid R, et al. Ticagrelor versus clopidogrel in the dual antiplatelet regimen for intracranial stenting or flow-diverter treatment for unruptured cerebral aneurysms: a single-center cohort study. AJNR Am J Neuroradiol. (2021) 42:1638–44. doi: 10.3174/ajnr.A7216
25. Wu Q, Shao Q, Li L, Liang X, Chang K, Li T, He Y. Prophylactic administration of tirofiban for preventing thromboembolic events in flow diversion treatment of intracranial aneurysms. J Neurointerv Surg. (2021) 13:835–40. doi: 10.1136/neurintsurg-2020-016878
26. Won SY, Seifert V, Dubinski D, Kashefiolasl S, Dinc N, Bruder M, et al. Short- and midterm outcome of ruptured and unruptured intracerebral wide-necked aneurysms with microsurgical treatment. Sci Rep. (2021) 11:4982. doi: 10.1038/s41598-021-84339-x
27. Yao X MJ, Li H, Shen H, Lu X, Chen G. Safety and efficiency of flow diverters for treating small intracranial aneurysms: a systematic review and meta-analysis. J Int Med Res. (2017) 45:11–21. doi: 10.1177/0300060516671600
28. Griessenauer CJ, Ogilvy CS, Foreman PM, Chua MH, Harrigan MR, He L, et al. Pipeline embolization device for small intracranial aneurysms: evaluation of safety and efficacy in a multicenter cohort. Neurosurgery. (2017) 80:579–87. doi: 10.1227/NEU.0000000000001377
29. Ryu CW, Park S, Shin HS, Koh JS. Complications in stent-assisted endovascular therapy of ruptured intracranial aneurysms and relevance to antiplatelet administration: a systematic review. AJNR Am J Neuroradiol. (2015) 36:1682–8. doi: 10.3174/ajnr.A4365
30. Fiorella D GL, Frame D, Arthur AS. How safe and effective are flow diverters for the treatment of unruptured small/medium intracranial aneurysms of the internal carotid artery? Meta-analysis for evidence-based performance goals. J Neurointerv Surg. (2020) 12:869–87. doi: 10.1136/neurintsurg-2019-015535
31. Jabbour P, Chalouhi N, Tjoumakaris S, Gonzalez LF, Dumont AS, Randazzo C, et al. The pipeline embolization device: learning curve and predictors of complications and aneurysm obliteration. Neurosurgery. (2013) 73:113–20. doi: 10.1227/01.neu.0000429844.06955.39
32. Liang XD, Wang ZL, Li TX, He YK, Bai WX, Wang YY, et al. Safety and efficacy of a new prophylactic tirofiban protocol without oral intraoperative antiplatelet therapy for endovascular treatment of ruptured intracranial aneurysms. J Neurointerv Surg. (2016) 8:1148–53. doi: 10.1136/neurintsurg-2015-012055
33. Geyik S, Yavuz K, Yurttutan N, Saatci I, Cekirge HS. Stent-assisted coiling in endovascular treatment of 500 consecutive cerebral aneurysms with long-term follow-up. AJNR Am J Neuroradiol. (2013) 34:2157–62. doi: 10.3174/ajnr.A3574
34. Becske T, Brinjikji W, Potts MB, Kallmes DF, Shapiro M, Moran CJ, et al. Long-term clinical and angiographic outcomes following pipeline embolization device treatment of complex internal carotid artery aneurysms: five-year results of the pipeline for uncoilable or failed aneurysms trial. Neurosurgery. (2017) 80:40–8. doi: 10.1093/neuros/nyw014
35. Kallmes DF, Ding YH, Dai D, Kadirvel R, Lewis DA, Cloft HJ. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke. (2007) 38:2346–52. doi: 10.1161/STROKEAHA.106.479576
36. Darsaut TE, Bing F, Salazkin I, Gevry G, Raymond J. Flow diverters failing to occlude experimental bifurcation or curved sidewall aneurysms: an in vivo study in canines. J Neurosurg. (2012) 117:37–44. doi: 10.3171/2012.4.JNS111916
37. Lin N, Brouillard AM, Krishna C, Mokin M, Natarajan SK, Sonig A, et al. Use of coils in conjunction with the pipeline embolization device for treatment of intracranial aneurysms. Neurosurgery. (2015) 76:142–9. doi: 10.1227/NEU.0000000000000579
38. Kang H, Luo B, Liu J, Zhang H, Li T, Song D, et al. Postoperative occlusion degree after flow-diverter placement with adjunctive coiling: analysis of complications. J Neurointerv Surg. (2022) 14:371–5. doi: 10.1136/neurintsurg-2021-017445
Keywords: flow diverter, intracranial aneurysms, unruptured, small, complications
Citation: Li L, Gao B-L, Shao Q-J, Zhang G-L, Wang Z-L, Li T-X and Zhu L-F (2022) Small Unruptured Intracranial Aneurysms Can Be Effectively Treated With Flow-Diverting Devices. Front. Neurol. 13:913653. doi: 10.3389/fneur.2022.913653
Received: 06 April 2022; Accepted: 29 April 2022;
Published: 30 May 2022.
Edited by:
Yu Zhou, Naval Military Medical University Shanghai, ChinaReviewed by:
Huibin Kang, Capital Medical University, ChinaCopyright © 2022 Li, Gao, Shao, Zhang, Wang, Li and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tian-Xiao Li, bGl0aWFueGlhb2RAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.