
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol., 03 May 2022
Sec. Neurological Biomarkers
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.895316
This article is part of the Research TopicPotential Biomarkers in Neurovascular DisordersView all 50 articles
Vascular cognitive impairment and dementia (VCID) is a neurodegenerative disease that is recognized as the second leading cause of dementia after Alzheimer's disease (AD). The underlying pathological mechanism of VCID include crebromicrovascular dysfunction, blood-brain barrier (BBB) disruption, neuroinflammation, capillary rarefaction, and microhemorrhages, etc. Despite the high incidence of VCID, no effective therapies are currently available for preventing or delaying its progression. Recently, pathophysiological microRNAs (miRNAs) in VCID have shown promise as novel diagnostic biomarkers and therapeutic targets. Studies have revealed that miRNAs can regulate the function of the BBB, affect apoptosis and oxidative stress (OS) in the central nervous system, and modulate neuroinflammation and neurodifferentiation. Thus, this review summarizes recent findings on VCID and miRNAs, focusing on their correlation and contribution to the development of VCID pathology.
Vascular cognitive impairment and dementia (VCID) is a term that ranges from mild cognitive impairment (MCI) to vascular dementia (VaD) and encompasses a continuum of cognitive disorders with cerebrovascular pathology (1). With an increase in the human lifespan, the number of individuals over the age of 60 in 2,050 is projected to be 2 billion (2), which will increase the demand for research on neurodegenerative diseases, especially those associated with aging. Age-related cerebrovascular factors are becoming recognized as a hallmark of VCID; thus, the number of patients affected by VCID is expected to exponentially increase in the upcoming decades (3) and be responsible for approximately 30% of the aging population living with dementia in Asia and developing countries (4).
VCID and dementia is a subtype of dementia affected by multiple factors, such as low educational attainment, female sex, stroke, and lifestyle (5). Current theories of the pathologies of VCID include cerebromicrovascular dysfunction, blood–brain barrier (BBB) disruption, neuroinflammation, capillary rarefaction, and microhemorrhages (3). However, none of the available pharmacological or preventative treatments decrease the development and progression of VCID. Improving our understanding of microRNAs (miRNAs) has provided strong epidemiological and experimental evidence that miRNAs are crucial for neuronal differentiation, survival, and activity (6). By inhibiting messenger RNA (mRNA) translation or promoting its degradation, miRNAs can regulate the expression levels of proteins and ultimately affect disease progression (7, 8). In the central nervous system (CNS), miRNAs can modulate the tight junctions of the BBB, affect the proliferation and differentiation of neuronal cells, and participate in the occurrence and development of neuroinflammation and oxidative stress (OS), resulting in neurodegenerative diseases and cognitive impairment and indicating that miRNAs have a more extensive influence on various brain diseases.
Biomarkers found in the CNS are typically present at relatively low concentrations in the BBB. The miRNAs are mainly transported within liposomes, high-density lipoproteins (HDLs), exosomes, and proteins that can protect them from degradation (9). Therefore, miRNAs are relatively stable compared to other biomarkers, and it is easy to monitor changes in their expression. Compared with Alzheimer's disease (AD), VCID is preventable and curable, suggesting that advanced diagnoses and reliable risk assessments will provide patients with more promising clinical outcomes. This review provides an up-to-date assessment of the role of miRNAs in VCID, from the series of depression of the BBB, apoptosis and OS, neuroinflammation, and neurodifferentiation (Figure 1).
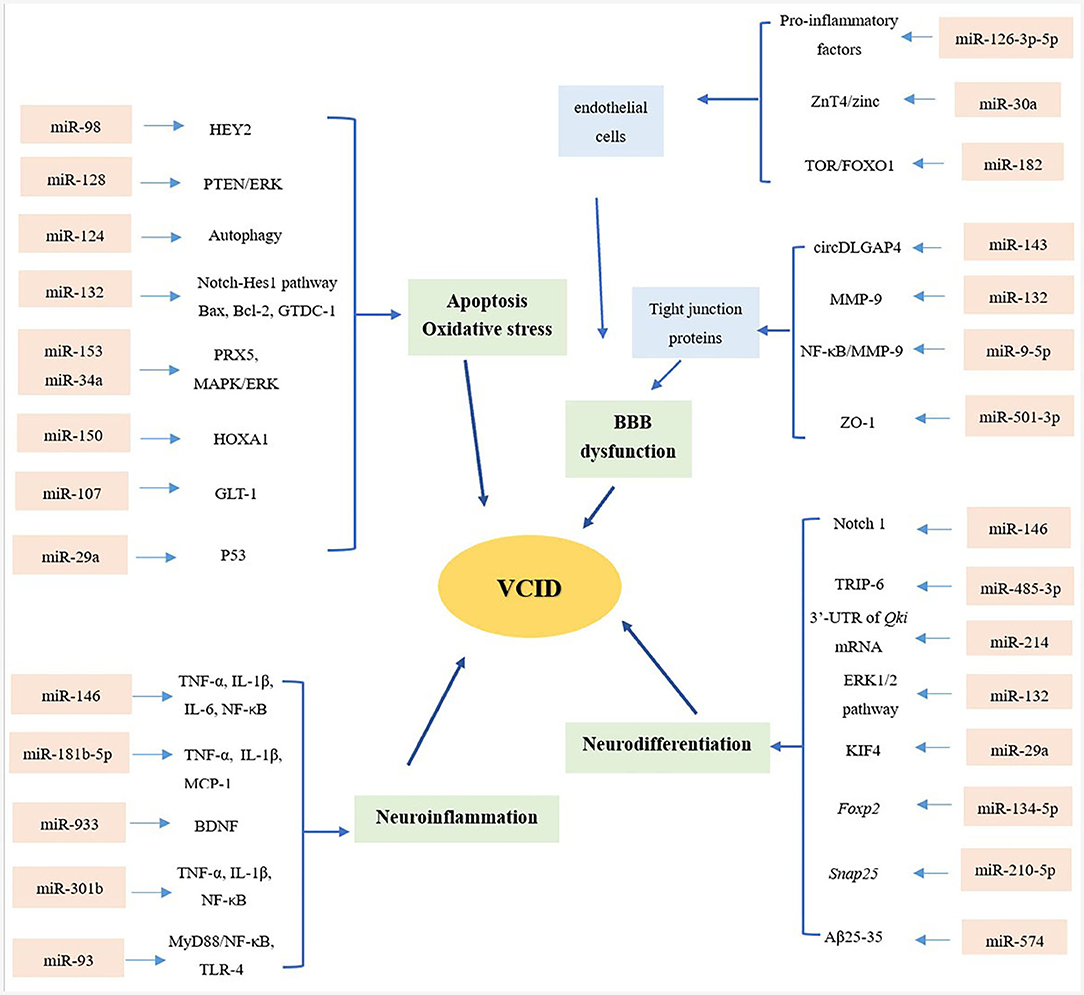
Figure 1. Overview of miRNAs and mechanisms of VCID. From the aspects of blood–brain barrier (BBB) dysfunction, apoptosis and oxidative stress, neuroinflammation and neurodifferentiation, microRNAs (miRNAs) playing multiple roles in the pathology of vascular cognitive impairment and dementia (VCID), providing a novel sight and promising targets in the diagnosis and treatment of VCID.
miRNAs are small non-coding RNAs of approximately 18–21 nucleotides that regulate gene expression by targeting mRNAs via cleavage and translational repression (10). The biogenesis of miRNAs starts with pri-miRNAs, which are transcribed by RNA polymerase II and converted into mature miRNA by RNase III enzymes such as Drosha and Dicer (11). Mature miRNAs leave the nucleus and are then combined with the Argonaute protein (AGO) or targeted mRNAs. It is understood that miRNAs can participate in and regulate molecular expression by interacting with 3′ untranslated regions (3′-UTRs) to post-transcriptionally affect the expression of target mRNA (11). Complete or partial function of miRNAs is also based on 3′-UTRs of target mRNAs (12). Several studies have shown that miRNAs may serve as novel therapeutic agents. While a single miRNA could target hundreds of mRNAs and influence the expression of genes involved in a functional interacting pathway (13), synergetic miRNAs share a common transcriptional regulatory mechanism on the levels of sequence, secondary structure, and transcriptional regulation (12). Depending on the construction of these miRNA–mRNA functional synergetic networks (MFSNs), the central role of these synergetic miRNAs can be analyzed in complex diseases (3). To further apply miRNAs in disease diagnosis and therapy, considerable research has been conducted to characterize their pathophysiological function.
The ability of miRNAs to target multiple genes within the signaling pathway makes them a promising target for modulation, and powerful regulators of cellular activities, such as cell differentiation, development, and apoptosis (14); synaptic homeostasis and plasticity processes (15); and angiogenesis in endothelial cells (ECs) (16) in CNS. In CNS, functional neurons are specialized and persistently renew the information required for constant neuronal adaptation to environmental clues (17), and the value of miRNAs has been recognized, especially for neural cells. As non-protein-coding molecules, miRNAs could modulate the function of tight junction proteins, ECs, astrocytes, and pericytes of BBB (18, 19), which help maintain the microenvironment of neural cells. To protect neural cells from apoptosis and OS, miRNAs can regulate the signaling pathway involved in modulating the proliferation or differentiation of cells (7, 20). Using public databases, such as the Gene Ontology (GO) annotation and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses, the principles of miRNA–mRNA interactions in signaling pathways were further characterized and provide a novel insight into the mechanisms of disease progression (21).
As the most important protector of neurons in the brain, BBB exists between the blood microcirculation system and the brain parenchyma (19) to restrict the invasion of toxic substances, immune cells, and pathogens, playing an irreplaceable role in maintaining CNS homeostasis and proper function (22). Tight junctions constructed by ECs form the basic BBB structure, along with the basement membrane (BM), astrocytes, and pericytes, that contribute to the support and regulatory function of the BBB (23). However, the structural and functional integrity of the BBB is easily degraded by neuroinflammation, age-related degeneration, and other risk factors, which contribute to the onset and progression of cerebrovascular changes and neurodegenerative pathologies (24, 25).
Scientists have made great efforts to understand the molecular structure and pathological changes in the BBB, and have found that, compared with other cytokines, exosomes enriched with proteins and miRNAs can be easily released into the extracellular space, with functions ranging from blood coagulation to cell-to-cell communication (26). Ischemia is one of the most pathological processes of VCID that results in BBB dysfunction via inflammation or OS under the regulation of miRNAs and other factors (27, 28). A recent study showed that circular RNA DLGAP4 (circDLGAP4), which is an endogenous sponge of miR-143, via regulating the tight-junction protein and mesenchymal cell marker to inhibit the endothelial–mesenchymal transition, circDLGAP4 can significantly attenuate infarct areas and BBB damage, and is proposed to partially decrease the incidence of dementia (29). The miR-132 could target matrix metalloproteinase 9 (MMP-9) and dysregulate its expression, acting protection role in reducing the degradation of tight-junction proteins VE-cadherin and β-catenin to maintain the integrity of BBB (30). In addition, miR-9-5p can also mitigate BBB damage by activating the Hedgehog pathway and inhibiting the nuclear factor (NF)-κB/MMP-9 pathway (31). As mentioned earlier, ECs form the basic structure of the BBB. Of note, miR-126 participates in the maintenance of BBB integrity by regulating the functional status of ECs and attenuating BBB disruption by suppressing pro-inflammatory cytokines (32). It has been certificated that both in vivo and in vitro, miR-98 and miR-126-3p/-5p could significantly reduce the brain infract volume and improved behavioral outcomes (27, 32). Moreover, tumor necrosis factor alpha (TNF-α) modulates cerebral tight junctions and affects the BBB via the regulation of laudin-5 and tight-junction protein 1 (ZO-1) through the TNF-α-miR-501-3p–ZO-1 axis, resulting in working memory deficits and white matter lesions (33). In addition, it was found that overexpression of miR-Let7A does not only prevent brain endothelial (bEnd.3) cell death and inhibit pro-inflammatory responses but also protects tight-junction proteins from degradation under high-glucose conditions, indicating that miR-Let7A may be a novel solution for controlling BBB degradation, especially in patients with concomitant diabetes mellitus (DM) (34). Furthermore, exact mechanisms of how miRNAs affect the BBB integrity and ECs function have also been carried out, and miR-30a and miR-182 could modulate BBB permeability, tight-junction protein loss, and ECs apoptosis via ZnT4/zinc signaling pathway and mTOR/FOXO1 pathway, respectively (35, 36). Further study of complex physiological processes between miRNAs and BBB, and how to apply these mechanisms in clinic treatment are warranted.
Abnormal apoptosis is a prerequisite for endothelial and neuronal cell damage, resulting in the onset and progression of VCID (37). Additionally, synergistic and additive interactions between apoptosis and other signaling pathways add to the symptoms of VCID. Dementia caused by multiple infarcts implies that if strokes are prevented, so is the VCID that results from cerebral infarcts (38). Identifying pathways that predominantly include miRNAs during apoptosis will contribute to a better understanding of the functional overlap across diseases. As one of the most abundant miRNAs in brain tissue, miR-124a can be transported into astrocytes through neuronal exosomes, significantly increasing the expression of protein excitatory amino acid transporter 2 (EAAT2, rodent analog GLT1) and modulating synaptic activation (Figure 2) (39). However, the exact role of miR-124 has not been systematically elucidated. In age-related ischemic encephalopathy (IE), miR-124 can improve the effects of cerebral ischemic reperfusion injury (CIRI) by regulating OS, autophagy, and neuroinflammation but plays a negative role in synaptic plasticity and axonal growth via apoptosis (40). A greater understanding of miR-124 will open new avenues for further intervention in VCID. Increasing evidence suggests that cognitive decline in the early stages of neurodegenerative diseases, such as VCID, is a consequence of changes in synaptic structure and function (15). Based on analyses using online tools and a luciferase reporter assay, miR-132 was shown to protect acetylcholine (Ach) from degradation by most fast enzymes like acetylcholinesterase (ACHE) found in the body, and that miR-132 could also exert favorable effects on CNS neurons via brain-derived neurotrophic factors (BDNF) (41). In contrast, another report showed that miR-132 negatively regulates neural stem cell (NSC) proliferation by affecting the cell cycle and apoptosis through the Notch-Hes1 pathway, Bax, Bcl-2, and glycosyl transferase-like domain containing−1 (GTDC-1), which could induce neural cell apoptosis and tau phosphorylation (Figure 2) (20, 42). Due to the complexity of miRNAs, the key miRNA in these types of pathways and the construction of a shared-miRNA network is imperative. Previous studies have investigated the implications of α-synuclein (α-Syn), especially in inducing mitochondrial fragmentation, OS, and autophagy, which could promote neuronal cell death after stroke. It has been reported that treatment with miR-7 mimics greatly reduces the post-ischemic induction of α-Syn, significantly decreases the lesion volume, and improves motor and cognitive functional recovery (43). In addition, miR-153 and miR-34a have confirmed roles in protecting neural cells from death and apoptosis by upregulating peroxiredoxin 5 (PRX5) and MAPK/ERK signaling pathways, respectively, reducing the cell cycle and leading to a reduction in cell proliferation (44, 45), which could ultimately mitigate the pathology of dementia. Moreover, compared with the miR-150 mimic negative group, miR-150 overexpression significantly aggravated cell apoptosis by inhibiting the expression of homeobox (HOX) A1, which aggravates hippocampal neuronal apoptosis and cognitive impairment (46).
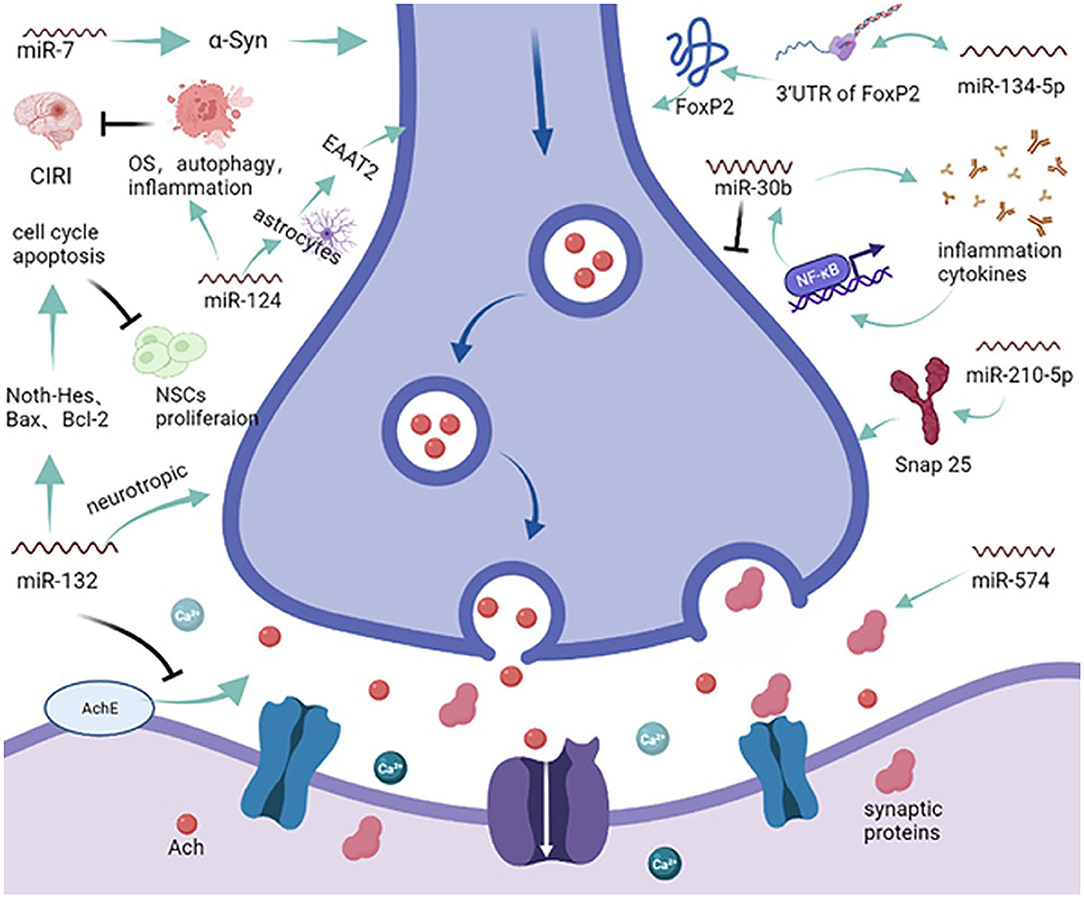
Figure 2. miRNAs and the synapse. MicroRNAs enveloped in exosomes can be released to target acetylcholine (Ach) and synaptic proteins, affecting the transmission of information at the synapse. miR-124 can modulate synaptic activation via the excitatory amino acid transporter 2 (EAAT2) and dysregulate oxidative stress autophagy to reduce cerebral ischemia reperfusion injury. Furthermore, miR-132 can modulate the degradation of Ach and dysregulate the proliferation of neural stem cells. On the other hand, miRNAs can also modulate synaptic neurodifferentiation via Snap 25, FxP2, and many other inflammatory cytokines.
In addition to apoptosis, OS is also considered as one of the significant events in the pathological cascade of dementia, causing mitochondrial dysfunction and protein misfolding (47). Many studies in this field have shown that there is a mutual correlation between OS and miRNAs, and OS affects the expression of miRNAs, which have a counteracting effect on genes involved in OS (6). In the CNS, ECs play an irreplaceable role in protecting the cerebrovascular system from conditions, such as OS erosion, inflammation, and diabetes; however, OS has been predicted to predispose neurons to death in both direct and indirect ways (6, 48). Attenuating OS responsiveness could specifically limit the risk factors of cerebrovascular disease and improve endothelial homeostasis in vascular depression and VCID (49). The miR-107c and miR-29a could target glutamate transporter-1 (GLT-1) to modulate excitotoxicity in the CNS; miR-107c could target GLT-1 directly to evaluate the glutamate accumulation and neuronal excitotoxicity, whereas miR-29a mainly acts on p53 upregulated modulator of apoptosis (PUMA), which could attenuate OS and protect neurons from dementia caused by ischemic injury (50). The miR-128 is enriched in the CNS and easily detected in circulating lymphocytes and can inhibit neuronal damage caused by oxygen and glucose deprivation/reoxygenation (OGD/R) via the PTEN and ERK pathways (51). The correlation between miR-124 level and lesion size on CT indicated that miR-124 could be released from damaged brain tissue in patients who died within 3 months after suffering from a stroke (52). In contrast, by binding to the regulatory factor X1 (RFX1) mRNA, miR-124 could increase the expression of RFX1, resulting in the suppression of apolipoprotein E (APOE) and cellular amyloid beta (Aβ) in microglia, which could undermine the cognitive behavior of dementia (53). Another area of research in this field tracked the expression level of miRNAs in 45 patients with MCI and AD; notably, miR-146a and miR-181a were significantly upregulated in patients with MCI who later converted to AD, which was related to Aβ and APOE ε4 allele presence (54). This indicates that miRNAs can also be an indicator of disease progression, providing an insight in disease prediction. Furthermore, according to the measurement of OS-related proteins, superoxide dismutase and Na+, K+, and ATP, Chen et al. found that miR-98 could bind to the enhancer of split (Hes) related with the YRPW motif protein 2 (HEY2) to inhibit the production of Aβ and improve OS and mitochondrial dysfunction, providing a novel basis for targeted therapy for dementia (55).
Dynamic changes in miRNAs regulate the expression of genes involved in cognitive processes such as learning and executive abilities. Although the pathophysiology of VCID remains largely unknown, considerable efforts have been focused on neuroinflammation. Neuroinflammation is a hallmark of many neurological disorders, and pro-inflammatory or anti-inflammatory miRNAs within CNS signaling pathways can greatly aggravate or mitigate the pathological consequences of neurodegenerative diseases (56, 57). It has been reported that anti-inflammatory miRNAs (miR-21, miR-124, and miR-146a) and pro-inflammatory miRNAs (miR-27b, miR-155, and miR-326) regulate neuroinflammation by down or upregulating endogenous levels of immune receptors such as toll-like receptors (TLRs) or misfolded proteins that accumulate in the extracellular space (58). Related experiments conducted in BV-2 microglial cells and mice showed that miR-146 could suppress the release of pro-inflammatory factors [TNF-α, interleukin (IL)-1β, and IL-6] and the expression of mRNA in targeted cells; the upregulation of miR-146 could not only suppress the NF-κB pathway and microglial activation in the hippocampus but also promote hippocampus-dependent learning and memory capability (59). In addition to miR-146, mouse model and bioinformatics studies confirmed that miR-181b-5p can repress the expression of pro-inflammatory mediators such as TNF-α, IL-1β, and monocyte chemoattractant protein (MCP)-1. Injection of an miR-181b-5p mimic into the hippocampus of mice significantly improved cognition (60). Moreover, middle cerebral artery occlusion (MCAO)-induced behavioral disability and microglial activation in the brain were greatly improved and inhibited by miRNA-210-LNA (miR-210 inhibitor) post-treatment, providing new insight into the molecular basis of a novel therapeutic strategy (61). As the cholesterol metabolite, 27-hydroxycholesterol (27-OHC), induces discrete or directional inflammatory factors in microvascular endothelial cells (human microvascular endothelial cells, HMVECs) and increases the expression of miR-933 and inflammatory cytokines, which are elevated in plasma from dementia patients, more than that, via facilitates permeability and directional secretion from ECs into the brain, miR-933 may act as a paracrine inhibitor of neuronal BDNF, which provides a useful neuroprotective properties (62). These results show how miRNA functions in neuroinflammation and VCID progression, providing a novel insight on possible VCID interventions.
Before VCID occurs, a sustained pro-inflammatory environment brought about by NF-κB leads to chronic reactive astrogliosis, undermining the white matter (WM) (63). By interacting with NF erythroid 2-related factor 2 (Nrf2), NF-κB can fine-tune the cellular oxidative and inflammatory balance and participate in multiple pathologies in VCID, such as restoration of endothelial function and neurovascular coupling, reduction of amyloidopathy, and protection of WM integrity (64). To further implement miRNAs in disease mechanisms, counter intervention between miRNAs and NF-κB is ongoing. With advances in understanding that acupuncture can alleviate cognitive degeneration in VCID, in rats treated with acupuncture, TLR-4 was greatly dysregulated, accompanied by a decrease in miR-93 and MyD88/NF-κB signaling pathway activation (65). Furthermore, miR-301b accelerated cognitive impairment in mice with depression-like behavior; overexpression of miR-301b activated the NF-κB signaling pathway and aggravated inflammation in hippocampus, which accompanied the release of TNF-α, IL-1β, and many other cytokines (66).
Vascular cognitive impairment and dementia typically refers to patients with both stroke and cognitive impairment. Recent studies have highlighted the roles of cerebrovascular injury, white matter tract integrity, microinfarcts, and secondary neurodegeneration in the development of VCID. The miRNAs modulate multiple biological functions, such as cell fate determination and differentiation. It has been found that the onset of cognitive impairment is accompanied by the senescence, loss, and neurogenesis decline of hippocampal neural stem cells (H-NSCs). Interestingly, embryonic stem cell-derived small extracellular vesicles (ESC-sEVs) can alleviate senescence and recover the compromised proliferation and differentiation capacity of H-NSCs via miR-17-5p, miR-18a-5p, miR-21-5p, and miR-29a-3p, which can inhibit the mammalian target of rapamycin complex 1 (mTORC1) activation (67).
Over the past 20 years, stem cell technology has become an increasingly helpful in the investigation and treatment of neurodegenerative diseases. NSCs participate in brain homeostasis and repair and show pleiotropic intrinsic properties, making them a promising candidate for the treatment of dementia (Figure 3) (68). Indeed, Notch signaling encodes a highly conserved cell-surface receptor that affects cell processes such as cell differentiation, cell apoptosis, and cell proliferation. In serum-free medium, miR-146 significantly promoted NSC proliferation by targeting the Notch 1 pathway but reduced the differentiation efficiency of glial cells (69). However, miR-485-3p has the complete opposite effect; miR-485-3p can reduce proliferation but can promote the differentiation of NSCs by decreasing TRIP-6 activity (70). Following the criteria and moderated t-statistics, miR-10a-5p was shown to attenuate the self-renewal of undifferentiated NSCs; however, similar to miR-574, miR-30c-5p, miR23-3p, miR130a-3p, and miR-17-5p miRNA families were predicted to decrease the expression of several genes associated with the differentiation of neurons, synapse formation, and neurite outgrowth (71). The miR-214 not only affects the differentiation of NSCs but also plays a key role that helps maintain the balance between proliferation and differentiation by binding the 3′-UTR of Qki mRNA, affecting downstream its signal transmission (72). Additionally, miR-132 is significantly overexpressed in differentiating NSCs and is accompanied by the activation of the ERK1/2 pathway, and it promotes glial cell differentiation via Mecp2 expression (20). During the process of neurodifferentiation, the level of miR-29a in plasma shows a time-dependent increase similar to that of Kruppel-like factor 4 (KlF4), which suggests that the regulation of miR-29a occurs through the KlF4 signaling pathway (73). This mechanism suggests that KIF4 may be used to promote miR-29a and provides a novel possibility for the treatment of VCID (73).
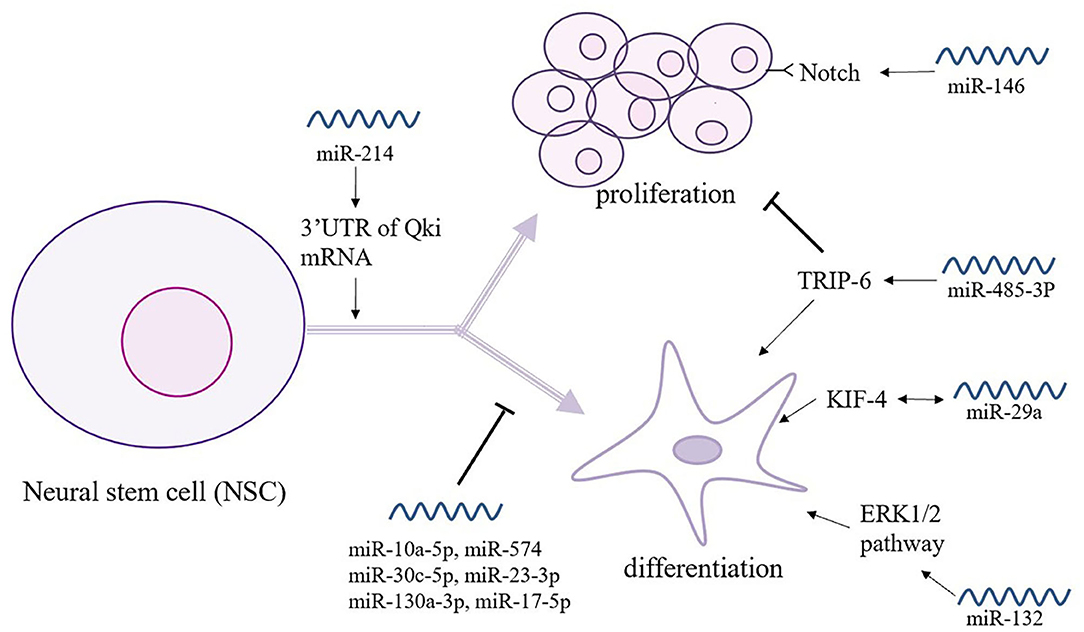
Figure 3. The relationship between miRNAs and neural stem cells (NSCs). NSCs play a key role in brain homeostasis and repair and show intrinsic pleiotropic properties. Thus, the intimate connections between NSCs and miRNAs are under study. As shown here, miR-146 and miR-585-3p play an opposite role in the proliferation and differentiation of NSCs. Moreover, a great number of miRNAs participates in the self-renewal and gene expression of NSCs.
A widely accepted theory is that memory deficiency associated with cognitive impairment results from synaptic dysfunction. Another focus of research in this field is the investigation of the correlation between miRNAs and synaptic proteins (Figure 2). It has been found that miR-134-5p can act on Forkhead box P2 (Foxp2) mRNA to affect its level of expression; however, silencing Foxp2 minimizes the effect of miR-134-5p on synaptic protein loss, which may prevent the development of cognitive impairment, especially in vocal learning (74). In addition, by comparing the expression level of miR-30b in APP transgenic (TG) and wild-type (WT) mice, miR-30b was significantly upregulated in TG mice, causing synaptic and cognitive dysfunction (75). Indeed, miR-30b is triggered by pro-inflammatory cytokines through NF-κB signaling, suggesting a feedback loop in the process of dementia. More specifically, real-time reverse transcription polymerase chain reaction (RT-PCR) revealed that overexpression of miR-210-5p can decrease the number of synapses in primary hippocampal neurons by targeting synaptosomal-associated protein, 25 kDa (Snap25) (76). In experimental trials that used APP/PS1 mice and WT mice, miR-574 could lower neuritin and synaptic protein expression in primary hippocampal neurons via targeting Aβ25-35, resulting aggravation of cognitive dysfunction in APP/PS1 mice compared with WT mice (77).
As the second most common cause of cognitive impairment, patients with VCID will be up to 150 million in 2,050. VCID has drawn the attention of researchers because it is preventable and curable; however, some neuroprotective agents have been reported to attenuate but not cure symptoms. Risk factors of VCID including protective factors, such as higher education, occupation and social networks, and others, increase the risk of dementia (38). According to the National Institute for Neurological Disorders and Stroke-Association International pour la Recherché et l'Enseignement en Neurosciences (NINDS-AIREN), the basic features of VCID include (1) acute impairment of memory and at least two other cognitive domains, (2) neuroimaging evidence of cerebrovascular lesions, and (3) evidence for a temporal relationship between stroke and cognitive loss (78). In recent years, miRNAs are regarded as cost-effective and non-invasive biomarkers in disease diagnosis and therapy response monitoring (79). Additionally, miRNAs are easily detected in biofluids like plasma and cerebrospinal fluid (CSF) due to their unique biological characteristics (15), which can provide biological and clinical breakthroughs.
To gain a better understanding of how miRNAs could support both diagnosis and therapy, studies of clinical patients and biological experiments have been conducted. Validation studies revealed that four miRNAs (miR-409-3p, miR-502-3p, miR-486-5p, and miR-451a) are potentially valuable biomarkers for identifying VCID with relatively high sensitivity and specificity (80). Moreover, combined receiver operating characteristic curve analysis of seven miRNAs revealed an area under the curve (AUC) of 0.64 with a sensitivity of 55.5% and specificity of 65.7%, whereas plasma miR-409-3p, miR-502-3p, miR-486-5p, and miR-451a could differentiate patients with VCID from healthy controls (80). To improve the diagnosis and anti-diastole level of dementia, the value of miRNAs in differential diagnosis protocols was determined. Through the measurement of miRNA expression levels in MCI, VCID, AD, and Parkinson's disease with dementia (PDD), miR-1, miR-384, and miR-19b-3p were identified as good diagnostic biomarkers and provided a novel insight in disease prevention (81, 82). Another study comparing different miRNAs expressed in AD, MCI, and healthy controls found that miR-455-3p, miR-4668-5p, miR-3613-3p, and miR-4674 were upregulated, whereas miR-6722 was downregulated in AD and MCI compared with healthy controls (83). More research is warranted concerning the clinical consequences of VCID and expression changes of miRNAs, especially in disease diagnosis and prediction (Table 1).
A large number of fundamental experiments have been conducted to gain a better understanding of their signaling pathways and the post-transcriptional mechanisms of miRNAs. Comparison of astrocytic and microglial activation, WM damage, water channels, and glymphatic dysfunction in mice with miR-126 deletion (miR-126 EC−/−), and control (miR-126 flox/flox), miR-126 EC−/−mice showed significantly decreased cerebral blood flow (CBF) and increased inflammation that was accompanied by poor performance and cognitive deficits (84). As a vasoconstrictor factor, endothelin-1 (ET-1) was increased in the plasma of patients after stroke and in the CSF of patients with VCID (86). An miR-125a inhibitor substantially upregulated the expression of ET-1, whereas miR-125a and the presence of the rs12976445 minor allele polymorphism downregulated the delivery of ET-1 to ECs (87). These results suggested that miR-125a, ET-1, and rs12976445 have a promising potential as pathological solutions for post-stroke dementia.
Apoptosis and proliferation have been implicated in many diseases, confirming the negative correlation between miRNAs and cognitive impairment. It is known that miR-191 can aggravate apoptosis and misregulate proliferation and migration; in vivo studies have shown that applying an miR-191 antagomir significantly attenuated infarction volume by mechanically targeting vascular endothelial zinc finger 1 (VEZF1) transcript (85). In addition, miR-196a and LRIG3 enhanced learning and memory by ameliorating injury to hippocampal neurons via the PI3K/Akt pathway (88). Although these studies represent only the tip of the iceberg, they provide a novel insight into the multiple miRNAs that can intervene in signaling pathway function and affect the outcomes of patients with different forms of dementia and cognitive impairment.
Various treatments and interventions have been reported to be effective for dementia; however, no therapeutic cures are currently available. According to previous reports, symptomatic and alternative therapies are effective; however, antipsychotic treatment is less satisfactory (89). For VCID caused by vascular factors that may be mixed with AD pathological changes, more attention should be focused on therapeutic agents for synaptic protection, anti-pathologic therapeutics, and effective management of vascular risk factors (90). However, acupuncture is a promising alternative therapy and may be an underlying TLR4 inhibitor for VCID (65). Preclinical experiments indicate the advantages of remote ischemic conditioning (RIC) for decreasing the recurrence of ischemic stroke, and repetitive treatments with RIC in patients for 6 months show satisfactory outcomes, especially in neuropsychological assessments (91). Currently, therapeutics based on miRNAs are mainly focused on miRNA mimics and inhibitors (antagomirs), respectively, to adjust the expression level of target genes (92). Mice with transient middle cerebral artery occlusion showed that pre- or post-ischemic treatment with an miR-7 mimic decreased lesion volume and improved motor and cognitive functional recovery (43). Biomarkers can aid in early diagnosis before the pathology occurs, limiting disease progression, and potentially indicating patient response to the treatment. Thus, refining the use of biomarkers will allow dementia treatment to enter the era of precision medicine (93).
This is an exciting time for miRNAs research because of the recent advancement and identification of miRNA genes, their expression patterns, and their regulatory targets in diseases such as VCID, AD, and other neurodegenerative diseases. The mechanisms discussed in this review provide prima facie evidence for the mutual effects of miRNAs on the pathogenesis of VCID. A vast number of miRNAs play roles in the mutual regulation in genes and proteins, and it has been reported that high- or low-expression level of miRNAs can improve or impair the pathological progress of diseases. Hence, we have highlighted the roles of miRNAs in the modulation of VCID. However, the clear interventions of miRNAs and VCID are still poorly reported, and further studies in this area are expected to emphasize the pathways and mechanisms that could improve disease and help develop VCID therapies.
WZ and MZ organized the content of the entire manuscript, wrote the sections, and were responsible for the figures. GZ, ZW, CW, and LS contributed substantially to the conception and design of the work. All authors read and approved the final manuscript.
This study was supported by the grant provided by the Major Chronic Disease Program of the Ministry of Science and Technology of China (No. 2018YFC1312301) and the General Program of the National Natural Science Foundation of China (No. 82071442), The First Hospital of Jilin University, China.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank Editage (https://www.editage.cn) for English language editing.
1. Nguyen DH, Cunningham JT, Sumien N. Estrogen receptor involvement in vascular cognitive impairment and vascular dementia pathogenesis and treatment. Geroscience. (2021) 43:159–66. doi: 10.1007/s11357-020-00263-4
2. Wortmann M. Dementia: a global health priority - highlights from an adi and world health organization report. Alzheimers Res Ther. (2012) 4:40. doi: 10.1186/alzrt143
3. Balasubramanian P, DelFavero J, Ungvari A, Papp M, Tarantini A, Price N, et al. Time-restricted feeding (Trf) for prevention of age-related vascular cognitive impairment and dementia. Aging Res Rev. (2020) 64:101189. doi: 10.1016/j.arr.2020.101189
4. Wolters FJ, Ikram MA. Epidemiology of vascular dementia. Arterioscler Thromb Vasc Biol. (2019) 39:1542–9. doi: 10.1161/ATVBAHA.119.311908
5. van der Flier WM, Skoog I, Schneider JA, Pantoni L, Mok V, Chen CLH, et al. Vascular cognitive impairment. Nat Rev Dis Primers. (2018) 4:18003. doi: 10.1038/nrdp.2018.3
6. Konovalova J, Gerasymchuk D, Parkkinen I, Chmielarz P, Domanskyi A. Interplay between micrornas and oxidative stress in neurodegenerative diseases. Int J Mol Sci. (2019) 20:23. doi: 10.3390/ijms20236055
7. Juzwik CA. S SD, Zhang Y, Paradis-Isler N, Sylvester A, Amar-Zifkin A, et al. Microrna dysregulation in neurodegenerative diseases: a systematic review. Prog Neurobiol. (2019) 182:101664. doi: 10.1016/j.pneurobio.2019.101664
8. Correia de Sousa M, Gjorgjieva M, Dolicka D, Sobolewski C, Foti M. Deciphering mirnas' action through mirna editing. Int J Mol Sci. (2019) 20:24. doi: 10.3390/ijms20246249
9. Tili E, Mezache L, Michaille JJ, Amann V, Williams J, Vandiver P, et al. Microrna 155 up regulation in the cns is strongly correlated to down's syndrome dementia. Ann Diagn Pathol. (2018) 34:103–9. doi: 10.1016/j.anndiagpath.2018.03.006
10. Wang F, Niu G, Chen X, Cao F. Molecular imaging of micrornas. Eur J Nucl Med Mol Imaging. (2011) 38:1572–9. doi: 10.1007/s00259-011-1786-0
11. Lee YS, Dutta A. Micrornas in cancer. Annu Rev Pathol. (2009) 4:199–227. doi: 10.1146/annurev.pathol.4.110807.092222
12. Bartel DP. Micrornas: genomics, biogenesis, mechanism, and function. Cell. (2004) 116:281–97. doi: 10.1016/S0092-8674(04)00045-5
13. Lu TX, Rothenberg ME. Microrna. J Allergy Clin Immunol. (2018) 141:1202–7. doi: 10.1016/j.jaci.2017.08.034
14. Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, Ghaffari SH. An overview of micrornas: biology, functions, therapeutics, analysis methods. J Cell Physiol. (2019) 234:5451–65. doi: 10.1002/jcp.27486
15. Siedlecki-Wullich D, Minano-Molina AJ, Rodriguez-Alvarez J. Micrornas as early biomarkers of alzheimer's disease: a synaptic perspective. Cells. (2021) 10:1. doi: 10.3390/cells10010113
16. Tiwari A, Mukherjee B, Dixit M. Microrna key to angiogenesis regulation: mirna biology and therapy. Curr Cancer Drug Targets. (2018) 18:266–77. doi: 10.2174/1568009617666170630142725
17. Wu J, He J, Tian X, Luo Y, Zhong J, Zhang H, et al. Microrna-9-5p alleviates blood-brain barrier damage and neuroinflammation after traumatic brain injury. J Neurochem. (2020) 153:710–26. doi: 10.1111/jnc.14963
18. Toyama K, Spin JM, Mogi M, Tsao PS. Therapeutic perspective on vascular cognitive impairment. Pharmacol Res. (2019) 146:104266. doi: 10.1016/j.phrs.2019.104266
19. Almutairi MM, Gong C, Xu YG, Chang Y, Shi H. Factors controlling permeability of the blood-brain barrier. Cell Mol Life Sci. (2016) 73:57–77. doi: 10.1007/s00018-015-2050-8
20. Chen D, Hu S, Wu Z, Liu J, Li S. The role of mir-132 in regulating neural stem cell proliferation, differentiation and neuronal maturation. Cell Physiol Biochem. (2018) 47:2319–30. doi: 10.1159/000491543
21. Liu B, Li J, Cairns MJ. Identifying mirnas, targets and functions. Brief Bioinform. (2014) 15:1–19. doi: 10.1093/bib/bbs075
22. Ma F, Zhang X, Yin KJ. Micrornas in central nervous system diseases: a prospective role in regulating blood-brain barrier integrity. Exp Neurol. (2020) 323:113094. doi: 10.1016/j.expneurol.2019.113094
23. Van Dyken P, Lacoste B. Impact of metabolic syndrome on neuroinflammation and the blood-brain barrier. Front Neurosci. (2018) 12:930. doi: 10.3389/fnins.2018.00930
24. Goodall EF, Leach V, Wang C, Cooper-Knock J, Heath PR, Baker D, et al. Age-associated mrna and mirna expression changes in the blood-brain barrier. Int J Mol Sci. (2019) 20:12. doi: 10.3390/ijms20123097
25. Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol. (2015) 7:a020412. doi: 10.1101/cshperspect.a020412
26. Chakraborty C, Sharma AR, Sharma G, Bhattacharya M, Lee SS. Micrornas: possible regulatory molecular switch controlling the bbb microenvironment. Mol Ther Nucleic Acids. (2020) 19:933–6. doi: 10.1016/j.omtn.2019.12.024
27. Bernstein DL, Zuluaga-Ramirez V, Gajghate S, Reichenbach NL, Polyak B, Persidsky Y, et al. Mir-98 reduces endothelial dysfunction by protecting blood-brain barrier (Bbb) and improves neurological outcomes in mouse ischemia/reperfusion stroke model. J Cereb Blood Flow Metab. (2020) 40:1953–65. doi: 10.1177/0271678X19882264
28. Vasudeva K, Munshi A. Mirna dysregulation in ischaemic stroke: focus on diagnosis, prognosis, therapeutic and protective biomarkers. Eur J Neurosci. (2020) 52:3610–27. doi: 10.1111/ejn.14695
29. Bai Y, Zhang Y, Han B, Yang L, Chen X, Huang R, et al. Circular rna dlgap4 ameliorates ischemic stroke outcomes by targeting mir-143 to regulate endothelial-mesenchymal transition associated with blood-brain barrier integrity. J Neurosci. (2018) 38:32–50. doi: 10.1523/JNEUROSCI.1348-17.2017
30. Zuo X, Lu J, Manaenko A, Qi X, Tang J, Mei Q, et al. Microrna-132 attenuates cerebral injury by protecting blood-brain-barrier in mcao mice. Exp Neurol. (2019) 316:12–9. doi: 10.1016/j.expneurol.2019.03.017
31. O'Carroll D, Schaefer A. General principals of mirna biogenesis and regulation in the brain. Neuropsychopharmacology. (2013) 38:39–54. doi: 10.1038/npp.2012.87
32. Pan J, Qu M, Li Y, Wang L, Zhang L, Wang Y, et al. Microrna-126-3p/-5p overexpression attenuates blood-brain barrier disruption in a mouse model of middle cerebral artery occlusion. Stroke. (2020) 51:619–27. doi: 10.1161/STROKEAHA.119.027531
33. Toyama K, Spin JM, Deng AC, Huang TT, Wei K, Wagenhauser MU, et al. Microrna-Mediated therapy modulating blood-brain barrier disruption improves vascular cognitive impairment. Arterioscler Thromb Vasc Biol. (2018) 38:1392–406. doi: 10.1161/ATVBAHA.118.310822
34. Song J, Yoon SR, Kim OY. Mir-Let7a controls the cell death and tight junction density of brain endothelial cells under high glucose condition. Oxid Med Cell Longev. (2017) 2017:6051874. doi: 10.1155/2017/6051874
35. Wang P, Pan R, Weaver J, Jia M, Yang X, Yang T, et al. Microrna-30a regulates acute cerebral ischemia-Induced blood-brain barrier damage through znt4/Zinc pathway. J Cereb Blood Flow Metab. (2021) 41:641–55. doi: 10.1177/0271678X20926787
36. Zhang T, Tian C, Wu J, Zhang Y, Wang J, Kong Q, et al. Microrna-182 exacerbates blood-brain barrier (Bbb) disruption by downregulating the mtor/Foxo1 pathway in cerebral ischemia. FASEB J. (2020) 34:13762–75. doi: 10.1096/fj.201903092R
37. Wang XX, Zhang B, Xia R, Jia QInflammation Y. Apoptosis and autophagy as critical players in vascular dementia. Eur Rev Med Pharmacol Sci. (2020) 24:9601–14. doi: 10.26355/eurrev_202009_23048
38. Iadecola C, Duering M, Hachinski V, Joutel A, Pendlebury ST, Schneider JA, et al. Vascular cognitive impairment and dementia: jacc scientific expert panel. J Am Coll Cardiol. (2019) 73:3326–44. doi: 10.1016/j.jacc.2019.04.034
39. Morel L, Regan M, Higashimori H, Ng SK, Esau C, Vidensky S, et al. Neuronal exosomal mirna-dependent translational regulation of astroglial glutamate transporter glt1. J Biol Chem. (2013) 288:7105–16. doi: 10.1074/jbc.M112.410944
40. Liu X, Feng Z, Du L, Huang Y, Ge J, Deng Y, et al. The potential role of microrna-124 in cerebral ischemia injury. Int J Mol Sci. (2019) 21:1. doi: 10.3390/ijms21010120
41. Yang FW, Wang H, Wang C, Chi GN. Upregulation of acetylcholinesterase caused by downregulation of microrna-132 is responsible for the development of dementia after ischemic stroke. J Cell Biochem. (2020) 121:135–41. doi: 10.1002/jcb.28985
42. Liu DY, Zhang L. Microrna-132 promotes neurons cell apoptosis and activates tau phosphorylation by targeting gtdc-1 in alzheimer's disease. Eur Rev Med Pharmacol Sci. (2019) 23:8523–32.
43. Kim T, Mehta SL, Morris-Blanco KC, Chokkalla AK, Chelluboina B, Lopez M, et al. The microrna mir-7a-5p ameliorates ischemic brain damage by repressing alpha-synuclein. Sci Signal. (2018) 11:560. doi: 10.1126/scisignal.aat4285
44. Li GF Li ZB, Zhuang SJ Li GC. Inhibition of microrna-34a protects against propofol anesthesia-Induced neurotoxicity and cognitive dysfunction via the mapk/Erk signaling pathway. Neurosci Lett. (2018) 675:152–9. doi: 10.1016/j.neulet.2018.03.052
45. Xu C, Wang C, Meng Q, Gu Y, Wang Q, Xu W, et al. Mir153 promotes neural differentiation in the mouse hippocampal ht22 cell line and increases the expression of neuronspecific enolase. Mol Med Rep. (2019) 20:1725–35. doi: 10.3892/mmr.2019.10421
46. Wei C, Xu X, Zhu H, Zhang X, Gao Z. Promotive role of microrna150 in hippocampal neurons apoptosis in vascular dementia model rats. Mol Med Rep. (2021) 23:4. doi: 10.3892/mmr.2021.11896
47. Nunomura A, Perry Rna G. and Oxidative Stress in Alzheimer's Disease: Focus on Micrornas. Oxid Med Cell Longev. (2020) 2020:2638130. doi: 10.1155/2020/2638130
48. Gocmez SS, Sahin TD, Yazir Y, Duruksu G, Eraldemir FC, Polat S, et al. Resveratrol prevents cognitive deficits by attenuating oxidative damage and inflammation in rat model of streptozotocin diabetes induced vascular dementia. Physiol Behav. (2019) 201:198–207. doi: 10.1016/j.physbeh.2018.12.012
49. Luca M, Luca A. Oxidative stress-Related endothelial damage in vascular depression and vascular cognitive impairment: beneficial effects of aerobic physical exercise. Oxid Med Cell Longev. (2019) 2019:8067045. doi: 10.1155/2019/8067045
50. Khoshnam SE, Winlow W, Farbood Y, Moghaddam HF, Farzaneh M. Emerging roles of micrornas in ischemic stroke: as possible therapeutic agents. J Stroke. (2017) 19:166–87. doi: 10.5853/jos.2016.01368
51. Liu P, Han Z, Ma Q, Liu T, Wang R, Tao Z, et al. Upregulation of microrna-128 in the peripheral blood of acute ischemic stroke patients is correlated with stroke severity partially through inhibition of neuronal cell cycle reentry. Cell Transplant. (2019) 28:839–50. doi: 10.1177/0963689719846848
52. Rainer TH, Leung LY, Chan CPY, Leung YK, Abrigo JM, Wang D, et al. Plasma mir-124-3p and mir-16 concentrations as prognostic markers in acute stroke. Clin Biochem. (2016) 49:663–8. doi: 10.1016/j.clinbiochem.2016.02.016
53. Feng CZ, Yin JB, Yang JJ, Cao L. Regulatory factor x1 depresses apoe-Dependent abeta uptake by mirna-124 in microglial response to oxidative stress. Neuroscience. (2017) 344:217–28. doi: 10.1016/j.neuroscience.2016.12.017
54. Ansari A, Maffioletti E, Milanesi E, Marizzoni M, Frisoni GB, Blin O, et al. Mir-146a and mir-181a are involved in the progression of mild cognitive impairment to alzheimer's disease. Neurobiol Aging. (2019) 82:102–9. doi: 10.1016/j.neurobiolaging.2019.06.005
55. Chen FZ, Zhao Y, Chen HZ. Microrna-98 reduces amyloid beta-Protein production and improves oxidative stress and mitochondrial dysfunction through the notch signaling pathway via hey2 in alzheimer's disease mice. Int J Mol Med. (2019) 43:91–102. doi: 10.3892/ijmm.2018.3957
56. Slota JA, Booth SA. Micrornas in neuroinflammation: implications in disease pathogenesis, biomarker discovery and therapeutic applications. Non-coding RNA. (2019) 5:2. doi: 10.3390/ncrna5020035
57. Nuzziello N, Liguori M. The microrna centrism in the orchestration of neuroinflammation in neurodegenerative diseases. Cells. (2019) 8:10. doi: 10.3390/cells8101193
58. Gaudet AD, Fonken LK, Watkins LR, Nelson RJ, Popovich PG. Micrornas: roles in regulating neuroinflammation. Neuroscientist. (2018) 24:221–45. doi: 10.1177/1073858417721150
59. Chen L, Dong R, Lu Y, Zhou Y, Li K, Zhang Z, et al. Microrna-146a protects against cognitive decline induced by surgical trauma by suppressing hippocampal neuroinflammation in mice. Brain Behav Immun. (2019) 78:188–201. doi: 10.1016/j.bbi.2019.01.020
60. Lu Y, Xu X, Dong R, Sun L, Chen L, Zhang Z, et al. Microrna-181b-5p attenuates early postoperative cognitive dysfunction by suppressing hippocampal neuroinflammation in mice. Cytokine. (2019) 120:41–53. doi: 10.1016/j.cyto.2019.04.005
61. Huang L, Ma Q, Li Y, Li B, Zhang L. Inhibition of microrna-210 suppresses pro-Inflammatory response and reduces acute brain injury of ischemic stroke in mice. Exp Neurol. (2018) 300:41–50. doi: 10.1016/j.expneurol.2017.10.024
62. Dias IHK, Brown CL, Shabir K, Polidori MC, Griffiths HR. Mirna 933 expression by endothelial cells is increased by 27-hydroxycholesterol and is more prevalent in plasma from dementia patients. J Alzheimers Dis. (2018) 64:1009–17. doi: 10.3233/JAD-180201
63. Saggu R, Schumacher T, Gerich F, Rakers C, Tai K, Delekate A, et al. Astroglial nf-Kb contributes to white matter damage and cognitive impairment in a mouse model of vascular dementia. Acta Neuropathol Commun. (2016) 4:76. doi: 10.1186/s40478-016-0350-3
64. Yang T, Zhang F. Targeting transcription factor nrf2 (Nuclear factor erythroid 2-related factor 2) for the intervention of vascular cognitive impairment and dementia. Arterioscler Thromb Vasc Biol. (2021) 41:97–116. doi: 10.1161/ATVBAHA.120.314804
65. Wang L, Yang JW, Lin LT, Huang J, Wang XR, Su XT, et al. Acupuncture attenuates inflammation in microglia of vascular dementia rats by inhibiting mir-93-mediated tlr4/Myd88/Nf-Kappab signaling pathway. Oxid Med Cell Longev. (2020) 2020:8253904. doi: 10.1155/2020/8253904
66. Tang CZ, Zhang DF, Yang JT, Liu QH, Wang YR, Wang WS. Overexpression of microrna-301b accelerates hippocampal microglia activation and cognitive impairment in mice with depressive-like behavior through the nf-Kappab signaling pathway. Cell Death Dis. (2019) 10:316. doi: 10.1038/s41419-019-1522-4
67. Hu G, Xia Y, Zhang J, Chen Y, Yuan J, Niu X, et al. Esc-Sevs rejuvenate senescent hippocampal nscs by activating lysosomes to improve cognitive dysfunction in vascular dementia. Adv Sci (Weinh). (2020) 7:1903330. doi: 10.1002/advs.201903330
68. Boese AC, Hamblin MH, Lee JP. Neural stem cell therapy for neurovascular injury in alzheimer's disease. Exp Neurol. (2020) 324:113112. doi: 10.1016/j.expneurol.2019.113112
69. Xiao WZ, Lu AQ, Liu XW, Li Z, Zi Y, Wang ZW. Role of mirna-146 in proliferation and differentiation of mouse neural stem cells. Biosci Rep. (2015) 35:5. doi: 10.1042/BSR20150088
70. Gu J, Shao R, Li M, Yan Q, Hu H. Mir-485-3p modulates neural stem cell differentiation and proliferation via regulating trip6 expression. J Cell Mol Med. (2020) 24:398–404. doi: 10.1111/jcmm.14743
71. Kumar S, Curran JE, DeLeon E, Leandro AC, Howard TE, Lehman DM, et al. Role of mirna-Mrna interaction in neural stem cell differentiation of induced pluripotent stem cells. Int J Mol Sci. (2020) 21:19. doi: 10.3390/ijms21196980
72. Shu P, Fu H, Zhao X, Wu C, Ruan X, Zeng Y, et al. Microrna-214 modulates neural progenitor cell differentiation by targeting quaking during cerebral cortex development. Sci Rep. (2017) 7:8014. doi: 10.1038/s41598-017-08450-8
73. Gao Y, Qiao H, Zhong T, Lu Z, Hou Y. Microrna29a promotes the neural differentiation of rat neural stem/progenitor cells by targeting klf4. Mol Med Rep. (2020) 22:1008–16. doi: 10.3892/mmr.2020.11177
74. Ragusa M, Bosco P, Tamburello L, Barbagallo C, Condorelli AG, Tornitore M, et al. Mirnas plasma profiles in vascular dementia: biomolecular data and biomedical implications. Front Cell Neurosci. (2016) 10:51. doi: 10.3389/fncel.2016.00051
75. Song Y, Hu M, Zhang J, Teng ZQ, Chen C. A novel mechanism of synaptic and cognitive impairments mediated via microrna-30b in alzheimer's disease. EBioMedicine. (2019) 39:409–21. doi: 10.1016/j.ebiom.2018.11.059
76. Ren Z, Yu J, Wu Z, Si W, Li X, Liu Y, et al. Microrna-210-5p contributes to cognitive impairment in early vascular dementia rat model through targeting snap25. Front Mol Neurosci. (2018) 11:388. doi: 10.3389/fnmol.2018.00388
77. Li F, Wei G, Bai Y, Li Y, Huang F, Lin J, et al. Microrna-574 is involved in cognitive impairment in 5-month-Old app/Ps1 mice through regulation of neuritin. Brain Res. (2015) 1627:177–88. doi: 10.1016/j.brainres.2015.09.022
78. Kalaria RN. Neuropathological diagnosis of vascular cognitive impairment and vascular dementia with implications for alzheimer's disease. Acta Neuropathol. (2016) 131:659–85. doi: 10.1007/s00401-016-1571-z
79. Ghoreishy A, Khosravi A, Ghaemmaghami A. Exosomal microrna and stroke: a review. J Cell Biochem. (2019) 120:16352–61. doi: 10.1002/jcb.29130
80. Prabhakar P, Chandra SR, Christopher R. Circulating micrornas as potential biomarkers for the identification of vascular dementia due to cerebral small vessel disease. Age Aging. (2017) 46:861–4. doi: 10.1093/ageing/afx090
81. Yang TT, Liu CG, Gao SC, Zhang Y, Wang PC. The serum exosome derived microrna-135a,−193b, and−384 were potential alzheimer's disease biomarkers. Biomed Environ Sci. (2018) 31:87–96. doi: 10.3967/bes2018.011
82. Swarup V, Hinz FI, Rexach JE, Noguchi KI, Toyoshiba H, Oda A, et al. Identification of evolutionarily conserved gene networks mediating neurodegenerative dementia. Nat Med. (2019) 25:152–64. doi: 10.1038/s41591-018-0223-3
83. Kumar S, Vijayan M, Reddy PH. Microrna-455-3p as a potential peripheral biomarker for alzheimer's disease. Hum Mol Genet. (2017) 26:3808–22. doi: 10.1093/hmg/ddx267
84. Yu P, Venkat P, Chopp M, Zacharek A, Shen Y, Ning R, et al. Role of microrna-126 in vascular cognitive impairment in mice. J Cereb Blood Flow Metab. (2019) 39:2497–511. doi: 10.1177/0271678X18800593
85. Du K, Zhao C, Wang L, Wang Y, Zhang KZ, Shen XY, et al. Mir-191 inhibit angiogenesis after acute ischemic stroke targeting vezf1. Aging (Albany NY). (2019) 11:2762–86. doi: 10.18632/aging.101948
86. Nakajima M, Morimoto S, Takamoto S, Kitano S, Fukuo K, Onishi T, et al. endothelin-1 in cerebrospinal fluid in elderly patients with hypertension and dementia. Hypertension. (1994) 24:97–100. doi: 10.1161/01.HYP.24.1.97
87. Tiedt S, Prestel M, Malik R, Schieferdecker N, Duering M, Kautzky V, et al. Rna-Seq identifies circulating mir-125a-5p, mir-125b-5p, and mir-143-3p as potential biomarkers for acute ischemic stroke. Circ Res. (2017) 121:970–80. doi: 10.1161/CIRCRESAHA.117.311572
88. Yang K, Feng S, Ren J, Zhou W. Upregulation of microrna-196a improves cognitive impairment and alleviates neuronal damage in hippocampus tissues of alzheimer's disease through downregulating lrig3 expression. J Cell Biochem. (2019) 120:17811–21. doi: 10.1002/jcb.29047
89. Perng CH, Chang YC, Tzang RF. The treatment of cognitive dysfunction in dementia: a multiple treatments meta-Analysis. Psychopharmacology (Berl). (2018) 235:1571–80. doi: 10.1007/s00213-018-4867-y
90. Sun MK. Potential therapeutics for vascular cognitive impairment and dementia. Curr Neuropharmacol. (2018) 16:1036–44. doi: 10.2174/1570159X15666171016164734
91. Liao Z, Bu Y, Li M, Han R, Zhang N, Hao J, et al. Remote ischemic conditioning improves cognition in patients with subcortical ischemic vascular dementia. BMC Neurol. (2019) 19:206. doi: 10.1186/s12883-019-1435-y
92. Sun P, Liu DZ, Jickling GC, Sharp FR, Yin KJ. Microrna-Based therapeutics in central nervous system injuries. J Cereb Blood Flow Metab. (2018) 38:1125–48. doi: 10.1177/0271678X18773871
Keywords: apoptosis, blood-brain barrier, miRNAs, neuroinflammation, neurodifferentiation, oxidative stress, vascular cognitive impairment, dementia
Citation: Zhai W, Zhao M, Zhang G, Wang Z, Wei C and Sun L (2022) MicroRNA-Based Diagnosis and Therapeutics for Vascular Cognitive Impairment and Dementia. Front. Neurol. 13:895316. doi: 10.3389/fneur.2022.895316
Received: 13 March 2022; Accepted: 28 March 2022;
Published: 03 May 2022.
Edited by:
Yuzhen Xu, Tongji University, ChinaReviewed by:
Yunpeng Cao, The First Affiliated Hospital of China Medical University, ChinaCopyright © 2022 Zhai, Zhao, Zhang, Wang, Wei and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Sun, c3VubGk5OUBqbHUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.