
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 26 May 2022
Sec. Neurological Biomarkers
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.893624
This article is part of the Research TopicPotential Biomarkers in Neurovascular DisordersView all 50 articles
 Yunfei Xu1,2,3,4
Yunfei Xu1,2,3,4 Haoduo Qiao1,2,3,4
Haoduo Qiao1,2,3,4 Shun Yang1
Shun Yang1 Lin Zhou1,2,3,4
Lin Zhou1,2,3,4 Yao Zhao1,2,3,4
Yao Zhao1,2,3,4 Qing Xu1,2,3,4
Qing Xu1,2,3,4 Shuying Miao4,5
Shuying Miao4,5 Dun Yuan1*
Dun Yuan1* Jie Zhao1*
Jie Zhao1* Ying Liu1,2,3,4*
Ying Liu1,2,3,4*Background and Purpose: Stroke is a serious fatal and disabling disease. Stroke-associated pneumonia (SAP) is the most common complication of stroke, which may further aggravate the stroke. The prevention and early prediction of SAP is a key clinical strategy. 15-hydroxyprostaglandin dehydrogenase (15-PGDH) is involved in pneumonia, while its relationship with SAP has yet to be determined. Therefore, we investigated the predictive value of 15-PGDH for SAP and visualized their relationship.
Methods: Stroke patients were recruited and divided into SAP group and Non-SAP group. Baseline demographic and clinical data were obtained from the medical record system, blood samples were collected to detect relevant variables and 15-PGDH levels. Patient characteristics were compared with a t-test. Binary logistic regression analysis was performed to determine the predictive value of 15-PGDH for SAP. Restricted cubic splines (RCS) were performed to visualize the relationship between 15-PGDH and SAP risk. Finally, the SAP patient characteristics between the severe group and mild group were compared.
Results: 50 patients were enrolled and divided into SAP group (n = 26) and Non-SAP group (n = 24). 15-PGDH in the SAP group was lower than that in the Non-SAP group (0.258 ± 0.275 vs. 0.784 ± 0.615, p = 0.025). Binary logistic regression analysis revealed that the lower 15-PGDH, the higher the risk of SAP (OR = 0.04, 95%CI, 0.010–0.157, p < 0.001). The RCS model showed the L-shaped relationship between 15-PGDH and SAP.
Conclusions: In stroke patients, serum 15-PGDH is a valuable biomarker for predicting SAP. There is an L-shaped relationship between the level of 15-PGDH and the risk of SAP.
Stroke is a common acute cerebrovascular disease, which is the second leading cause of death and the third-leading cause of death and disability combined globally (1). The high mortality rate of stroke is largely attributable to its complications. Stroke-associated pneumonia (SAP) is the most common complication of stroke, occurring most often within the first week in 8.6% to 14.3% of stroke patients (2–4). Stroke activates inflammatory cascades in the brain, such as the excessive release of cytokines, then stimulates the central immune system to produce anti-inflammatory signals. Subsequently, the efferent vagus nerve is activated and inhibits cytokine production, ultimately leading to the suppression of systemic immunity. Stroke-induced immunosuppression increases the susceptibility of stroke patients to infection, accompanied by decreased pulmonary clearance and impaired secretion drainage caused by the alteration in tracheal endothelia after stroke, ultimately leading to the occurrence of SAP (5–7). At the same time, risk factors such as stroke severity, advanced age, hypertension, and smoking history also contribute to the occurrence of SAP (8, 9). SAP further increases the severity of the stroke, lengthens hospital stay, increases financial burden, worsens the functional outcome, and increases patient mortality (9, 10). Prevention and early diagnosis or prediction of SAP is an important clinical strategy. However, current methods are less specific and sensitive, making the early diagnosis of SAP become a big difficulty (3, 11). There is still great value in exploring the early markers of SAP.
Prostaglandin E2 (PGE2) is a key mediator of inflammation, its elevation is a response to inflammation (12). 15-hydroxyprostaglandin dehydrogenase (15-PGDH) is the sole degradation enzyme of PGE2 by oxidizing it to inactive 15-keto PGE2 (13). The decrease of 15-PGDH can lead to an increase in PGE2, which has been observed in stroke (14). Meanwhile, 15-PGDH is generally identified as a tumor suppressor, such as lung cancer, breast cancer, colorectal cancer, etc (15–17). In addition, 15-PGDH is also involved in lipopolysaccharide-induced acute kidney injury, pulmonary fibrosis, tissue repair, and other pathological processes (18–20). Interestingly, a previous study reported that in pulmonary infection caused by pseudomonas aeruginosa, inhibition of PGE2 overproduction shows a protective effect, in part by raising the level of 15-PGDH (21). However, no report has revealed the level of 15-PGDH in SAP and the relationship between 15-PGDH and SAP. Therefore, we will determine the relationship between 15-PGDH and SAP, and determine whether it can be used as an early marker to predict SAP and provide a basis for early diagnosis of SAP.
This was a retrospective study, all the patients with hemorrhagic stroke or ischemic stroke were recruited from the Department of Neurosurgery, Xiangya Hospital, Central South University between February 2019 to June 2021. This research was approved by the ethics committee of the Xiangya Hospital of Central South University and the human ethics identification number is 2021101119. All participants involved in this study provided written informed consent.
The inclusion criteria of stroke patients were as follows: (1) age≥18 years; (2) stroke onset within 48 h; (3) acute stroke confirmed by brain magnetic resonance imaging (MRI) or brain computerized tomography (CT). The exclusion criteria were as follows: (1) active infection or pneumonia before admission; (2) patients treated with nonsteroidal anti-inflammatory drugs; (3) patients with incomplete clinical records. Besides, the diagnosis of SAP was performed by two experienced neurologists based on the modified Centers for Disease Control and Prevention criteria of hospital- acquired pneumonia (22), assisted with clinical and laboratory parameters of respiratory infection and was confirmed by chest X-ray or CT (23).
54 Patients Were Recruited, 4 Patients Were Excluded, and 50 Patients Were Included in This Study. Further, 26 Patients Were Diagnosed in the SAP Group and 24 Patients Were Diagnosed in the Non-SAP Group (Figure 1). The SAP Group Was Evaluated by Pneumonia Severity Index (PSI) for Severity and Was Divided Into Severe Group (PSI>90) and the Mild Group (PSI ≤ 90) (24).
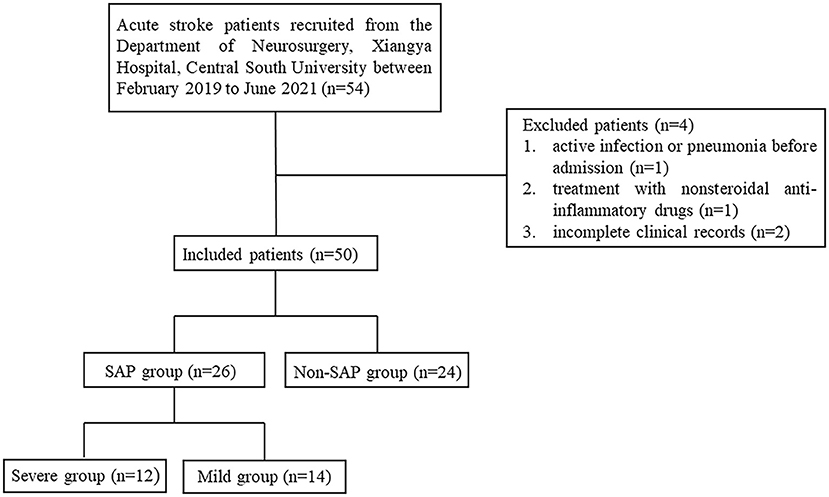
Figure 1. Flowchart of case recruitment. SAP, stroke-associated pneumonia; PSI, pneumonia severity index.
Baseline demographic and clinical data were obtained from the medical record system, mainly including age, sex, risk factors or past medical history such as history of smoking and drinking, hypertension, hyperlipidemia, coronary heart disease, chronic obstructive pulmonary disease (COPD), diabetes mellitus, and nephropathy, as well as ICU admission, hospitalization time, stroke subtype. Glasgow coma score (GCS) was used to assess the severity of stroke at admission. Blood samples were collected the morning after admission and tested for the counts of white blood cell (WBC), red blood cell (RBC), hemoglobin, platelets, neutrophils, and lymphocytes. Further, after SAP diagnosis, PSI was used to assess the severity of SAP.
Blood samples were collected the morning after admission. After centrifugation at 2000 g for 10 min, serum was isolated and frozen at −80°C for later measurement. A Human 15-PGDH/HPGD enzyme-linked immunosorbent assay (ELISA) kit (Cat No. ELH-15PGDH-1, RayBiotech) was used to detect the levels of serum 15-PGDH. Detect and calculate the results according to the instructions of the kit.
All statistical analyses were performed using Statistical Package for the Social Sciences version 26 (SPSS) and R (version 4.1.2). Normally distributed continuous variables were presented as the mean ± standard deviation, while other continuous variables were presented as the median and interquartile range (IQR). Continuous variables were analyzed by t-test or the Mann–Whitney U-test. Classified data were presented as percentages and analyzed by the chi-square test or Fisher exact test. Binary Logistic regression analysis was used to confirm the contribution of risk factors to SAP occurrence. The receiver operating characteristic (ROC) curve was used to determine the discrimination ability of 15-PGDH levels to predict SAP. The area under the curve (AUC) was determined from the ROC curve analysis. Restricted cubic splines (RCS) with four knots were performed to visualize the relationship between 15-PGDH and SAP risk. All statistical analyses were two-sided and p < 0.05 was set to be a statistical difference.
From February 2019 to June 2021, 54 stroke patients were recruited from the Department of Neurosurgery, Xiangya Hospital, Central South University. While 4 patients were excluded, 1 patient due to active infection or pneumonia before admission, 1 patient due to treatment with non-steroidal anti-inflammatory drugs, and 2 patients due to incomplete clinical records. Finally, 50 patients were included in the study. Twenty Six patients were assigned to the SAP group (52%) and 24 to the Non-SAP group (48%). Further, according to PSI, the SAP group was further divided into severe group (n = 12) and mild group (n = 14). These results were summarized in Figure 1.
As shown in Table 1, age (p = 0.011), smoking (p = 0.037), the counts of WBC (p = 0.034) and neutrophils (p = 0.012) and hospitalization time (p = 0.039) in SAP group were significantly higher than those in Non-SAP group, and GCS score (p = 0.029), the counts of thrombocytes (p = 0.032) and lymphocytes (p = 0.021) in SAP group were significantly lower than those in Non-SAP group. While other variables including sex, drinking, hypertension, hyperlipidemia, coronary heart disease, COPD, diabetes mellitus, nephropathy, stroke type, the counts of RBC and hemoglobin were not statistically different. Notably, the level of 15-PGDH (p = 0.025) in the SAP group was significantly lower than that in the Non-SAP group, indicating that there may be some relationship between 15-PGDH and SAP, which is what we will analyze next.
A binary logistic regression analysis was performed to determine which variables could be potential for predicting SAP. As shown in Table 2, higher age (OR = 1.057, 95%CI = 1.026–1.090, p < 0.001), smoking (OR = 4.375, 95%CI = 2.214–9.010, p < 0.001), WBC (OR = 1.283, 95%CI = 1.128–1.459, p < 0.001), neutrophil (OR = 1.088, 95%CI = 1.015–1.166, p = 0.017) and lower GCS (OR = 0.754, 95%CI = 0.658–0.864, p < 0.001), thrombocytes (OR = 0.992, 95%CI = 0.988–0.996, p < 0.001), lymphocytes (OR = 0.575, 95%CI = 0.354–0.933, p = 0.025) were risk factors of SAP.
Notably, lower 15-PGDH (OR = 0.040, 95%CI = 0.010–0.157, p < 0.001) was also a risk factor for SAP. Further, according to the ROC curve in Figure 2, the AUC of 15-PGDH for predicting SAP was 0.836, 95%CI = 0.751–0.922, p < 0.001. Besides, the optimal cutoff value of 15-PGDH for SAP was 0.186 ng/mL, with a specificity of 100% and a sensitivity of 59.1%.
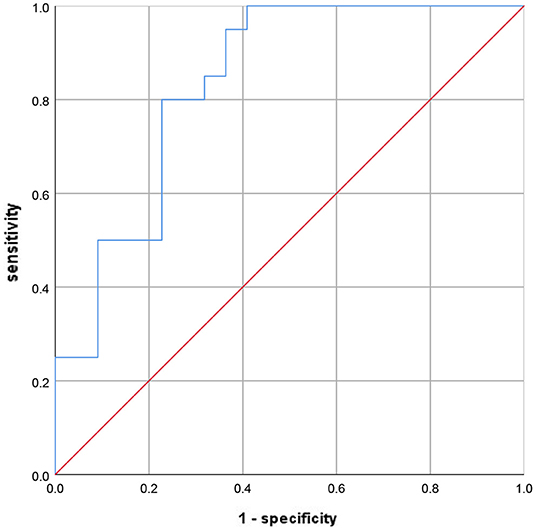
Figure 2. The ROC curve to evaluate the diagnostic value of 15-PGDH for SAP. ROC, receiver operating characteristic; SAP, stroke-associated pneumonia; 15-PGDH, 15-hydroxyprostaglandin dehydrogenase.
To further visualize the relationship between 15-PGDH and SAP risk, restricted cubic spline was next performed. As shown in the Figure 3, the lowest level of 15-PGDH represented the highest risk of SAP. With the increase of 15-PGDH, the risk of SAP decreases continuously. In particular, 0.37 ng/ mL was a point of interest. When it was lower than 0.37 ng/ mL, the risk of SAP increased sharply with the decrease of 15-PGDH, while when it was higher than 0.37 ng/ mL, the risk of SAP increased relatively gentle.
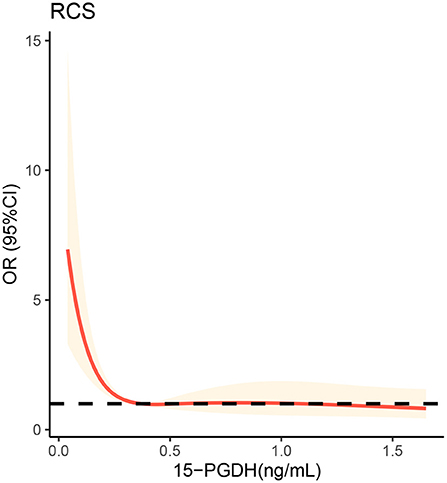
Figure 3. Restricted cubic spline analysis to evaluate the association between serum 15-PGDH levels and risk of SAP. SAP, stroke-associated pneumonia; OR, odds ratio; 95%CI, 95% confidence interval; 15-PGDH, 15-hydroxyprostaglandin dehydrogenase.
According to PSI, the SAP group was further divided into severe group (n = 12) and mild group (n = 14). There were some differences between the two groups in terms of patient characteristics in Table 3. Patients in the severe group observed higher age (p < 0.001), WBC count (p = 0.017) and neutrophils count (p = 0.018) than those in the mild group.
Two important findings are presented in this study. First, lower serum 15-PGDH increases the risk of SAP in stroke patients. Second, there is an L-shaped relationship between 15-PGDH and SAP risk. Once 15-PGDH is lower than the critical value, SAP risk will be significantly increased.
Stroke is an acute neurovascular disease with high morbidity, mortality and disability. SAP is the most common complication of stroke, usually manifests within 7 days after the onset of stroke (3). The complication of SAP further aggravates the severity of stroke, prolonged hospitalization, worsen the prognosis, and increase mortality (6, 10). The occurrence of SAP increases the difficulty of stroke treatment. Therefore, the prevention and early prediction or diagnosis of SAP should not be ignored in the treatment of stroke patients. Our study demonstrates that advanced age, smGoking, higher WBC and neutrophils, and lower GCS, thrombocytes, and lymphocytes are all risk factors for SAP. In addition, previous studies have found that dysphagia (25), aspiration (26), invasive treatments, antacids, hypertension, diabetes, pulmonary diseases and so on are also risk factors for SAP (27). Prevention and early diagnosis of so many risk factors are an important part of stroke and SAP management.
Inflammation-related indicators are the most important predictive markers of SAP and contribute greatly to the early diagnosis of SAP. Monocyte to lymphocyte ratio (28), neutrophil to lymphocyte ratio (24) and platelet to lymphocyte ratio (29) have been proven to be valuable predictors of SAP markers. PGE2 is an important inflammatory mediator involved in many inflammatory processes. As the degradation enzyme of PGE2, 15-PGDH is potential to become one predictive marker of SAP. In our study, serum 15-PGDH level in patients with SAP were significantly lower than those without SAP. Binary logistic regression analysis proved that low 15-PGDH was a risk factor for SAP. With an AUC of 0.836, 15-PGDH is undoubtedly a valuable predictor of SAP. Furthermore, RCS visualizes the relationship between 15-PGDH and SAP risk as L-shaped, which may contribute significantly to the prediction and treatment of SAP. It should be noted that the 15-PGDH concentration of 0.37 ng/mL is a critical threshold. When 15-PGDH is lower than 0.37 ng/mL, its decrease will sharply increase the risk of SAP, while when higher than 0.37 ng/mL, this trend is very gentle. Therefore, when assessing the risk of SAP, on the premise of avoiding the reduction of 15-PGDH, it is more important to ensure that 15-PGDH should not be lower than 0.37 ng/mL, which may be critical for SAP prevention.
Admittedly, there are some limitations to our research. First, since our study is a retrospective study, it is difficult to draw a causal relationship between 15-PGDH and SAP, which requires further verification by subsequent studies. Second, we did not collect enough patients, which may lead to partial results bias. For example, the incidence of SAP was higher than previously reported. Third, although we compared the characteristics of SAP patients in the severe group and mild group, it is unclear whether the level of 15-PGDH is predictive of the severity of SAP. Furthermore, we did not analyze patients with different stroke types separately, and there may be some different findings.
In stroke patients, serum 15-PGDH is a valuable biomarker for predicting SAP. There is an L-shaped relationship between the level of 15-PGDH and the risk of SAP. Once lower than 0.37 ng/mL, the risk of SAP will increase rapidly.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Ethics Committee of the Xiangya Hospital of Central South University. The patients/participants provided their written informed consent to participate in this study.
DY, YL, and JZ designed and directed the project. SY collected clinical samples. HQ collected baseline demographic and clinical data. YX, LZ, YZ, and QX performed the experiments. YX wrote the manuscript.
This work was supported by the National Natural Science Foundation of China (Nos. 82172147, 81571880, 81373147, 30901555, 30972870, and 81360080), Natural Science Foundation of Hunan Province (2021JJ30900 and 2016JJ2157), and the Fundamental Research Funds for the Central Universities of Central South University (2020zzts222). All funding bodies were involved in the study design, collection, analysis, interpretation of data, and writing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the patients for granting permission to publish this information.
15-PGDH, 15-hydroxyprostaglandin dehydrogenase; AUC, area under the curve; COPD, chronic obstructive pulmonary disease; CI, confidence interval; CT, computerized tomography; ELISA, enzyme-linked immunosorbent assay; GCS, Glasgow coma score; IQR, interquartile range; MRI, magnetic resonance imaging; OR, odds ratio; PGE2, prostaglandin E2; PSI, pneumonia severity index; RBC, red blood cell; RCS, restricted cubic splines; ROC, receiver operating characteristic; SAP, stroke-associated pneumonia; WBC, white blood cell.
1. Global regional and and national burden of stroke and its risk factors 1990-2019: 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. 2021 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
2. Cugy E, Sibon I. Stroke-Associated pneumonia risk score: validity in a French stroke unit. J Stroke Cerebrovasc Dis. (2017) 26:225–9. doi: 10.1016/j.jstrokecerebrovasdis.2016.09.015
3. Kishore AK, Vail A, Chamorro A, Garau J, Hopkins SJ, Di Napoli M, et al. How is pneumonia diagnosed in clinical stroke research? a systematic review and meta-analysis. Stroke. (2015) 46:1202–9. doi: 10.1161/STROKEAHA.114.007843
4. Chen Y, Yang H, Wei H, Chen Y, Lan M. Stroke-associated pneumonia: a bibliometric analysis of worldwide trends from 2003 to 2020. Medicine (Baltimore). (2021) 100:e27321. doi: 10.1097/MD.0000000000027321
5. Johnston KC Li JY, Lyden PD, Hanson SK, Feasby TE, Adams RJ, et al. Medical and neurological complications of ischemic stroke: experience from the RANTTAS trial. RANTTAS Investigators Stroke. (1998) 29:447–53. doi: 10.1161/01.str.29.2.447
6. Finlayson O, Kapral M, Hall R, Asllani E, Selchen D, Saposnik G. Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology. (2011) 77:1338–45. doi: 10.1212/WNL.0b013e31823152b1
7. Faura J, Bustamante A, Miró-Mur F, Montaner J. Stroke-Induced immunosuppression: implications for the prevention and prediction of post-stroke infections. J Neuroinflammation. (2021) 18:127. doi: 10.1186/s12974-021-02177-0
8. Suda S, Aoki J, Shimoyama T, Suzuki K, Sakamoto Y, Katano T, et al. Stroke-associated infection independently predicts 3-month poor functional outcome and mortality. J Neurol. (2018) 265:370–5. doi: 10.1007/s00415-017-8714-6
9. Teh WH, Smith CJ, Barlas RS, Wood AD, Bettencourt-Silva JH, Clark AB, et al. Impact of stroke-associated pneumonia on mortality, length of hospitalization, and functional outcome. Acta Neurol Scand. (2018) 138:293–300. doi: 10.1111/ane.12956
10. Smith CJ, Bray BD, Hoffman A, Meisel A, Heuschmann PU, Wolfe CD, et al. Can a novel clinical risk score improve pneumonia prediction in acute stroke care? a UK multicenter cohort study. J Am Heart Assoc. (2015) 4:e001307. doi: 10.1161/JAHA.114.001307
11. Warusevitane A, Karunatilake D, Sim J, Smith C, Roffe C. Early diagnosis of pneumonia in severe stroke: clinical features and the diagnostic role of C-reactive protein. PLoS ONE. (2016) 11:e0150269. doi: 10.1371/journal.pone.0150269
12. Mahesh G, Anil Kumar K, Reddanna P. Overview on the discovery and development of anti-inflammatory drugs: should the focus be on synthesis or degradation of PGE(2)? J Inflamm Res. (2021) 14:253–63. doi: 10.2147/JIR.S278514
13. Shin MK, Koh Y, Vázquez-Rosa E, Cintrón-Pérez C, Markowitz S, Pieper AA. Inhibition of 15-hydroxyprostaglandin dehydrogenase protects a mouse model of Alzheimer's disease without affecting amyloid pathology. Alzheimers Dement. (2021) 17 (Suppl. 3):e052500. doi: 10.1002/alz.052500
14. Xu Y, Liu Y, Li K, Miao S, Lv C, Wang C, et al. Regulation of PGE(2) pathway during cerebral ischemia reperfusion injury in Rat. Cell Mol Neurobiol. (2021) 41:1483–96. doi: 10.1007/s10571-020-00911-5
15. Wang W, Yang C, Deng H. Overexpression of 15-hydroxyprostaglandin dehydrogenase inhibits A549 lung adenocarcinoma cell growth via inducing cell cycle arrest and inhibiting epithelial-mesenchymal transition. Cancer Manag Res. (2021) 13:8887–900. doi: 10.2147/CMAR.S331222
16. Volpato M, Cummings M, Shaaban AM, Abderrahman B, Hull MA, Maximov PY, et al. Downregulation of 15-hydroxyprostaglandin dehydrogenase during acquired tamoxifen resistance and association with poor prognosis in ERα-positive breast cancer. Explor Target Antitumor Ther. (2020) 1:355–71. doi: 10.37349/etat.2020.00021
17. Zhang S, Liu J, Yang M, Fan D, Gao J, Liu X, et al. Single nucleotide polymorphism rs2555639 in 15-PGDH and colorectal cancer metastasis. J buon. (2019) 24:1507–11.
18. Miao S, Lv C, Liu Y, Zhao J, Li T, Wang C, et al. Pharmacologic blockade of 15-PGDH protects against acute renal injury induced by LPS in mice. Front Physiol. (2020) 11:138. doi: 10.3389/fphys.2020.00138
19. Smith JNP, Witkin MD, Jogasuria AP, Christo KF, Raffay TM, Markowitz SD, et al. Therapeutic targeting of 15-PGDH in murine pulmonary fibrosis. Sci Rep. (2020) 10:11657. doi: 10.1038/s41598-020-68336-0
20. Palla AR, Ravichandran M, Wang YX, Alexandrova L, Yang AV, Kraft P, et al. Inhibition of prostaglandin-degrading enzyme 15-PGDH rejuvenates aged muscle mass and strength. Science. (2021) 371:abc8059. doi: 10.1126/science.abc8059
21. Mao YX, Xu JF, Seeley EJ, Tang XD, Xu LL, Zhu YG, et al. Adipose tissue-derived mesenchymal stem cells attenuate pulmonary infection caused by pseudomonas aeruginosa via inhibiting overproduction of prostaglandin E2. Stem Cells. (2015) 33:2331–42. doi: 10.1002/stem.1996
22. Smith CJ, Kishore AK, Vail A, Chamorro A, Garau J, Hopkins SJ, et al. Diagnosis of stroke-associated pneumonia: recommendations from the pneumonia in stroke consensus group. Stroke. (2015) 46:2335–40. doi: 10.1161/STROKEAHA.115.009617
23. Shi G, Li M, Zhou R, Wang X, Xu W, Yang F, et al. Procalcitonin related to stroke-associated pneumonia and clinical outcomes of acute ischemic stroke after IV rt-PA treatment. Cell Mol Neurobiol. (2021). doi: 10.1007/s10571-020-01031-w
24. Nam KW, Kim TJ, Lee JS, Kwon HM, Lee YS, Ko SB, et al. High neutrophil-to-lymphocyte ratio predicts stroke-associated pneumonia. Stroke. (2018) 49:1886–92. doi: 10.1161/STROKEAHA.118.021228
25. Eltringham SA, Kilner K, Gee M, Sage K, Bray BD, Smith CJ, et al. Factors associated with risk of stroke-associated pneumonia in patients with dysphagia: a systematic review. Dysphagia. (2020) 35:735–44. doi: 10.1007/s00455-019-10061-6
26. Hannawi Y, Hannawi B, Rao CP, Suarez JI, Bershad EM. Stroke-associated pneumonia: major advances and obstacles. Cerebrovasc Dis. (2013) 35:430–43. doi: 10.1159/000350199
27. Liu DD, Chu SF, Chen C, Yang PF, Chen NH, He X. Research progress in stroke-induced immunodepression syndrome (SIDS) and stroke-associated pneumonia (SAP). Neurochem Int. (2018) 114:42–54. doi: 10.1016/j.neuint.2018.01.002
28. Cheng HR, Song JY, Zhang YN, Chen YB, Lin GQ, Huang GQ, et al. High monocyte-to-lymphocyte ratio is associated with stroke-associated pneumonia. Front Neurol. (2020) 11:575809. doi: 10.3389/fneur.2020.575809
Keywords: 15-PGDH, stroke, pneumonia, PGE2, predictor
Citation: Xu Y, Qiao H, Yang S, Zhou L, Zhao Y, Xu Q, Miao S, Yuan D, Zhao J and Liu Y (2022) 15-Hydroxyprostaglandin Dehydrogenase Is a Predictor of Stroke-Associated Pneumonia. Front. Neurol. 13:893624. doi: 10.3389/fneur.2022.893624
Received: 10 March 2022; Accepted: 27 April 2022;
Published: 26 May 2022.
Edited by:
Wen-Jun Tu, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaReviewed by:
Erming Zeng, Nanchang University, ChinaCopyright © 2022 Xu, Qiao, Yang, Zhou, Zhao, Xu, Miao, Yuan, Zhao and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying Liu, bGl1MTk3N3lpbmdAMTI2LmNvbQ==; Jie Zhao, c3RlZWx6akBjc3UuZWR1LmNu; Dun Yuan, eXVhbmR1bmxwQGhvdG1haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.