
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 23 June 2022
Sec. Movement Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.895272
This article is part of the Research Topic Insights in Movement Disorders: 2021 View all 11 articles
 Yan Liang†
Yan Liang† Junyu Lin†
Junyu Lin† Yanbing Hou
Yanbing Hou Lingyu Zhang
Lingyu Zhang Ruwei Ou
Ruwei Ou Chunyu Li
Chunyu Li Qianqian Wei
Qianqian Wei Bei Cao
Bei Cao Kuncheng Liu
Kuncheng Liu Zheng Jiang
Zheng Jiang Tianmi Yang
Tianmi Yang Jing Yang
Jing Yang Meng Zhang
Meng Zhang Simin Kang
Simin Kang Yi Xiao
Yi Xiao Qirui Jiang
Qirui Jiang Jing Yang
Jing Yang Wei Song
Wei Song Xueping Chen
Xueping Chen Bi Zhao
Bi Zhao Ying Wu
Ying Wu Huifang Shang*
Huifang Shang*Purpose: The study aimed to evaluate the health-related quality of life (HRQoL) measured by the five-level EuroQol-5 dimensions (EQ-5D-5L) in patients with cervical dystonia, and to explore the determinants of HRQoL in patients with cervical dystonia.
Methods: EQ-5D-5L health state profiles were converted into a single aggregated “health utility” score. A calibrated visual analog scale (EQ VAS) was used for self-rating of current health status. Multiple linear regression analysis was used to explore the factors associated with HRQoL in cervical dystonia.
Results: A total of 333 patients with cervical dystonia were enrolled in the analysis, with an average age of 44.3 years old. The most common impaired dimension of health was anxiety/depression (73.6%), followed by pain/discomfort (68.2%) and usual activities (48%). The median health utility score was 0.80, and the median EQ VAS score was 70.2. Multivariate linear regression analysis indicated that disease duration and the scores of the Hamilton Depression Rating Scale (HDRS), Pittsburgh sleep quality index (PSQI), Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS) Part I, and TWSTRS Part III were associated with the health utility scores. After adjusting other parameters, the TWSTRS Part III score and the HDRS score were significantly associated with the EQ VAS scores (p < 0.05).
Conclusion: This study evaluated HRQoL in patients with cervical dystonia using the Chinese version of the EQ-5D-5L scale. We found that, besides motor symptoms, non-motor symptoms, including depression, pain, and sleep quality, could be greater determinants of HRQoL in patients with cervical dystonia. Management of non-motor symptoms, therefore, may help improve HRQoL in patients with cervical dystonia.
Cervical dystonia (CD) is one of the most common focal dystonias characterized by involuntary contractions of cervical muscles, leading to abnormal movements and posture of head (1). Besides the motor symptoms, non-motor symptoms, such as anxiety, depression, sleep disorders, and pain, are also very common in patients with CD (2). While CD is not a life-threatening disease, it can affect activities of daily living, decrease the quality of life (3), and even cause disability of patients (4).
Most of the previous studies assessed the quality of life in CD using the craniocervical dystonia questionnaire-24 (CDQ 24) or the Short Form-36 Health Survey (SF-36). Additionally, they had usually small sample sizes and yielded inconsistent results. For example, motor severity has been reported to correlate with poor quality of life in CD in some studies (5–7), but not in other studies (8–13).
The five-level EuroQol5-dimensions questionnaire (EQ-5D-5L) is a standardized and more convenient tool to evaluate the health-related quality of life (HRQoL) worldwide (14). It has been extensively used in neurological diseases, such as Parkinson's disease (15), amyotrophic lateral sclerosis (16), and multiple sclerosis (17). The utility values of EQ-5D-5L for Chinese were established in 2017 (18).
Therefore, the aim of this study was to assess HRQoL in patients with CD using the EQ-5D-5L scale in a large Chinese cohort and to explore the determinants of HRQoL in CD.
We performed a cross-sectional study. All the patients were recruited from the Department of Neurology of West China Hospital of Sichuan University. The patients were diagnosed as CD by neurologists specialized in movement disorders. Only the patients with isolated cervical dystonia were included in the analysis. The patients who had concominant blepharospasm, oromandibular dystonia or dystonia in the limbs or trunk besides CD were excluded in the current study. The study was approved by the Ethics Committee of West China Hospital of Sichuan University (No. 2022-260). All the participants have signed informed consent.
We collected demographic and clinical data of all the participants, including sex, age, age of the onset, and disease duration. All the participants underwent a face-to-face interview by trained movement disorder specialists. Motor and non-motor symptoms were assessed using standard scales. Motor severity was assessed using the Toronto Western Spasmodic Torticollis Rating Scale Part I (TWSTRS-I). Depression was assessed using the Hamilton Depression Rating Scale-24 (HDRS-24) (19). Anxiety was assessed using the Hamilton Anxiety Rating Scale (HARS) (20). Excessive daytime sleepiness was assessed using the Epworth Sleepiness Scale (ESS) scale (21). Sleep quality was assessed using the Pittsburgh sleep quality index (PSQI) scale (22). The global cognitive function was assessed using the Montreal Cognitive Assessment (MoCA) scale (23). The Toronto Western Spasmodic Torticollis Rating Scale Part III (TWSTRS-III) was used to assess pain severity. The Toronto Western Spasmodic Torticollis Rating Scale Part II (TWSTRS-II) was used to assess the activities of daily living.
The HRQoL was assessed using the EQ-5D-5L. EQ-5D-5L comprises two parts. The first part of the EQ-5D-5L assesses five dimensions of health, namely, mobility (MO), self-care (SC), usual activities (UA), pain/discomfort (PD), and anxiety/depression (AD). Each dimension has five levels, namely, no problems, slight problems, moderate problems, severe problems, and extreme problems. The scores of these five problems can be converted into a single aggregated “health utility” score according to the Chinese version of the population-based utility values (18). The second part of the EQ-5D-5L is a self-rating calibrated visual analog scale (EQ VAS), with a range of 0 to 100. Score 0 indicates worst possible health state, while score 100 indicates best possible health state.
All continuous variables were presented as the mean and standard deviation (SD), and all categorical variables were presented as numbers and percentages. Spearman's correlation analyses were conducted to explore relationships between EQ-5D-5L values (health utility scores and EQ VAS scores) and clinical variables (sex, age, age of the onset, disease duration, scores of TWSTRS-I, TWSTRS-II, TWSTRS-III, HDRS-24, HARS, ESS, PSQI, and MoCA). The multivariate linear regression model was used to explore the factors correlated with the health utility scores and EQ VAS scores of EQ-5D-5L in CD. The health utility scores and EQ VAS scores of EQ-5D-5L were used as dependent variables.
All analyses were performed using the Statistical Package for the Social Sciences (SPSS) version 22.0, and the R. two-tailed p-values of < 0.05 were considered statistically significant.
A total of 333 patients with CD (118 males) were included in the study. The average age of the patients was 44.3 (SD, 13.3) at the baseline, with a mean disease duration of 3.7 (SD, 5.7) years (Table 1).
The median health utility score was 0.80, and the median EQ VAS score was 70.2 for the total patients with CD. Levels 2–5 were considered as impaired for each dimension. The most common impaired dimension of health was anxiety/depression (73.6%), followed by pain/discomfort (68.2%), usual activities (48%), mobility (33.9%), and self-care (20.4%) (Figure 1).
Spearman's correlation analyses showed that the health utility scores were significantly associated with disease duration (r = 0.168, p = 0.002), the TWSTRS-I score (r = −0.314, p < 0.001), the TWSTRS-II score (r = −0.625, p < 0.001), the TWSTRS-III score (r = −0.424, p < 0.001), the HARS score (r = −0.511, p < 0.001), the HDRS score (r = −0.590, p < 0.001), and the PSQI score (r = −0.269, p < 0.001). The EQ VAS scores were significantly associated with disease duration (r = 0.110, p = 0.045), the TWSTRS-I score (r = −0.141, p = 0.010), the TWSTRS-II score (r = −0.353, p < 0.001), the TWSTRS-III score (r = −0.249, p < 0.001), the HARS score (r = −0.409, p < 0.001), the HDRS score (r = −0.484, p < 0.001), and the PSQI score (r = −0.258, p < 0.001; Table 2).
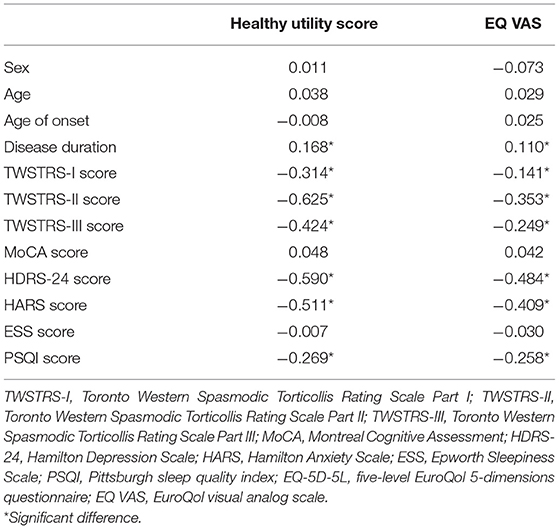
Table 2. Spearman's correlation analyses of the EQ-5D-5L healthy utility score and the EQ VAS score in patients with cervical dystonia.
The multivariate linear regression analysis showed that disease duration (β = 0.086, p = 0.036) and the scores of the HDRS (β = −0.458, p < 0.001), the PSQI (β = −0.094, p = 0.035), the TWSTRS Part I (β = −0.216, p < 0.001), and the TWSTRS Part III (β = −0.215, p < 0.001) were associated with the EQ-5D-5L health utility scores. After adjusting other parameters, the TWSTRS Part III score (β = −0.124, p < 0.012) and the HDRS score (β = −0.459, p < 0.001) were significantly associated with the EQ VAS scores (p < 0.05; Table 3).
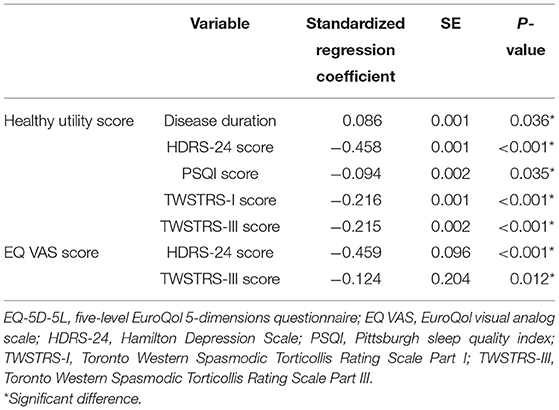
Table 3. Stepwise linear regression analysis of the total EQ-5D-5L healthy utility score and the total EQ VAS score in patients with cervical dystonia.
The current study describes the HRQoL profile in patients with CD in a large Chinese cohort using the EQ-5D-5L scale. The results showed that anxiety/depression (73.6%) and pain/discomfort (68.2%) were the highest reported dimensions impaired in patients with CD. In addition, multivariate linear regression analysis showed that EQ-5D-5L health utility scores were associated with disease duration, motor severity, and non-motor symptoms, including pain, depression, and sleep quality, while EQ VAS scores were only associated with non-motor symptoms, including pain and depression.
As with our results, non-motor symptoms have been widely reported to play an important role in the decreased quality of life in isolated dystonia, including CD (12, 24, 25). Approximately 55~90% of the patients with CD have been reported to suffer from pain (1, 26, 27). In the current study, pain/discomfort was reported by 68.2% of the patients with CD. Inconsistent with our results, pain has also been identified to affect the quality of life in patients with CD in several studies (7–9, 11–13). The pain in CD can be relieved by botulinum toxin injection (28). However, the mechanism of pain in patients with CD remains largely unknown. The probable mechanisms include both muscle-based and non-muscle-based mechanisms, such as network changes in the basal ganglia (29).
Depression was another determinant of decreased HRQoL in patients with CD identified in the current study. Mood disorders have been reported to be important determinants of poor quality of life in patients with CD in many previous studies (5, 8–11, 13). Depression is common in patients with CD. A recent meta-analysis has yielded depression prevalence of 31.5% in patients with CD (30). In the current study, anxiety/depression was reported by 68.2% of the patients with CD, indicating that the rate of psychiatric comorbidities in CD might be underestimated. Impairment of the dopaminergic system might be an explanation of the development of depression in patients with CD (31).
In line with our results, sleep disorder has also been found to affect the quality of life in patients with CD by a previous study (10). Sleep disorder is also a very common nonmotor symptom in CD (32). Nearly half of the patients with CD have been found to have poor sleep quality (33, 34), which was in accordance with our results (49.8%). Patients with CD with sleep disorders also had a higher pain burden than those with normal sleep (12).
Several studies reported that motor severity was not associated with quality of life in patients with CD (8–13). However, other studies came to the opposite conclusion (5–7). In addition, a study found that motor symptoms had a small influence only on the physical functioning domain of the HRQoL in CD (24). In the current study, motor severity was associated with EQ-5D-5L health utility scores, but not with EQ VAS scores according to the multivariate linear regression analyses. Therefore, the role of motor severity in the HRQoL of CD needs to be validated by more studies in the future.
The results of the current study offered some indications for the strategies of the decreased quality of life in patients with CD. For example, botulinum toxin injection benefits for both pain and motor severity of CD (28), and it has also been reported to help improve the HRQoL in patients with CD (35). In addition, as non-motor symptoms played an important role in the decreased HRQoL in patients with CD, dealing with these non-motor symptoms might be a good choice for improving the HRQoL in CD.
However, several limitations should be acknowledged in the current study. The first limitation was the lack of the healthy controls. The second limitation was that the treatment choices were not included in the analyses.
In conclusion, our study evaluated the HRQoL in patients with CD using the Chinese version of the EQ-5D-5L scale. The results revealed that, besides motor symptoms, non-motor symptoms, including depression, pain, and sleep quality, could be greater determinants of HRQoL in patients with CD, indicating that management of non-motor symptoms may help improve HRQoL in patients with CD.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Ethics Committee of West China Hospital of Sichuan University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
YL and JL contributed to conception, organization and execution, data collection and statistical analysis, and drafting the manuscript. YH, LZ, CL, QW, BC, KL, ZJ, TY, JY (12th author), MZ, SK, YX, QJ, JY (17th author), WS, XC, BZ, and YW contributed to execution and data collection. RO contributed to conception, organization, execution, and data collection. HS contributed to conception and organization, manuscript review and critique, and was responsible for overall content as the guarantor. All authors contributed to the article and approved the submitted version.
This study was supported by the 1.3.5 project for disciplines of excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (Grant No. 2019HXFH016).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank all the participants of this study.
1. Jankovic J, Leder S, Warner D, Schwartz K. Cervical dystonia: clinical findings and associated movement disorders. Neurology. (1991) 41:1088–91. doi: 10.1212/WNL.41.7.1088
2. Ray S, Pal PK, Yadav R. Non-motor symptoms in cervical dystonia: a review. Ann Indian Acad Neurol. (2020) 23:449–57. doi: 10.4103/aian.AIAN_287_20
3. Tomic S, Petkovic I, Pucic T, Resan B, Juric S, Rotim T. Cervical dystonia and quality of life. Acta Neurol Belg. (2016) 116:589–92. doi: 10.1007/s13760-016-0634-1
4. van den Dool J, Tijssen MA, Koelman JH, Engelbert RH, Visser B. Determinants of disability in cervical dystonia. Parkinsonism Relat Disord. (2016) 32:48–53. doi: 10.1016/j.parkreldis.2016.08.014
5. Skogseid IM, Malt UF, Roislien J, Kerty E. Determinants and status of quality of life after long-term botulinum toxin therapy for cervical dystonia. Eur J Neurol. (2007) 14:1129–37. doi: 10.1111/j.1468-1331.2007.01922.x
6. Queiroz MR, Chien HF, Barbosa ER. Quality of life in individuals with cervical dystonia before botulinum toxin injection in a Brazilian tertiary care hospital. Arq Neuropsiquiatr. (2011) 69:900–4. doi: 10.1590/S0004-282X2011000700010
7. Werle RW, Takeda SY, Zonta MB, Guimaraes AT, Teive HA. The physical, social and emotional aspects are the most affected in the quality of life of the patients with cervical dystonia. Arq Neuropsiquiatr. (2014) 72:405–10. doi: 10.1590/0004-282X20140044
8. Smit M, Kuiper A, Han V, Jiawan VC, Douma G, van Harten B, et al. Psychiatric co-morbidity is highly prevalent in idiopathic cervical dystonia and significantly influences health-related quality of life: results of a controlled study. Parkinsonism Relat Disord. (2016) 30:7–12. doi: 10.1016/j.parkreldis.2016.06.004
9. Smit M, Kamphuis ASJ, Bartels AL, Han V, Stewart RE, Zijdewind I, et al. Fatigue, sleep disturbances, and their influence on quality of life in cervical dystonia patients. Mov Disord Clin Pract. (2017) 4:517–23. doi: 10.1002/mdc3.12459
10. Han V, Skorvanek M, Smit M, Turcanova Koprusakova M, Hoekstra T, van Dijk JP, et al. Prevalence of non-motor symptoms and their association with quality of life in cervical dystonia. Acta Neurol Scand. (2020) 142:613–22. doi: 10.1111/ane.13304
11. Ndukwe I, O'Riordan S, Walsh CB, Hutchinson M. Trust the patient not the doctor: the determinants of quality of life in cervical dystonia. Front Neurol. (2020) 11:991. doi: 10.3389/fneur.2020.00991
12. Klingelhoefer L, Kaiser M, Sauerbier A, Untucht R, Wienecke M, Mammadova K, et al. Emotional well-being and pain could be a greater determinant of quality of life compared to motor severity in cervical dystonia. J Neural Transm (Vienna). (2021) 128:305–14. doi: 10.1007/s00702-020-02274-z
13. Monaghan R, Cogley C, Burke T, McCormack D, O'Riordan S, Ndukwe I, et al. Non-motor features of cervical dystonia: cognition, social cognition, psychological distress and quality of life. Clin Park Relat Disord. (2021) 4:100084. doi: 10.1016/j.prdoa.2020.100084
14. Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. (2011) 20:1727–36. doi: 10.1007/s11136-011-9903-x
15. Alvarado-Bolanos A, Cervantes-Arriaga A, Rodriguez-Violante M, Llorens-Arenas R, Calderon-Fajardo H, Millan-Cepeda R, et al. Convergent validation of EQ-5D-5L in patients with Parkinson's disease. J Neurol Sci. (2015) 358:53–7. doi: 10.1016/j.jns.2015.08.010
16. Wei QQ, Hou Y, Chen Y, Ou R, Cao B, Zhang L, et al. Health-related quality of life in amyotrophic lateral sclerosis using EQ-5D-5L. Health Qual Life Outcomes. (2021) 19:181. doi: 10.1186/s12955-021-01822-9
17. Zhang Y, Taylor BV, Simpson SJr, Blizzard L, Campbell JA, Palmer AJ, et al. Feelings of depression, pain and walking difficulties have the largest impact on the quality of life of people with multiple sclerosis, irrespective of clinical phenotype. Mult Scler. (2021) 27:1262–75. doi: 10.1177/1352458520958369
18. Luo N, Liu G, Li M, Guan H, Jin X, Rand-Hendriksen K. Estimating an EQ-5D-5L value set for China. Value Health. (2017) 20:662–9. doi: 10.1016/j.jval.2016.11.016
19. Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. (1967) 6:278–96. doi: 10.1111/j.2044-8260.1967.tb00530.x
20. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. (1959) 32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x
21. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. (1991) 14:540–5. doi: 10.1093/sleep/14.6.540
22. Buysse DJ, Reynolds 3rd CF, Monk TH, Berman SR, Kupfer DJ. The pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. (1989) 28:193–213. doi: 10.1016/0165-1781(89)90047-4
23. Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. (2005) 53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x
24. Smit M, Bartels AL, Kuiper A, Kamphuis ASJ, Han V, Tijssen MAJ. The frequency and self-perceived impact on daily life of motor and non-motor symptoms in cervical dystonia. Mov Disord Clin Pract. (2017) 4:750–4. doi: 10.1002/mdc3.12510
25. Junker J, Berman BD, Hall J, Wahba DW, Brandt V, Perlmutter JS, et al. Quality of life in isolated dystonia: non-motor manifestations matter. J Neurol Neurosurg Psychiatry. (2021) doi: 10.1136/jnnp-2020-325193. [Epub ahead of print].
26. Chan J, Brin MF, Fahn S. Idiopathic cervical dystonia: clinical characteristics. Mov Disord. (1991) 6:119–26. doi: 10.1002/mds.870060206
27. Charles PD, Adler CH, Stacy M, Comella C, Jankovic J, Manack Adams A, et al. Cervical dystonia and pain: characteristics and treatment patterns from CD PROBE (cervical dystonia patient registry for observation of onabotulinumtoxina efficacy). J Neurol. (2014) 261:1309–19. doi: 10.1007/s00415-014-7343-6
28. Rodrigues FB, Duarte GS, Marques RE, Castelao M, Ferreira J, Sampaio C, et al. Botulinum toxin type a therapy for cervical dystonia. Cochrane Database Syst Rev. (2020) 11:CD003633. doi: 10.1002/14651858.CD003633.pub4
29. Rosales RL, Cuffe L, Regnault B, Trosch RM. Pain in cervical dystonia: mechanisms, assessment and treatment. Expert Rev Neurother. (2021) 21:1125–34. doi: 10.1080/14737175.2021.1984230
30. Medina Escobar A, Pringsheim T, Goodarzi Z, Martino D. The prevalence of depression in adult onset idiopathic dystonia: systematic review and metaanalysis. Neurosci Biobehav Rev. (2021) 125:221–30. doi: 10.1016/j.neubiorev.2021.02.036
31. Zoons E, Tijssen MAJ, Dreissen YEM, Speelman JD, Smit M, Booij J. The relationship between the dopaminergic system and depressive symptoms in cervical dystonia. Eur J Nucl Med Mol Imaging. (2017) 44:1375–82. doi: 10.1007/s00259-017-3664-x
32. Hertenstein E, Tang NK, Bernstein CJ, Nissen C, Underwood MR, Sandhu HK. Sleep in patients with primary dystonia: a systematic review on the state of research and perspectives. Sleep Med Rev. (2016) 26:95–107. doi: 10.1016/j.smrv.2015.04.004
33. Paus S, Gross J, Moll-Muller M, Hentschel F, Spottke A, Wabbels B, et al. Impaired sleep quality and restless legs syndrome in idiopathic focal dystonia: a controlled study. J Neurol. (2011) 258:1835–40. doi: 10.1007/s00415-011-6029-6
34. Eichenseer SR, Stebbins GT, Comella CL. Beyond a motor disorder: a prospective evaluation of sleep quality in cervical dystonia. Parkinsonism Relat Disord. (2014) 20:405–8. doi: 10.1016/j.parkreldis.2014.01.004
Keywords: cervical dystonia, HRQoL, EQ-5D-5L, non-motor symptoms, pain, depression
Citation: Liang Y, Lin J, Hou Y, Zhang L, Ou R, Li C, Wei Q, Cao B, Liu K, Jiang Z, Yang T, Yang J, Zhang M, Kang S, Xiao Y, Jiang Q, Yang J, Song W, Chen X, Zhao B, Wu Y and Shang H (2022) Health-Related Quality of Life in Cervical Dystonia Using EQ-5D-5L: A Large Cross-Sectional Study in China. Front. Neurol. 13:895272. doi: 10.3389/fneur.2022.895272
Received: 13 March 2022; Accepted: 27 May 2022;
Published: 23 June 2022.
Edited by:
Maria Fiorella Contarino, Leiden University Medical Center, NetherlandsReviewed by:
Cristian Falup-Pecurariu, Transilvania University of Braşov, RomaniaCopyright © 2022 Liang, Lin, Hou, Zhang, Ou, Li, Wei, Cao, Liu, Jiang, Yang, Yang, Zhang, Kang, Xiao, Jiang, Yang, Song, Chen, Zhao, Wu and Shang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huifang Shang, aGZzaGFuZzIwMDJAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.