- 1Suzhou Medical College of Soochow University, Suzhou, China
- 2Center for Rehabilitation Medicine, Department of Neurology, Zhejiang Provincial People's Hospital (Affiliated People's Hospital, Hangzhou Medical College), Hangzhou, China
Objectives: We aimed to investigate the association between post-procedure cerebral blood flow velocity (CBFV) and severity of brain edema in patients with acute ischemic stroke (AIS) who received early endovascular therapy (EVT).
Methods: We retrospectively included patients with AIS who received EVT within 24 h of onset between February 2016 and November 2021. Post-procedure CBFV of the middle cerebral artery was measured in the affected and the contralateral hemispheres using transcranial Doppler ultrasound. The severity of brain edema was measured using the three-level cerebral edema grading from the Safe Implementation of Thrombolysis in Stroke-Monitoring Study, with grades 2–3 indicating severe brain edema. The Association between CBFV parameters and severity of brain edema was analyzed.
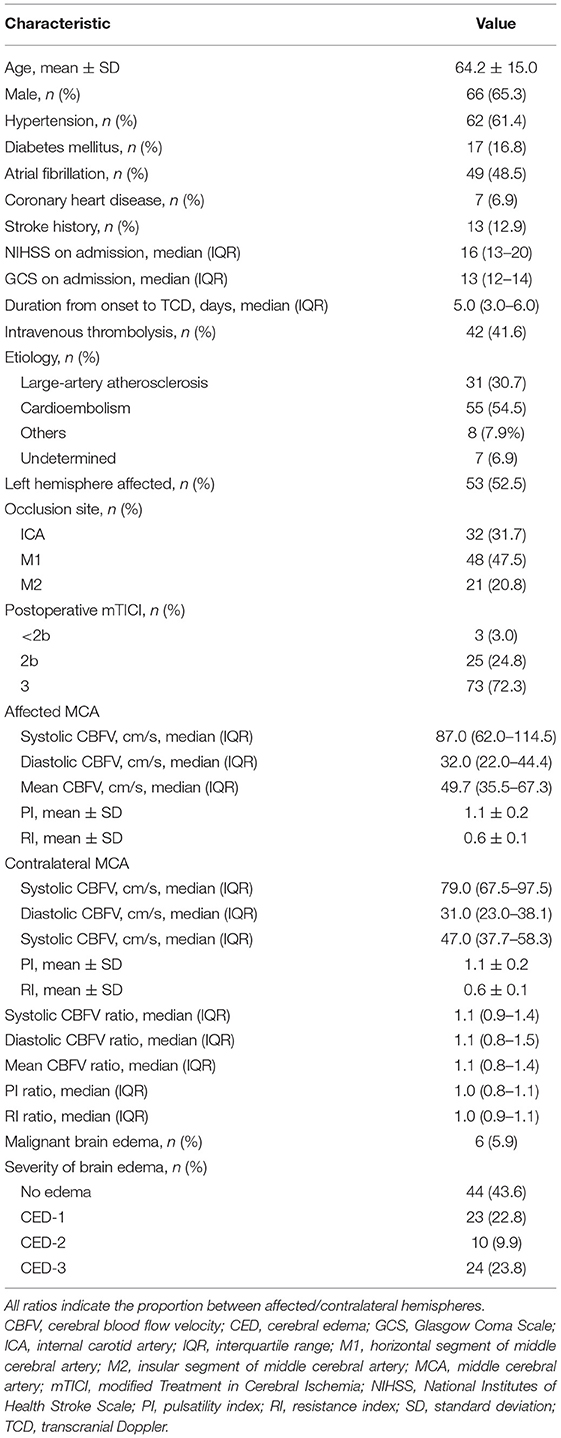
Results: A total of 101 patients (mean age 64.2 years, 65.3% male) were included, of whom 56.3% (57/101) suffered brain edema [grade 1, 23 (22.8%); grade 2, 10 (9.9%); and grade 3, 24 (23.8%)]. Compared to patients with non-severe brain edema, patients with severe brain edema had lower affected/contralateral ratios of systolic CBFV (median 1 vs. 1.2, P = 0.020) and mean CBFV (median 0.9 vs. 1.3, P = 0.029). Multivariate logistic regression showed that severe brain edema was independently associated with affected/contralateral ratios of systolic CBFV [odds ratio (OR) = 0.289, 95% confidence interval (CI): 0.069–0.861, P = 0.028] and mean CBFV (OR = 0.278, 95% CI: 0.084–0.914, P = 0.035) after adjusting for potential confounders.
Conclusion: Post-procedure affected/contralateral ratio of CBFV may be a promising predictor of brain edema severity in patients with AIS who received early EVT.
Introduction
Brain edema is one of the most devastating complications after acute ischemic stroke (AIS) (1). Malignant brain edema (MBE) is the most severe type of brain edema, and it usually occurs following the occlusion of the internal carotid artery (ICA) or the middle cerebral artery (MCA) (2). MBE is characterized by a disastrous clinical course, with a mortality rate reaching 80% in conservatively treated patients (3). Since effective treatment options are limited once MBE occurs, early prediction and identification of patients at risk of severe brain edema is essential to improve prognosis (4).
Endovascular therapy (EVT) is the most effective treatment for patients with large vessel occlusion (5). Recent studies showed that revascularization was associated with a reduced risk of MBE (4) and that EVT was inversely associated with the use of decompressive craniectomy for patients with AIS (6). However, about 20% of patients with large vessel occlusion still developed MBE despite successful recanalization (7, 8). Whether post-procedure factors could guide the early prediction of brain edema severity is still unknown for patients receiving EVT.
Transcranial Doppler (TCD) as a non-invasive approach for measuring cerebral blood flow velocity (CBFV) and estimating increased intracranial pressure at the bedside is widely used in neurocritical care (9, 10). CBFV reflects real-time cerebral hemodynamics in patients with AIS; however, the value of CBFV in predicting brain edema has been poorly studied (11). Therefore, in this study, we aimed to investigate the value of post-procedure CBFV in predicting the severity of brain edema in patients with AIS who received early EVT.
Materials and Methods
Patient Selection
Patients with AIS who were admitted to Zhejiang Provincial People's Hospital (Hangzhou, China) from February 2016 to November 2021 and received early EVT were prospectively included in our study. Stroke was diagnosed according to the World Health Organization criteria, and ischemic stroke was confirmed by brain computed tomography (CT) or magnetic resonance imaging (MRI). We included patients who received EVT for occlusion of the MCA or ICA within 24 h of stroke onset. We excluded patients who had more than one EVT within 1 week of stroke onset, who had no imaging scan or TCD examination within 1 week of EVT, or who underwent post-procedure TCD after the last CT scan.
This study was approved by the Ethics Committee of Zhejiang Provincial People's Hospital. Informed consent was obtained from patients or their relatives.
Clinical Management and Data Collection
Upon admission, a brain non-contrast CT scan was performed for every patient with AIS. Patients who were suspected of large vessel occlusion were further evaluated by CT angiography and CT perfusion scanning (Aquilion/ONE TSX-301A, Toshiba, Tokyo, Japan). We conformed to the latest guidelines for the selection of candidates for early EVT (5, 12). The final decision to perform EVT was made after discussing it with the patient's families. In our center, EVT includes mechanical thrombectomy with a stent retriever, direct aspiration, and angioplasty/stenting. Which procedure to perform is determined by the neuro-interventionalist in charge.
From all patients, we collected demographic information, time of onset, past medical history, and stroke severity on admission. Stroke severity was measured using the National Institutes of Health Stroke Scale (NIHSS) (13) and Glasgow Coma Scale (GCS) (14). The state of post-procedure recanalization was evaluated using the modified Treatment in Cerebral Ischemia (mTICI) score (15). Hemorrhagic transformation was determined using the European Cooperative Acute Stroke Study (ECASS) criteria (16), which comprised hemorrhagic infarction and parenchymal hemorrhage.
Post-procedure CBFV of the MCA was measured using TCD (EMS-9PB, Delica, Shenzhen, China) within seven days of stroke onset. We used a 2-MHz probe to obtain the CBFV of bilateral MCAs through the temporal bone window, with a depth of 45–60 mm (9). Systolic, diastolic, and mean CBFVs were recorded. We performed a brain CT scan immediately after EVT. We strictly maintained post-procedure blood pressure under 140/90 mmHg in all patients receiving EVT. If angioplasty or stenting was performed during EVT, blood pressure was further controlled around 110–120/70–80 mmHg. If the patient met indications for decompressive craniectomy, neurosurgeons were consulted.
Osmotic therapy was prescribed when the radiological sign of space-occupying brain edema was observed. Mannitol and/or glycerol infusion was the most used regimen. The dosage of osmotic therapy was determined by the neurologist in charge. Follow-up brain CT/MRI was scheduled on the first, third, and seventh days after EVT, or in case of neurological worsening.
Outcome Measures
Brain edema was defined as effacement of cortical sulci or the ventricular system due to compression of adjacent brain tissue (17). The severity of brain edema was further assessed using the cerebral edema (CED) grading from the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST) (18), a three-level scale that classifies brain edema into CED-1 (focal brain edema up to one-third of the hemisphere), CED-2 (brain edema greater than one-third of the hemisphere), and CED-3 (brain edema with midline shift, MLS). Typical imaging observations of different severity grades of brain edema are shown in Figure 1.
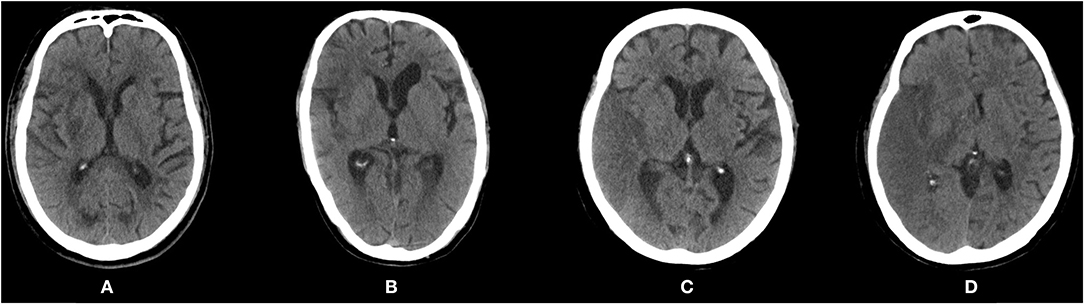
Figure 1. Post-procedure computed tomography scans showing different severity of brain edema according to the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST). (A) CED-0: no edema. (B) CED-1: focal brain edema up to one-third of the hemisphere. (C) CED-2: brain edema greater than one-third of the hemisphere. (D) CED-3: brain edema with midline shift. CED, cerebral edema.
The primary outcome of this study was severe brain edema (SBE), defined as brain edema of grades CED-2 or CED-3. The secondary outcome was MBE, defined as clinical deterioration (decrease in NIHSS ≥ 4 points or decrease in NIHSS item 1a of ≥1 point), together with radiological signs of space-occupying edema (MLS ≥ 5 mm) within seven days of onset (4). MLS was defined as the distance of septum pellucidum displacement at the level of the Foramen of Monro (19).
Statistical Analyses
All statistical analyses were performed using SPSS version 23.0 (IBM, Armonk, NY, USA). We reported the mean ± standard deviation (SD) or median with interquartile range (IQR) for reporting continuous variables, and number with percentage for categorical variables. All ratios presented throughout the article indicate the proportion of the parameter in the affected hemisphere compared with the contralateral hemisphere.
Baseline variables were compared between groups with different severity of brain edema (SBE vs. non-SBE, MBE vs. non-MBE). Analysis of variance (ANOVA) or Mann-Whitney U test were used to compare continuous variables, and χ2 or Fisher's exact test were used to compare categorical variables as appropriate. Multivariate logistic regression was applied to examine the association between CBFV and severity of brain edema after adjustment for potential confounders. Multivariate analysis was conducted using variables that were associated with P < 0.10 in the univariate analysis, as well as variables previously linked to brain edema (4, 20, 21). Consequently, we selected age, NIHSS score, and atrial fibrillation as potential confounders. Correlations between TCD parameters and severity of MLS were assessed using Spearman's correlation coefficients. A two-sided P < 0.05 was considered statistically significant.
Results
Patients' Demographic and Clinical Characteristics
Details of patient inclusion are shown in Figure 2. Five hundred and fifty-nine patients received early EVT from February 2016 to November 2021 in our center. We excluded 92 patients for basilar artery occlusion, 5 patients for anterior cerebral artery occlusion, 250 patients for no post-procedure TCD, 80 patients for no TCD examination within 1 week of EVT, and 21 patients for incomplete data. Finally, a total of 101 patients were included in the analysis; 65.3% (66/101) were male and the mean age was 64.2 years.
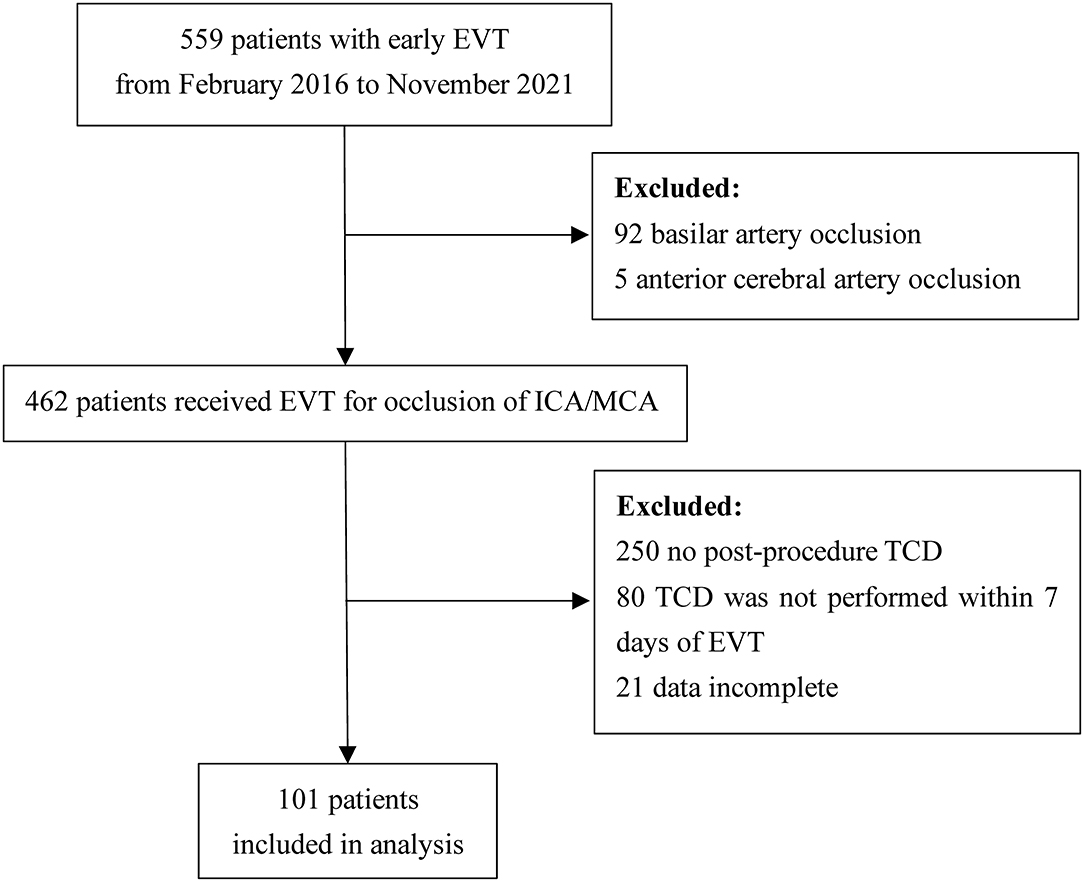
Figure 2. Flow diagram of patient inclusion for the study. EVT, endovascular therapy; ICA, internal carotid artery; MCA, middle cerebral artery; TCD, transcranial Doppler.
The occluded artery of the index stroke was the ICA in 31.7% (32/101) of patients, the M1 segment of MCA in 47.5% (48/101), and the M2 segment of MCA in 20.8% (21/101). The median time from onset to TCD examination was 5 days (IQR: 3–6 days). Median systolic CBFV was 87 cm/s, median diastolic CBFV was 32 cm/s, and mean CBFV of the MCA on the affected side was 49.7 cm/s. The hemorrhagic transformation occurred in 42.6% (43/101) of patients within seven days of onset. Hemorrhagic infarction grade 1 affected 4% (4/101); hemorrhagic infarction grade 2, 19.8% (20/101); parenchymal hemorrhage grade 1, 14.9% (15/101); and parenchymal hemorrhage grade 2, 4% (4/101). Other baseline characteristics are shown in Table 1.
Brain edema was present on follow-up imaging in 56.3% (57/101) of included patients. CED-1 was present in 23 (22.8%) of included patients; CED-2, 10 (9.9%); and CED-3, 24 (23.8%). For patients with CED-3, the median value of MLS was 4 mm (IQR: 3–7 mm). Six patients developed MBE within seven days of stroke onset.
Comparison of CBFV Between Patients Who Are Non-SBE and Patients With SBE
Compared to patients who are non-SBE (CED 0–1), patients with SBE (CED 2–3) had lower ratio of systolic CBFV (median: 1 vs. 1.2, P = 0.020), and lower ratio of mean CBFV (median: 0.9 vs. 1.3, P = 0.029). Similar trends were observed for the ratio of diastolic CBFV, but the intergroup difference did not reach statistical significance. Other baseline characteristics were comparable between the two groups (Table 2).
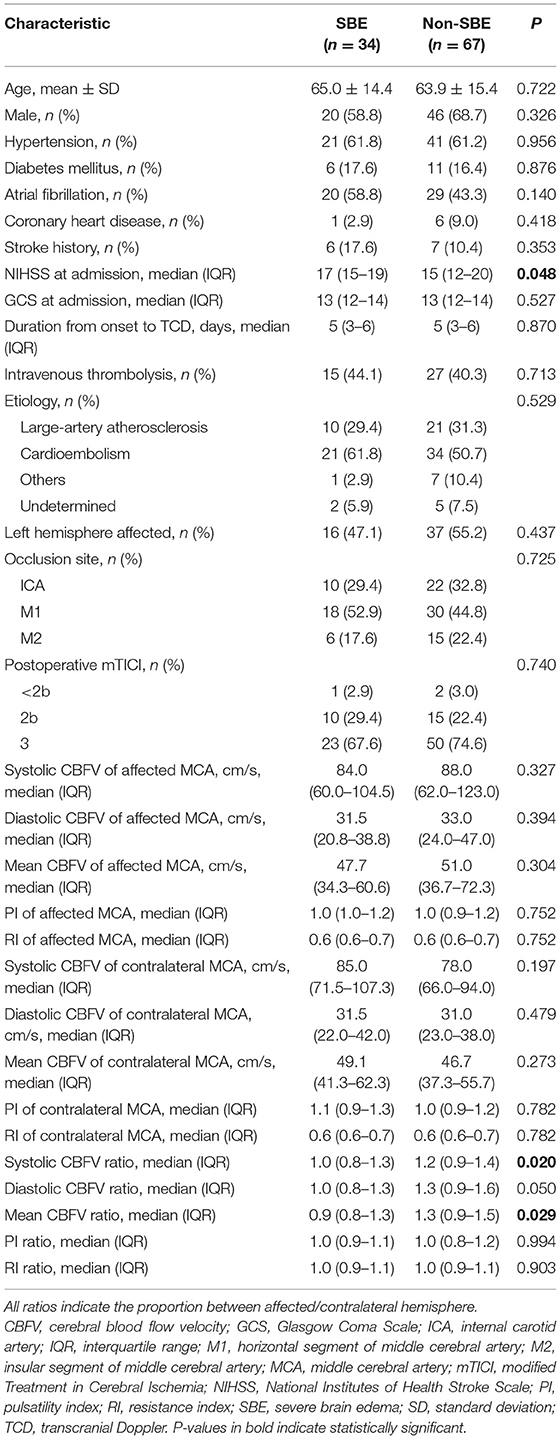
Table 2. Comparison of baseline characteristics between patients with or without severe brain edema (SBE).
After adjusting for potential confounders, including age, admission NIHSS score, and atrial fibrillation, we found increased risk of SBE to be independently associated with lower ratio of systolic CBFV (OR = 0.289, 95% CI: 0.069–0.861, P = 0.028) and lower ratio of mean CBFV (OR = 0.278, 95% CI: 0.084–0.914, P = 0.035) (Table 3). Among patients with MLS (CED-3), severity of MLS correlated with CBFV (systolic: r = −0.467, P = 0.021; diastolic: r = −0.465, P = 0.022; mean:
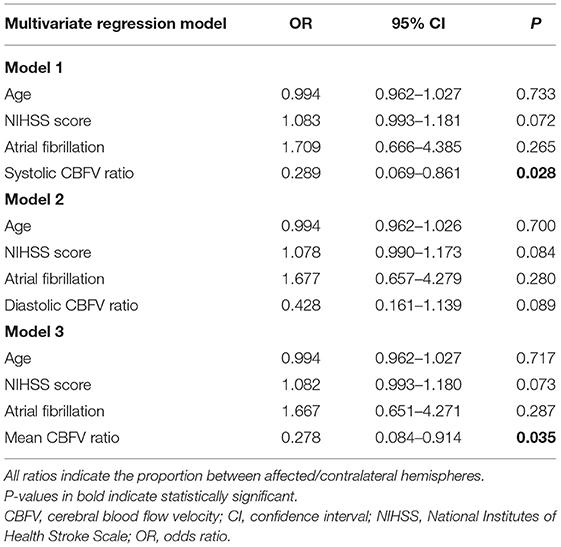
Table 3. Multivariate analysis of the association between the ratio of cerebral blood flow velocity and severe brain edema.
r = −0.481, P = 0.018) in the affected MCA and ratio of diastolic CBFV (r = −0.436, P = 0.033) (Table 4; Figure 3).
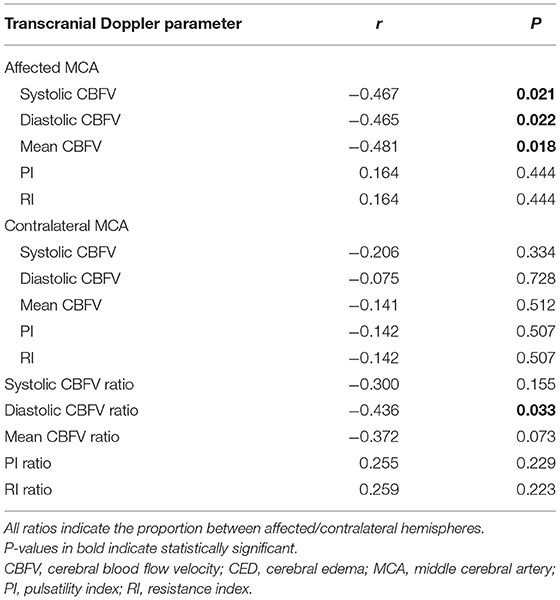
Table 4. Correlation between transcranial Doppler parameters and severity of midline shift in patients with CED-3 (n = 24).
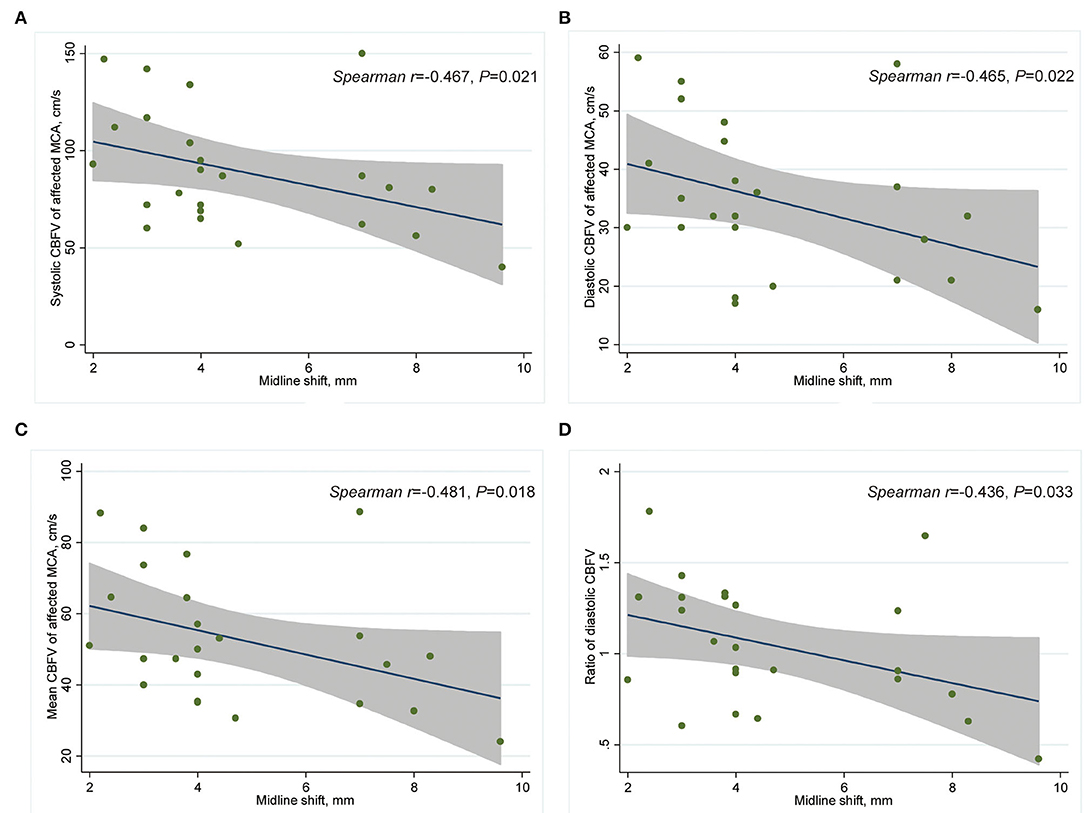
Figure 3. Scatter plot showing correlation between CBFV and MLS in patients with CED-3 (n = 24). (A) Systolic CBFV of the affected MCA and MLS; (B) diastolic CBFV of the affected MCA and MLS; (C) mean CBFV of the affected MCA and MLS; (D) and affected/contralateral ratio of diastolic CBFV and MLS. CBFV, cerebral blood flow velocity; CED, cerebral edema; MCA, middle cerebral artery; MLS, midline shift.
Comparison of CBFV Between Patients Who Are Non-MBE and Patients With MBE
Compared to patients with non-MBE, patients with MBE had a lower ratio of systolic CBFV (0.8 vs. 1.1, P = 0.049) and a lower ratio of mean CBFV (0.8 vs. 1.1, P = 0.041) (Table 5). The multivariate analysis could not be performed due to the small number of patients in the MBE group.
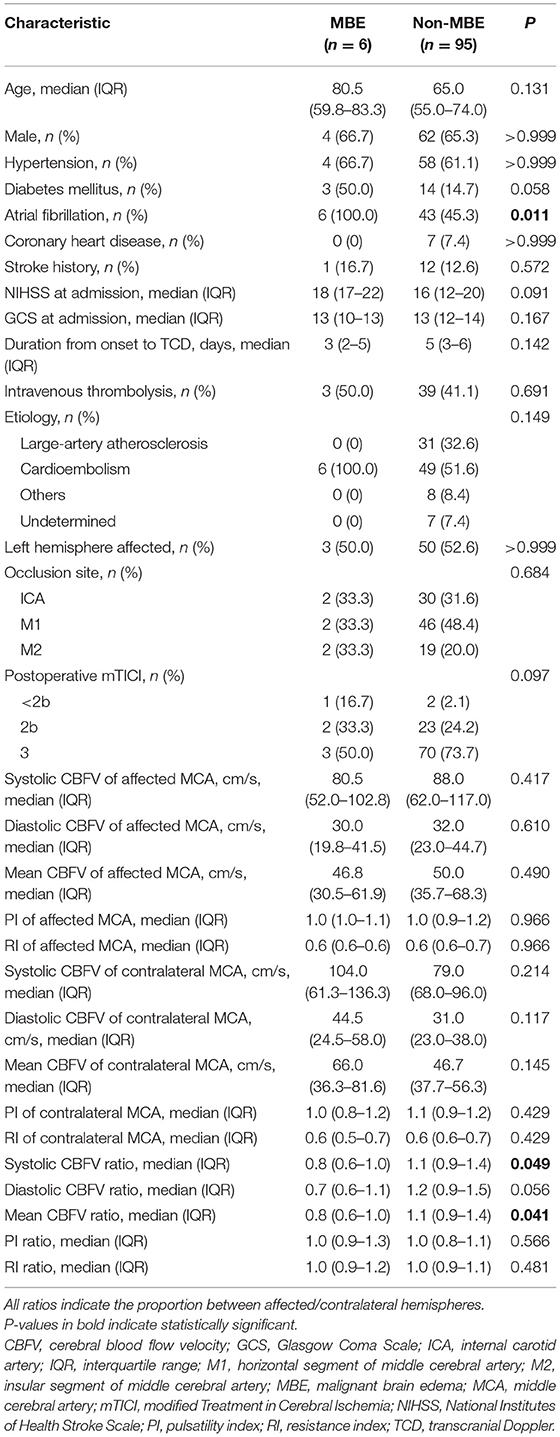
Table 5. Comparison of baseline characteristics between patients with or without malignant brain edema (MBE).
Comparison of CBFV Between Hemorrhagic Transformation Subgroups
Compared to patients with hemorrhagic infarction, patients with parenchymal hemorrhage had a higher ratio of diastolic CBFV (1.3 vs. 0.9, P = 0.043), while values of CBFV were similar between patients with or without hemorrhagic transformation.
Discussion
Patients with AIS with large vessel occlusion are at high risk of developing MBE even after EVT. However, reliable markers for the prediction of post-EVT brain edema severity are lacking. Thus, here, we aimed to investigate the association between post-procedure CBFV measured by TCD and the severity of brain edema to evaluate the prognostic capacity of CBFV. In a Chinese cohort of patients, we found that a lower ratio of affected/contralateral CBFV was independently associated with an increased risk of severe brain edema.
In AIS research, TCD has traditionally been used as a real-time screening tool for recanalization, perfusion state, and microemboli during EVT (22, 23), but studies on the association between TCD parameters and brain edema are limited. One study (24) assessed cerebral autoregulation using CBFV and arterial blood pressure in 46 patients with MCA infarction and found that impaired cerebral autoregulation was associated with brain edema on admission and 24 h after admission. In contrast, in this study, we assessed direct TCD parameters, which are more widely used in clinical practice than analysis of cerebral autoregulation.
In our study, a lower ratio of CBFV in the affected MCA relative to the contralateral MCA was associated with a higher risk of SBE. On the contrary, one study (25) included 185 patients with large vessel occlusion who received EVT, and found that a pathologically high ratio of peak systolic velocity (in the recanalized/contralateral MCA) was associated with 3-month poor outcomes, although the incidence of brain edema was not reported. Notably, the mean peak systolic velocity ratio was highest immediately after EVT and decreased with time in this study (25).
The detrimental effects of high CBFV could be explained by secondary injury due to cerebral hyperperfusion syndrome (26), while hypoperfusion itself is a strong risk factor for ischemia damage (27). Consistent with this, a study on traumatic brain injury showed that deviation of optimal cerebral perfusion pressure in either direction was associated with poor outcomes (28). Besides, contradictory results were observed when CBFV was measured at different times after EVT, suggesting that differences in when TCD was evaluated may be another reason for the observed disparity among studies. In a study of 123 patients with AIS receiving early EVT (29), the elevation of mean blood flow index (recanalized/contralateral MCA) within 24 h of EVT (mean time: 6.6 h) was associated with a higher risk of hemorrhagic transformation and unfavorable outcomes. While another study of 31 patients with AIS receiving EVT (30) reported that acceleration of CBFV within seven days (mean: 3.4 days) of mechanical thrombectomy was not associated with clinical deterioration.
Among TCD parameters, pulsatility index (PI) has been correlated with intracranial pressure in patients with traumatic brain injury (31). One study (32) investigated the association between PI generated from the contralateral MCA and MLS in patients with large MCA infarction. Researchers found that baseline PI values and their increase correlated with MLS on day 3, suggesting that higher PI was an indicator of increased intracranial pressure in AIS (32). In our study, however, PI values were similar between the SBE and non-SBE groups, which indicated that the relative decrease of CBFV in the affected hemisphere was not caused by the edema itself. We speculate that ischemia and mechanical injury to the vascular endothelium may cause dysfunction of cerebral autoregulation, which further aggravates ischemic damage and leads to brain edema (24, 33, 34).
Notably, although 33.7% of included patients in our study had SBE, only six of them developed MBE, which has a more profound influence on clinical outcomes (2). In our previous systematic review, the median time for detection of MBE in existing studies was 6 days (4). Here, the median time from onset to MBE was 5 days (2.8–5.3 days) and the median time from onset to TCD was 2.5 days (1.5–4.8 days) for patients with MBE. In an early post-mortem study (35), the extent of MLS reached its peak about 4 days after onset. A sonographic study detected a gradual increase in MLS during the first 4 days after hemispheric infarction (36). Therefore, CBFV measured by TCD early after EVT may offer valuable information for predicting space-occupying brain edema and subsequent MBE. However, this hypothesis could not be confirmed in the current study due to the small number of MBE cases.
Our study presents several limitations. First, the retrospective design might cause a selection bias, which may mean that some patients with severe edema did not undergo TCD because of their unstable condition. Second, the timing of TCD evaluations was not standardized. On the other hand, these two limitations may mean that our results better reflect actual clinical practice. Measuring CBFV immediately after EVT, and then, dynamically thereafter could help illuminate the natural course of brain edema following EVT and offer more information for clinical decisions. Prospective, multi-center studies with larger samples and quantitative analysis of brain edema are needed (37). A nomogram or grading scale derived from multivariate models is also needed to evaluate the reliability and strength of CBFV in predicting brain edema.
Conclusion
A lower affected/contralateral ratio of CBFV in MCA within seven days of EVT may be associated with an increased risk of severe brain edema in patients with AIS. Post-procedure CBFV measured by TCD may be a predictor of severe brain edema.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Zhejiang Provincial People's Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
HW, JP, and RY carried out the studies, participated in collecting data, and drafted the manuscript. YG and RY performed the statistical analysis and participated in its design. TW participated in the acquisition, analysis, or interpretation of data and drafted the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Projects of Medical and Health Science and Technology in Zhejiang Province of China (2019RC099, 2019KY010, 2021KY509, and 2022KY573) and the Scientific Research Fund of Zhejiang Provincial Education Department (Y201942650).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Balami JS, Chen RL, Grunwald IQ, Buchan AM. Neurological complications of acute ischaemic stroke. Lancet Neurol. (2011) 10:357–71. doi: 10.1016/S1474–4422(10)70313–6
2. Huttner HB, Schwab S. Malignant middle cerebral artery infarction: clinical characteristics, treatment strategies, and future perspectives. Lancet Neurol. (2009) 8:949–58. doi: 10.1016/S1474–4422(09)70224–8
3. Berrouschot J, Sterker M, Bettin S, Koster J, Schneider D. Mortality of space-occupying ('malignant') middle cerebral artery infarction under conservative intensive care. Intensive Care Med. (1998) 24:620–3. doi: 10.1007/s001340050625
4. Wu S, Yuan R, Wang Y, Wei C, Zhang S, Yang X, et al. Early prediction of malignant brain edema after ischemic stroke. Stroke. (2018) 49:2918–27. doi: 10.1161/STROKEAHA.118.022001
5. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
6. Khattar NK, Ugiliweneza B, Fortuny EM, Adams SW, Meyer KS, Sharma M, et al. Inverse national trends in decompressive craniectomy versus endovascular thrombectomy for stroke. World Neurosurg. (2020) 138:e642–51. doi: 10.1016/j.wneu.2020.03.022
7. Wang C, Zhu Q, Cui T, Wang L, Yang T, Hao Z, et al. Early prediction of malignant edema after successful recanalization in patients with acute ischemic stroke. Neurocrit Care. (2021) 36:822–30. doi: 10.1007/s12028–021-01380–4
8. Broocks G, Hanning U, Flottmann F, Schönfeld M, Faizy TD, Sporns P, et al. Clinical benefit of thrombectomy in stroke patients with low ASPECTS is mediated by oedema reduction. Brain. (2019) 142:1399–407. doi: 10.1093/brain/awz057
9. D'Andrea A, Conte M, Cavallaro M, Scarafile R, Riegler L, Cocchia R, et al. Transcranial doppler ultrasonography: from methodology to major clinical applications. World J Cardiol. (2016) 8:383–400. doi: 10.4330/wjc.v8.i7.383
10. Robba C, Cardim D, Sekhon M, Budohoski K, Czosnyka M. Transcranial Doppler: a stethoscope for the brain-neurocritical care use. J Neurosci Res. (2018) 96:720–30. doi: 10.1002/jnr.24148
11. Zhang Z, Pu Y, Mi D, Liu L. Cerebral hemodynamic evaluation after cerebral recanalization therapy for acute ischemic stroke. Front Neurol. (2019) 10:719. doi: 10.3389/fneur.2019.00719
12. Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, Jauch EC, et al. 2015 American Heart Association/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. (2015) 46:3020–35. doi: 10.1161/STR.0000000000000074
13. Brott T, Adams HP, Olinger CP, Marler JR, Barsan WG, Biller J, et al. Measurements of acute cerebral infarction - a clinical examination scale. Stroke. (1989) 20:864–70. doi: 10.1161/01.STR.20.7.864
14. Teasdale G, Jennett B. Assessment of coma and impaired consciousness - practical scale. Lancet. (1974) 2:81–4. doi: 10.1016/S0140–6736(74)91639–0
15. Wintermark M, Albers GW, Broderick JP, Demchuk AM, Fiebach JB, Fiehler J, et al. Acute stroke imaging research roadmap II. Stroke. (2013) 44:2628–39. doi: 10.1161/STROKEAHA.113.002015
16. Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA. (1995) 274:1017–25. doi: 10.1001/jama.274.13.1017
17. Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group Alberta Stroke Programme Early CT Score. Lancet. (2000) 355:1670–4. doi: 10.1016/S0140–6736(00)02237–6
18. Thorén M, Azevedo E, Dawson J, Egido JA, Falcou A, Ford GA, et al. Predictors for cerebral edema in acute ischemic stroke treated with intravenous thrombolysis. Stroke. (2017) 48:2464–71. doi: 10.1161/STROKEAHA.117.018223
19. Liao CC, Chen YF, Xiao F. Brain midline shift measurement and its automation: a review of techniques and algorithms. Int J Biomed Imaging. (2018) 2018:4303161. doi: 10.1155/2018/4303161
20. Sun W, Li G, Song Y, Zhu Z, Yang Z, Chen Y, et al. A web based dynamic MANA Nomogram for predicting the malignant cerebral edema in patients with large hemispheric infarction. BMC Neurol. (2020) 20:360. doi: 10.1186/s12883–020-01935–6
21. Greenland S. Modeling and variable selection in epidemiologic analysis. Am J Public Health. (1989) 79:340–9. doi: 10.2105/AJPH.79.3.340
22. Tsivgoulis G, Ribo M, Rubiera M, Vasdekis SN, Barlinn K, Athanasiadis D, et al. Real-time validation of transcranial Doppler criteria in assessing recanalization during intra-arterial procedures for acute ischemic stroke: an international, multicenter study. Stroke. (2013) 44:394–400. doi: 10.1161/STROKEAHA.112.675074
23. Rubiera M, Cava L, Tsivgoulis G, Patterson DE, Zhao L, Zhang Y, et al. Diagnostic criteria and yield of real-time transcranial Doppler monitoring of intra-arterial reperfusion procedures. Stroke. (2010) 41:695–9. doi: 10.1161/STROKEAHA.109.565762
24. Castro P, Azevedo E, Serrador J, Rocha I, Sorond F. Hemorrhagic transformation and cerebral edema in acute ischemic stroke: Link to cerebral autoregulation. J Neurol Sci. (2017) 372:256–61. doi: 10.1016/j.jns.2016.11.065
25. Baracchini C, Farina F, Palmieri A, Kulyk C, Pieroni A, Viaro F, et al. Early hemodynamic predictors of good outcome and reperfusion injury after endovascular treatment. Neurology. (2019) 92:e2774–83. doi: 10.1212/WNL.0000000000007646
26. van Mook WN, Rennenberg RJ, Schurink GW, van Oostenbrugge RJ, Mess WH, Hofman PA, et al. Cerebral hyperperfusion syndrome. Lancet Neurol. (2005) 4:877–88. doi: 10.1016/S1474–4422(05)70251–9
27. Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol. (1998) 55:1475–82. doi: 10.1001/archneur.55.11.1475
28. Aries MJ, Czosnyka M, Budohoski KP, Steiner LA, Lavinio A, Kolias AG, et al. Continuous determination of optimal cerebral perfusion pressure in traumatic brain injury. Crit Care Med. (2012) 40:2456–63. doi: 10.1097/CCM.0b013e3182514eb6
29. Kneihsl M, Niederkorn K, Deutschmann H, Enzinger C, Poltrum B, Fischer R, et al. Increased middle cerebral artery mean blood flow velocity index after stroke thrombectomy indicates increased risk for intracranial hemorrhage. J Neurointerv Surg. (2018) 10:882–7. doi: 10.1136/neurintsurg-2017–013617
30. Perren F, Kargiotis O, Pignat JM, Pereira VM. Hemodynamic changes may indicate vessel wall injury after stent retrieval thrombectomy for acute stroke. J Neuroimaging. (2018) 28:412–5. doi: 10.1111/jon.12513
31. Moreno JA, Mesalles E, Gener J, Tomasa A, Ley A, Roca J, et al. Evaluating the outcome of severe head injury with transcranial Doppler ultrasonography. Neurosurg Focus. (2000) 8:e8. doi: 10.3171/foc.2000.8.1.1702
32. Asil T, Uzunca I, Utku U, Berberoglu U. Monitoring of increased intracranial pressure resulting from cerebral edema with transcranial Doppler sonography in patients with middle cerebral artery infarction. J Ultrasound Med. (2003) 22:1049–53. doi: 10.7863/jum.2003.22.10.1049
33. Guo ZN, Shao A, Tong LS, Sun W, Liu J, Yang Y, et al. The role of nitric oxide and sympathetic control in cerebral autoregulation in the setting of subarachnoid hemorrhage and traumatic brain injury. Mol Neurobiol. (2016) 53:3606–15. doi: 10.1007/s12035–015-9308-x
34. Dohmen C, Bosche B, Graf R, Reithmeier T, Ernestus RI, Brinker G, et al. Identification and clinical impact of impaired cerebrovascular autoregulation in patients with malignant middle cerebral artery infarction. Stroke. (2007) 38:56–61. doi: 10.1161/01.STR.0000251642.18522.b6
35. Shaw CM, Alvord EC, Berry RG. Swelling of the brain following ischemic infarction with arterial occlusion. Arch Neurol. (1959) 1:161–77. doi: 10.1001/archneur.1959.03840020035006
36. Gerriets T, Stolz E, Modrau B, Fiss I, Seidel G, Kaps M, et al. Sonographic monitoring of midline shift in hemispheric infarctions. Neurology. (1999) 52:45–9. doi: 10.1212/WNL.52.1.45
Keywords: ischemic stroke, endovascular therapy, edema, cerebral blood flow velocity, transcranial Doppler
Citation: Pan J, Wu H, Wu T, Geng Y and Yuan R (2022) Association Between Post-procedure Cerebral Blood Flow Velocity and Severity of Brain Edema in Acute Ischemic Stroke With Early Endovascular Therapy. Front. Neurol. 13:906377. doi: 10.3389/fneur.2022.906377
Received: 28 March 2022; Accepted: 17 June 2022;
Published: 18 July 2022.
Edited by:
Peiyu Huang, Zhejiang University, ChinaReviewed by:
Haihui Jiang, Peking University Third Hospital, ChinaTu Wei, Second Affiliated Hospital of Nanchang University, China
Copyright © 2022 Pan, Wu, Wu, Geng and Yuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruozhen Yuan, eXVhbnJ6ODIyQHNpbmEuY29t; Yu Geng, Z2VuZ3kyMDA0QDEyNi5jb20=
†These authors have contributed equally to this work
 Jie Pan
Jie Pan Huadong Wu
Huadong Wu Tingting Wu
Tingting Wu Yu Geng
Yu Geng Ruozhen Yuan
Ruozhen Yuan