
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 22 June 2022
Sec. Neurological Biomarkers
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.893401
This article is part of the Research TopicPotential Biomarkers in Neurovascular DisordersView all 50 articles
 Fei-Hong Wang1†
Fei-Hong Wang1† Long-Yan Meng1†
Long-Yan Meng1† Tong-Ya Yu1
Tong-Ya Yu1 Yan Tan1
Yan Tan1 Hui Quan1
Hui Quan1 Jia-Yu Hu1
Jia-Yu Hu1 Qing-Ke Bai2*
Qing-Ke Bai2* Jun-Chao Xie1*
Jun-Chao Xie1* Yan-Xin Zhao1*
Yan-Xin Zhao1*Background: Abdominal obesity and adipocytokines are closely related to atherosclerosis, and adiponectin level is considered one of the important clinical indicators. This study aimed to analyze the associations of abdominal visceral fat content and adiponectin level with intracranial atherosclerotic stenosis (ICAS).
Methods: A total of 186 patients were enrolled in this study. Patients were distributed into ICAS and non-ICAS by the degree of artery stenosis. Plasma adiponectin levels and the ratio of visceral adipose tissue (VAT) to subcutaneous adipose tissue (SAT) were measured. The related factors of intracranial atherosclerotic stenosis were determined using multivariable logistic regression analysis.
Results: The VAT/SAT ratio (OR, 26.08; 95% CI, 5.92–114.83; p < 0.001) and adiponectin (OR, 0.61; 95% CI, 0.44–0.84; p = 0.002) were found to be the independent predictors of ICAS in a multivariable logistic regression analysis. The prevalence of ICAS increased (T1: 27.4%; T2: 50.0%; T3: 75.8%) as the VAT/SAT ratio tertile increased (p < 0.001). The prevalence of ICAS decreased (T1: 72.6%; T2: 54.8%; T3: 25.8%) as the adiponectin tertile increased (p < 0.001). In ROC curves analysis, VAT/SAT ratio had a sensible accuracy for the prediction of ICAS. The optimal cut-off value of VAT/SAT ratio to predict ICAS in this study was 1.04 (AUC: 0.747; p < 0.001; sensitivity: 67.4%; specificity: 74.7%). The optimal adiponectin cutoff was 3.03 ug/ml (AUC: 0.716; p < 0.001; sensitivity:75.8%; specificity: 61.5%).
Conclusion: Higher VAT/SAT ratio and lower plasma adiponectin levels were closely related to the increased risk of intracranial atherosclerotic stenosis.
Ischemic stroke caused by intracranial atherosclerotic stenosis is frequent in the Asian, African–American, and Hispanic populations (1). Large artery intracranial occlusive disease (LAICOD) has become the most common subtype of stroke in the world (2). Stroke is the leading cause of death in China, with a high recurrence rate (3). ICAS is an important predictor of stroke and its recurrence (4, 5). Looking for the relevant factors of ICAS is of great significance for the prediction and early intervention of stroke.
Several studies previously investigated traditional risk factors associated with ICAS including sex, age, metabolic syndrome (MetS), hyperlipidemia, hypertension, diabetes, smoking, and hyperhomocysteinemia (6). Lipid metabolism disorders including elevated low-density lipoprotein cholesterol (LDL-C) and cholesterol levels are the most important modifiable factors for increased risk of recurrent stroke and ICAS-related vascular events (4). Obesity is closely related to cardiovascular events (7). However, little is known about the relationship between obesity and intracranial atherosclerosis. Several obesity-related parameters, such as waist circumference, body mass index (BMI), and waist to hip ratio (WHR) have shown a weaker correlation with intracranial atherosclerosis (8). Abdominal obesity may be more likely to cause atherosclerosis than systemic obesity (9). Measurement of central obesity, especially VAT, may be more closely related to intracranial atherosclerosis. The increase of visceral fat and the decrease of subcutaneous fat accumulation may be positively correlated to atherosclerosis (10). Adiponectin, secreted by adipocytes, is a unique adipokine that has anti-inflammatory, antioxidant, anti-atherosclerotic effects and also a significant inhibitory effect on obesity (11–13). A recent study showed that the adiponectin-transfected endothelial progenitor cells protected T2DM rats from cerebral ischemia-reperfusion injury by increasing angiogenesis (14). Previous reports have shown that the levels of circulating adiponectin are reduced in patients with obesity, T2DM, coronary artery diseases, carotid artery stenosis, and atherosclerotic plaques (15, 16). However, there are few investigations on the relationship between adiponectin and intracranial atherosclerosis. Therefore, this study aimed to investigate the relationship between visceral fat, adiponectin, and intracranial atherosclerosis.
This was a single-center cross-sectional study with 226 consecutive patients recruited from September 2018 to June 2019 at Shanghai Tenth People's Hospital. Patients were admitted if they satisfied the following conditions: Age ≥ 45 years, complete abdominal non-enhanced CT (NECT), and CT angiography (CTA)/magnetic resonance angiography (MRA) of cerebral arteries. If the patient was < 45 years old, had acute cerebral infarction, missed key clinical data, had a history of severe abdominal organ disease or previous abdominal surgery, they would be excluded. All patients gave their informed permission. Gender, age, smoking history, low-density lipoprotein cholesterol, diabetes, hypertension, and coronary heart disease were acquired as baseline characteristics of classical cerebrovascular risk factors. The patients fasted overnight and had 5 ml venous blood drawn the next morning. Within 4 h after anticoagulation with 2% EDTA, the blood was centrifuged at 3,000 rpm for 15 min. Plasma was kept at −80°C in a refrigerator. An enzyme-linked immunosorbent assay (ELISA) kit from MultiSciences in China was used to determine the concentration of plasma adiponectin. Self-reported history of hypertension, use of anti-hypertensive medication before admission, and the diagnosis of hypertension after discharge are all considered hypertension. Diabetes mellitus (DM) was identified as a self-reported history of diabetes, diabetes medication, a glycated hemoglobin level of more than 7%, or a diabetes diagnosis at discharge. Smokers were defined as those who smoked for 6 months and had more than one cigarette per day, while heavy drinkers were defined as those whose average daily alcohol consumption was 2 units for men or 1 unit for women (17). For statistical purposes, we referred to some relevant references (18–20) and defined tertiles for adiponectin and VAT/SAT ratio separately as follows: T1, low-adiponectin (0.83–2.09 ug/ml); T2, middle-adiponectin (2.10–3.41 ug/ml); T3, high- adiponectin (3.42–6.38ug/ml); T1, low-VAT/SAT (0.30–0.84); T2, middle-VAT/SAT (0.85–1.13); T3, high-VAT/SAT (1.14–2.06).
A 16-section NECT (uCT 510, United Imaging Healthcare) was used to perform abdominal CT images (120 kVp, 100 mAs, 5 mm slide thickness). The intracranial arteries were examined using computed tomography angiography (CTA) to see whether there were any atherosclerotic lesions. Participants with renal insufficiency or a contraindication to contrast agents had their cerebral arteries examined with MRA to see if they had atherosclerotic stenosis. MRA was done using a 3.0T Verio Scanner (Siemens, Erlangen, Germany).
The cerebral atherosclerotic stenosis of each major intracranial artery was examined by two professional radiologists who were not given any personal information about the individuals. If a conflict arises, it is handled by consultation. Severe stenosis occurs when the lumen diameter of an intracranial artery is decreased by 50%. A stenosis of 50% or more in the large intracranial arteries, including intracranial segments of the internal carotid artery (ICA), anterior cerebral artery (ACA), middle cerebral artery (MCA), posterior cerebral artery (PCA), basilar artery (BA), and intracranial segments of vertebral artery (VA) was classified as the ICAS. The warfarin–aspirin symptomatic intracranial disease (WASID) study criteria were used to measure intracranial artery stenosis. The percentage of stenosis in an intracranial artery was calculated using the following formula: the percentage of intracranial atherosclerotic stenosis = {1–[(diameter of narrowest segment)/(diameter of normal segment)]} × 100%, where the diameter of the narrowest segment is the diameter of the artery at the site of the most severe stenosis, and diameter of the normal segment is the diameter of the proximal normal artery (21).
The abdominal circumference was measured at the level of the navel. At the level of the navel, ImageJ 1.52a (National Institute of Health, USA) was used to calculate VAT, SAT, and total adipose tissue (TAT) (22). The attenuation threshold was −190 to −30 Hu. The region of interest (ROI) for subcutaneous adipose was drawn along the outline of the abdominal skin, and ROI for visceral adipose was drawn along the inner edge of the abdominal muscles and the anterior edge of the spine, and then the full pixel area within the attenuation of the region of interest was measured. TAT was calculated by VAT plus SAT (23). The VAT/SAT ratio was determined using these two parameters. The liver/spleen (L/S) attenuation ratio <1 was used to characterize non–alcoholic fatty liver disease (NAFLD). Regions of interest with an area of over 2 cm2 were placed at different axial levels of the liver, while the spleen was in the same axial image correspondingly, vessels and biliary structures in the liver were avoided (22).
We used the χ2 test and Fisher's exact categorical variables test, as well as independent samples t-tests or Mann-Whitney U tests, depending on the distribution of continuous variables, to perform univariate analyses of demographic characteristics, vascular risk factors, laboratory indices, and imaging characteristics of patients in the ICAS and non-ICAS groups. The mean ± standard deviation or median with interquartile range is used to represent continuous variables. The Shapiro–Wilk test was employed to determine the distribution's normality. After examining standard cerebrovascular risk variables in the study, multivariable logistic regression analysis was used to discover the independent risk factors of intracranial atherosclerosis. The connection between abdominal fat-related measures and adiponectin levels was assessed using the Spearman correlation coefficient. Subjects were placed into three groups with the same sample size based on the tertiles of plasma adiponectin level or VAT/SAT. The prevalence of ICAS was evaluated in each tertile of plasma adiponectin level or VAT/SAT. The efficacy of VAT/SAT and adiponectin to predict cerebral atherosclerosis was assessed using the receiver-operating characteristic (ROC) curve. To distinguish their prediction powers, researchers employed the MedCalc statistical software. The value p < 0.05 was regarded as statistically significant, and SPSS v25 was used for statistical analysis (IBM Corporation, New York, United States).
Due to a lack of key clinical data, a history of severe abdominal organ disease or previous abdominal surgery, 40 patients were removed from the data analysis. Finally, 186 patients (59.1% men; mean age: 67.19 ± 8.68 years) were enrolled in the study, including the non-ICAS group (n = 91; 48.9%) and the ICAS group (n = 95; 51.1%) (Table 1). When compared to the non-ICAS group, patients in the ICAS group had a larger VAT/SAT ratio (1.15 ± 0.30 vs. 0.89 ± 0.25, p < 0.001), a greater prevalence of NAFLD (37.9% vs. 19.8%, p = 0.007), a higher prevalence of diabetes (55.8% vs. 30.8%, p = 0.001), higher elevated fibrinogen levels (2.96 ± 1.03 vs. 2.55 ± 0.77, p = 0.002), and lower adiponectin levels (2.43 ± 0.99 vs. 3.36 ± 1.27, p < 0.001). However, there was no statistical significance in coronary heart disease, carotid intima-media thickness (IMT), carotid plaque, hypertension, triglyceride level, or LDL-C level between the two groups (p>0.05 for all). The lipid ratio is considered a good parameter for identifying ICAS (24). Referring to their research, we also included LDL-C/HDL-C, TC/HDL-C, TG/HDL-C, non-HDL-C/HDL-C in the analysis, and there was no statistical difference between the two groups (Table 1). Univariate analysis revealed that the ICAS group had a substantially larger abdominal perimeter, VAT area, and VAT/SAT than the non-ICAS group [90.77 ± 10.37 vs. 87.69 ± 10.61, p = 0.046; 184.53 ± 57.58 vs. 154.27 ± 52.30, p < 0.001; 1.15 ± 0.30 vs. 0.89 ± 0.25, p < 0.001] (Table 1). In multivariate logistic regression analysis, after controlling for potential confounders, we discovered that the VAT/SAT ratio (OR, 26.08; 95% CI, 5.92–114.83; p < 0.001), NAFLD (OR, 2.95; 95% CI, 1.33–6.50; p = 0.008), fibrinogen levels (OR, 1.80; 95% CI, 1.12–2.88; p = 0.014), diabetes mellitus (OR, 2.51; 95% CI, 1.22–5.18; p = 0.013), and comparatively low adiponectin levels (OR, 0.61; 95% CI, 0.44–0.84; p = 0.002) were independent predictors of increased ICAS risk (Table 2).
We used tertiles to categorize all VAT/SAT ratios and adiponectin levels to further investigate the relationship between ICAS, VAT/SAT, and adiponectin (Figure 1). The prevalence of ICAS increased (T1: 27.4%; T2: 50.0%; T3: 75.8%) as VAT/SAT ratio tertile increased (p < 0.001). The prevalence of ICAS decreased (T1: 72.6%; T2: 54.8%; T3: 25.8%) as adiponectin tertile increased (p < 0.001).
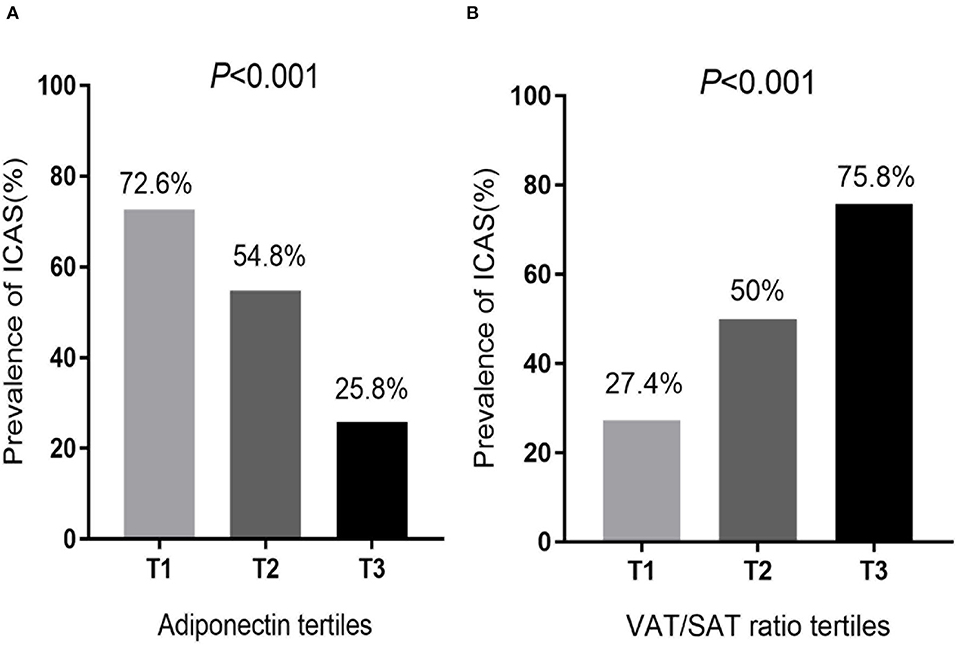
Figure 1. Comparison of (A) adiponectin tertiles and (B) VAT/SAT ratio tertiles determined according to the prevalence of ICAS. (A) Participants were divided into 3 groups, T1: low-adiponectin (0.83–2.09 ug/ml), T2: middle-adiponectin (2.10–3.41 ug/ml), and T3: high-adiponectin (3.42–6.38 ug/ml). (B) Participants were divided into 3 groups, T1: low-VAT/SAT (0.30–0.84), T2: middle-VAT/SAT (0.85–1.13), and T3: high-VAT/SAT (1.14–2.06). VAT, Visceral adipose tissue; SAT, Subcutaneous adipose tissue; ICAS, Intracranial atherosclerotic stenosis.
To investigate the relationship between adiponectin and obesity, we performed a Spearman correlation analysis of adiponectin and abdominal fat-related parameters (Figure 2; Table 3). VAT/SAT ratio (R = −0.337, p < 0.001) was negatively correlated with adiponectin compared with VAT (R = −0.239, p = 0.001), abdominal circumference (R = −0.170, p = 0.021), and BMI (R = −0.155, p = 0.034).
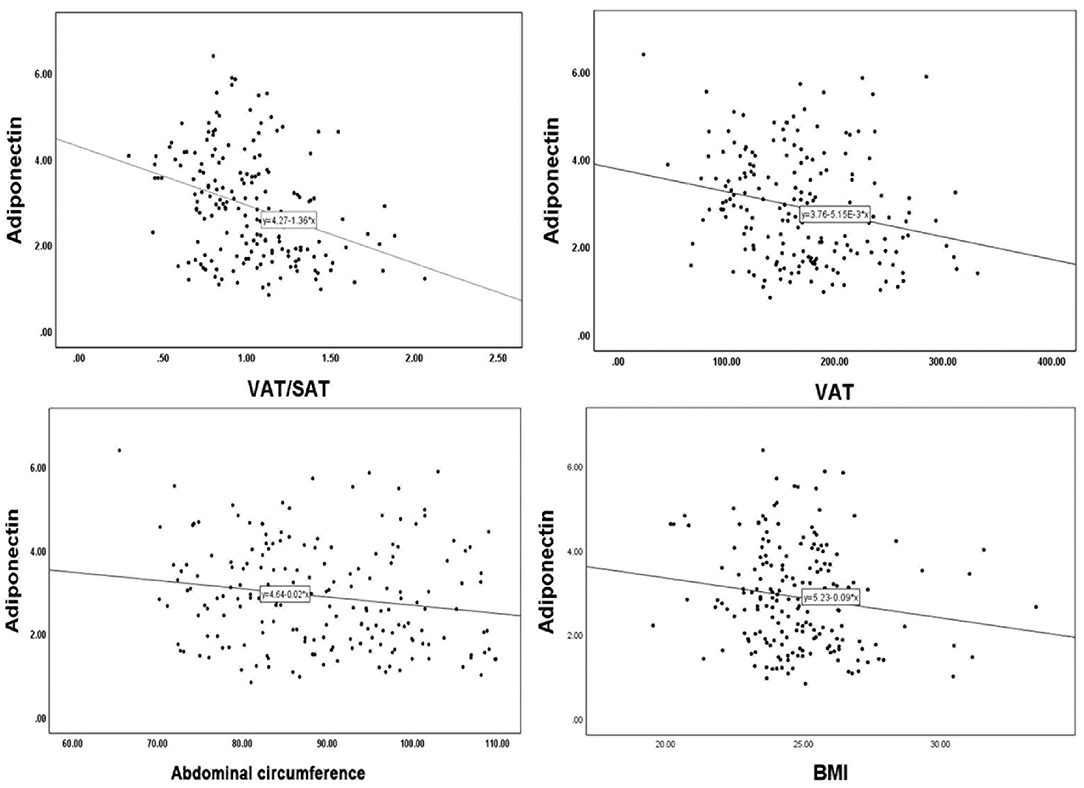
Figure 2. Linear correlation analysis of adiponectin with VAT/SAT, VAT, abdominal circumference, BMI. VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue; BMI, body mass index.
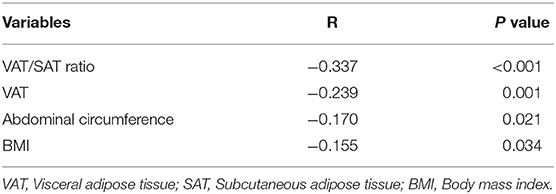
Table 3. Spearman correlation analyses between adiponectin and VAT/SAT, VAT, abdominal circumference, and BMI.
The ROC curve analysis illustrated the distinctive ability of independent risk factors to predict ICAS (Figure 3; Table 4). VAT/SAT ratio had sensible accuracy for the prediction of ICAS (AUC: 0.747; p < 0.001; sensitivity: 67.4%; specificity: 74.7%). The optimal cut-off value of VAT/SAT ratio was 1.04. The optimal adiponectin cutoff to predict ICAS in this study was 3.03 ug/ml (AUC: 0.716; p < 0.001; sensitivity: 75.8%; specificity: 61.5%).
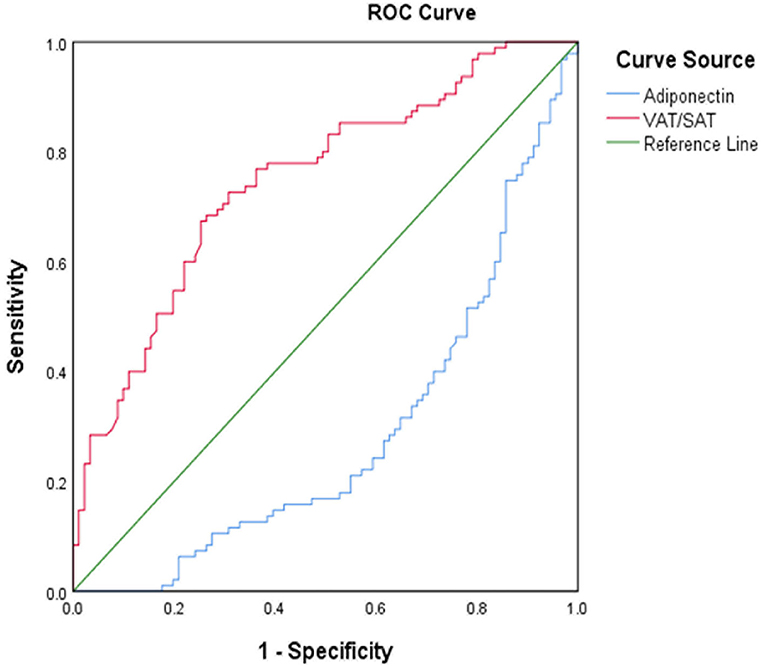
Figure 3. ROC curve for VAT/SAT and adiponectin for distinguish between ICAS and non-ICAS. ROC, receiver operating characteristic curve; VAT, visceral adipose tissue; SAT, subcutaneous adipose tissue; ICAS, intracranial atherosclerotic stenosis.
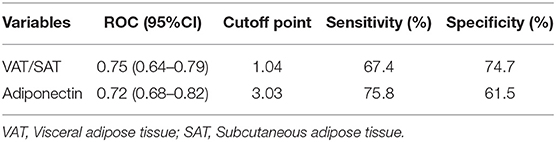
Table 4. ROC curve for predicting ICAS and cutoff points for maximum sum of sensitivity and specificity.
In this study, we explored the relationships between abdominal visceral fat content and clinical characteristics in non-ICAS and ICAS patients. We found that male gender, VAT area, VAT/SAT ratio, abdominal circumference, diabetes mellitus, creatinine level, fibrinogen level, glycated hemoglobin level, NAFLD, and adiponectin level were predictors of ICAS. VAT/SAT, NAFLD, diabetes mellitus, fibrinogen level, and lower adiponectin level were found to be independent risk factors for ICAS in a multivariable logistic regression analysis. Obesity is considered a risk factor for atherosclerosis (25). VAT contributed not only to general but also to the advancement of subclinical atherosclerosis, especially in ladies. Both previous research and our findings indicate that the particular part of VAT participated in the early advancement of atherosclerosis (26). In a general population sample from Norway, adiposity has consistently been associated with carotid atherosclerotic plaque burden (27). A small number of studies have investigated the association of BMI with ICAS, although they failed to demonstrate a significant association. However, as a measure of adiposity, BMI has limitations. Abdominal circumference is a sign of abdominal obesity, which is more closely related to subclinical atherosclerosis than BMI (28). Abdominal adiposity is associated with insulin resistance and atherosclerosis (29). It is speculated that the increased risk of abdominal obesity may be caused by the accumulation of large amounts of visceral fat tissue. However, abdominal parameters cannot be used to distinguish VAT from SAT, as the former has different biological characteristics and functions (30). Hence, we measured the abdominal fat including TAT, VAT, and SAT using abdominal CT. Previous research has suggested that the absolute value of VAT and SAT, as well as their ratio, may play a significant role in the progression of abdominal atherosclerosis (26). In our study, there was no statistical difference between the two groups in terms of the BMI, TAT area, and SAT.
Notably, VAT area, abdominal circumference, and VAT/SAT ratio are higher in the ICAS group than in the non-ICAS group. After adjusting the traditional cardiovascular risk factors, the VAT/SAT ratio remains an independent predictor. Previous studies have shown that VAT is metabolically active and causes chronic systemic inflammation through the secretion of adipokines, which is an important cause of insulin resistance caused by obesity (31, 32). Several studies have indicated that SAT has a protective effect against insulin sensitivity. When compared to the absolute value of VAT or SAT, the VAT/SAT ratio may be a better predictor of abdominal fat distribution.
Clinical research published over the last few decades has shown higher fibrinogen levels in people with atherosclerotic disease (33). Serum fibrinogen levels over a certain threshold may raise the risk of vascular disease by increasing viscosity, stimulating fibrin formation, or increasing platelet interaction. More inflammatory cytokines are produced as a result of a dysfunctional interaction between fat cells and tissue macrophages, which is linked to obesity (33, 34). Fibrinogen is closely linked with subclinical atherosclerosis in obese individual (35). Increased fibrinogen levels throughout time are closely linked to the occurrence of early carotid lesions, notably plaques. Recent studies have shown that in the Han population, higher fibrinogen levels are also independently related to the risk and severity of coronary atherosclerosis (36). We discovered that a high fibrinogen level was an independent predictor of ICAS in this study. Fibrinogen is a powerful predictor of ischemic stroke, according to some studies, although it does not indicate the type or clinical outcomes of stroke (37). We suggest that high fibrinogen levels may be a predictor of a large atherosclerotic stroke.
Adiponectin is an adipocyte secretory protein whose levels in the blood are reduced in obese and diabetic individuals. It may be beneficial to atherosclerosis, inflammation, insulin resistance pathways, and lipid disorders, making it an appealing biomarker and therapeutic target for cardiometabolic diseases such as coronary heart disease, non–cardiac vascular diseases, MetS, NAFLD, and T2DM (15). Okauchi et al. pointed out that the serum adiponectin concentration is related to BMI, WC, and VAT of middle-aged people (38). The concentration of adiponectin in circulation is negatively correlated with body fat, especially visceral fat (39). In our study, we found that VAT/SAT ratio was more negatively correlated with adiponectin than VAT, BMI, and abdominal circumference (Figure 2; Table 3). Many studies showed that visceral fat accumulation and decreased plasma adiponectin concentration were associated with insulin sensitivity, insulin resistance, and vascular inflammation (40–42). Patients with large artery atherosclerosis (LAA) strokes reported lower levels of serum adiponectin than those who experienced non-LAA strokes (43). Bang OY et al. found that adiponectin levels were associated with intracranial atherosclerosis. Moreover, adiponectin levels varied by stroke subtype, with the ICAS group having the lowest (44). Their results are similar to our current findings that the lower plasma adiponectin levels were related to the increased risk of intracranial atherosclerotic stenosis (ICAS). Here, we find that besides adiponectin, the VAT/SAT ratio appears to have a strong predictor for ICAS. Although a majority of clinical studies analyzed and compared total adiponectin levels and found the potential role of adiponectin in cardio-cerebrovascular diseases including cerebral infarction disease, it is also important to further explore the role of each isoform of adiponectin.
Adiponectin as a secreted multimeric protein has three isoforms: low molecular weight (LMW), medium molecular weight (MMW), and high molecular weight (HWM) (45). HMW adiponectin is the most active oligomeric form of adiponectin. There is evidence that circulating levels of total and HWM adiponectin are associated with coronary heart disease, ischemic stroke, and peripheral arterial disease (46). Besides, the research reported a positive association between LMW adiponectin and all-cause mortality in the elderly, which was thought contingent on unbalanced circulating levels of adiponectin isoforms (47). Noriko Tagawa et al. found that decreased total adiponectin, HMW adiponectin, and LMW adiponectin may be associated with atherosclerotic infarction, and that serum LMW adiponectin level was a credible biomarker for cerebrovascular disorders (48).
There are several limitations that need to be emphasized. Firstly, longitudinal observations are required to evaluate the longitudinal effects of visceral fat in the progression of ICAS. Secondly, because of the small sample size, there was no further precise classification of the atherosclerosis stenosis and ≥50% was used to determine significant stenosis in this study. Thirdly, previous studies have shown that the apo B/apo A-I ratio is considered to be an excellent surrogate for the prediction of vascular disease risk, such as ICAS (24, 49). Initially we wanted to include this parameter in the study, but for some economic reasons, our center does not routinely screen the two indicators of apo B and apo A-I. Such lipid parameters need to be further explored in a clinical or community study with a larger sample. Finally, the relationship between different isoforms of adiponectin and ICAS has not been studied here partly due to the difficulty in measuring each adiponectin isoform, and we will explore it in the future.
In conclusion, the higher VAT/SAT ratio and lower plasma adiponectin levels were closely related to the increased risk of ICAS. The traditional cardiovascular risk factor, diabetes mellitus, is still considered an independent predictor of ICAS. Adiponectin may have a protective effect against atherosclerosis. The VAT/SAT ratio appears to have a strong predictive ability for ICAS.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of Shanghai Tenth People's Hospital. The patients/participants provided their written informed consent to participate in this study.
Y-XZ, J-CX, and Q-KB conceived and designed the study. HQ and J-YH collected the clinical data. T-YY performed statistical analyses. F-HW and L-YM analyzed the data and drafted the paper and YT helped. All authors read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
The present study was supported by the Science and Technology Commission of Shanghai Municipality (Grant Number: 20ZR1443500), the Featured Clinical Discipline Project of Shanghai Pudong (PWYst2018-01), Key Discipline Group Construction Project of Shanghai Pudong (PWZxq2017-02), the Clinical Research Project of Shanghai Tenth People's Hospital (YNCR2C030), and Clinical Research Project of Shanghai Municipal Health Commission (201940452).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors are grateful to the study participants.
1. Wong L. Global burden of intracranial atherosclerosis. Int J Stroke. (2006) 1:158–9. doi: 10.1111/j.1747-4949.2006.00045.x
2. Gorelick P, Wong K, Bae H, Pandey D. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. (2008) 39:2396–9. doi: 10.1161/STROKEAHA.107.505776
3. Miao H, Yang Y, Wang H, Huo L, Wang M, Zhou Y, et al. Intensive lipid-lowering therapy ameliorates asymptomatic intracranial atherosclerosis. Aging Dis. (2019) 10:258–66. doi: 10.14336/AD.2018.0526
4. Chimowitz M, Lynn M, Howlett-Smith H, Stern B, Hertzberg V, Frankel M, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. New Eng J Med. (2005) 352:1305–16. doi: 10.1056/NEJMoa043033
5. Kern R, Steinke W, Daffertshofer M, Prager R, Hennerici M. Stroke recurrences in patients with symptomatic vs asymptomatic middle cerebral artery disease. Neurology. (2005) 65:859–64. doi: 10.1212/01.wnl.0000175983.76110.59
6. Ding X, Li C, Yu K, Gao A, Xiao L, Peng F, et al. Different risk factors between intracranial and extracranial atherosclerotic stenosis in Asian population: a systematic review and meta-analysis. Int J Neurosci. (2014) 124:834–40. doi: 10.3109/00207454.2013.879580
7. Kim S, Després J, Koh K. Obesity and cardiovascular disease: friend or foe? Eur Heart J. (2016) 37:3560–8. doi: 10.1093/eurheartj/ehv509
8. Ma Y, Leng X, Dong Y, Xu W, Cao X, Ji X, et al. Risk factors for intracranial atherosclerosis: a systematic review and meta-analysis. Atherosclerosis. (2019) 281:71–7. doi: 10.1016/j.atherosclerosis.2018.12.015
9. Fox C, Massaro J, Hoffmann U, Pou K, Maurovich-Horvat P, Liu C, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the framingham heart study. Circulation. (2007) 116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355
10. Bouchi R, Takeuchi T, Akihisa M, Ohara N, Nakano Y, Nishitani R, et al. High visceral fat with low subcutaneous fat accumulation as a determinant of atherosclerosis in patients with type 2 diabetes. Cardiovasc Diabetol. (2015) 14:136. doi: 10.1186/s12933-015-0302-4
11. Wong Y, Tse H. Circulating biomarkers for cardiovascular disease risk prediction in patients with cardiovascular disease. Front Cardiovasc Med. (2021) 8:713191. doi: 10.3389/fcvm.2021.713191
12. Reiterer M, Rajan M, Gómez-Banoy N, Lau J, Gomez-Escobar L, Ma L, et al. Hyperglycemia in acute COVID-19 is characterized by insulin resistance and adipose tissue infectivity by SARS-CoV-2. Cell Metab. (2021) 33:2174–88. doi: 10.1016/j.cmet.2021.10.014
13. Aronne L, Brown W, Isoldi K. Cardiovascular disease in obesity: A review of related risk factors and risk-reduction strategies. J Clin Lipidol. (2007) 1:575–82. doi: 10.1016/j.jacl.2007.10.005
14. Wang M, Li Y, Zhang R, Zhang S, Feng H, Kong Z, et al. Adiponectin-transfected endothelial progenitor cells have protective effects after 2-hour middle-cerebral artery occlusion in rats with type 2 diabetes mellitus. Front Neurol. (2021) 12:630681. doi: 10.3389/fneur.2021.630681
15. Katsiki N, Mantzoros C, Mikhailidis D. Adiponectin, lipids and atherosclerosis. Curr Opin Lipidol. (2017) 28:347–54. doi: 10.1097/MOL.0000000000000431
16. Moradi N, Fouani F, Vatannejad A, Bakhti Arani A, Shahrzad S, Fadaei R. Serum levels of asprosin in patients diagnosed with coronary artery disease (CAD): a case-control study. Lipids Health Dis. (2021) 20:88. doi: 10.1186/s12944-021-01514-9
17. Wang Y, Zhao X, Liu L, Soo Y, Pu Y, Pan Y, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese intracranial atherosclerosis (CICAS) study. Stroke. (2014) 45:663–9. doi: 10.1161/STROKEHA.113.003508
18. Altman DG, Bland JM. Statistics notes: quartiles, quintiles, centiles, and other quantiles. BMJ. (1994) 309:996. doi: 10.1136/bmj.309.6960.996
19. Kaess B, Pedley A, Massaro J, Murabito J, Hoffmann U, Fox C. The ratio of visceral to subcutaneous fat, a metric of body fat distribution, is a unique correlate of cardiometabolic risk. Diabetologia. (2012) 55:2622–30. doi: 10.1007/s00125-012-2639-5
20. Ho D, Cook N, Britton K, Kim E, Creager M, Ridker P, et al. High-molecular-weight and total adiponectin levels and incident symptomatic peripheral artery disease in women: a prospective investigation. Circulation. (2011) 124:2303–11. doi: 10.1161/CIRCULATIONAHA.111.045187
21. Samuels O, Joseph G, Lynn M, Smith H, Chimowitz M. A standardized method for measuring intracranial arterial stenosis. AJNR Am J neuroradiol. (2000) 21:643–6.
22. Gao Y, Wang Y, Lu C, Zeng C, Chang D, Ju S. Correlations between the abdominal fat-related parameters and severity of coronary artery disease assessed by computed tomography. Quant Imaging Med Surg. (2018) 8:579–87. doi: 10.21037/qims.2018.07.06
23. Fox C, Hwang S, Massaro J, Lieb K, Vasan R, O'Donnell C, et al. Relation of subcutaneous and visceral adipose tissue to coronary and abdominal aortic calcium (from the Framingham Heart Study). Am J Cardiol. (2009) 104:543–7. doi: 10.1016/j.amjcard.2009.04.019
24. Yang W, Li R, Shen Y, Wang X, Liu Q, Wang H, et al. Importance of lipid ratios for predicting intracranial atherosclerotic stenosis. Lipids Health Dis. (2020) 19:160. doi: 10.1186/s12944-020-01336-1
25. Chapman M, Sposito A. Hypertension and dyslipidaemia in obesity and insulin resistance: pathophysiology, impact on atherosclerotic disease and pharmacotherapy. Pharmacol Ther. (2008) 117:354–73. doi: 10.1016/j.pharmthera.2007.10.004
26. Gast K, den Heijer M, Smit J, Widya R, Lamb H, de Roos A, et al. Individual contributions of visceral fat and total body fat to subclinical atherosclerosis: the NEO study. Atherosclerosis. (2015) 241:547–54. doi: 10.1016/j.atherosclerosis.2015.05.026
27. Imahori Y, Mathiesen E, Leon D, Hopstock L, Hughes A, Johnsen S, et al. The contribution of obesity to carotid atherosclerotic plaque burden in a general population sample in Norway: the Tromsø study. Atherosclerosis. (2018) 273:15–20. doi: 10.1016/j.atherosclerosis.2018.04.014
28. Recio-Rodriguez J, Gomez-Marcos M, Patino-Alonso M, Agudo-Conde C, Rodriguez-Sanchez E, Garcia-Ortiz L. Abdominal obesity vs general obesity for identifying arterial stiffness, subclinical atherosclerosis and wave reflection in healthy, diabetics and hypertensive. BMC Cardiovasc Disord. (2012) 12:3. doi: 10.1186/1471-2261-12-3
29. Gast K, Smit J, den Heijer M, Middeldorp S, Rippe R, le Cessie S, et al. Abdominal adiposity largely explains associations between insulin resistance, hyperglycemia and subclinical atherosclerosis: the NEO study. Atherosclerosis. (2013) 229:423–9. doi: 10.1016/j.atherosclerosis.2013.05.021
30. Després J, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. (2006) 444:881–7. doi: 10.1038/nature05488
31. Wensveen F, Jelenčić V, Valentić S, Šestan M, Wensveen T, Theurich S, et al. NK cells link obesity-induced adipose stress to inflammation and insulin resistance. Nat Immunol. (2015) 16:376–85. doi: 10.1038/ni.3120
32. Sequeira I, Yip W, Lu L, Jiang Y, Murphy R, Plank L, et al. Visceral adiposity and glucoregulatory peptides are associated with susceptibility to type 2 diabetes: the TOFI_Asia study. Obesity. (2020) 28:2368–78. doi: 10.1002/oby.22994
33. Li T, Wang F, Peng R, Pei S, Hou Z, Lu B, et al. Sex-related differences in the association between plasma fibrinogen and non-calcified or mixed coronary atherosclerotic plaques. Biol Sex Differ. (2018) 9:51. doi: 10.1186/s13293-018-0210-x
34. Stranahan A. Visceral adiposity, inflammation, and hippocampal function in obesity. Neuropharmacology. (2021) 205:108920. doi: 10.1016/j.neuropharm.2021.108920
35. Menti E, Zaffari D, Galarraga T, Lessa J, Pontin B, Pellanda L, et al. Early markers of atherosclerotic disease in individuals with excess weight and dyslipidemia. Arq Bras Cardiol. (2016) 106:457–63. doi: 10.5935/abc.20160060
36. Zhang Y, Zhu C, Guo Y, Xu R, Li S, Dong Q, et al. Higher fibrinogen level is independently linked with the presence and severity of new-onset coronary atherosclerosis among Han Chinese population. PLoS ONE. (2014) 9:e113460. doi: 10.1371/journal.pone.0113460
37. Peycheva M, Deneva T, Zahariev Z. The role of fibrinogen in acute ischaemic stroke. Neurol Neurochir Pol. (2021) 55:74–80. doi: 10.5603/PJNNS.a2020.0094
38. Okauchi Y, Kishida K, Funahashi T, Noguchi M, Ogawa T, Ryo M, et al. Changes in serum adiponectin concentrations correlate with changes in BMI, waist circumference, and estimated visceral fat area in middle-aged general population. Diabetes Care. (2009) 32:e122. doi: 10.2337/dc09-1130
39. Maeda N, Funahashi T, Matsuzawa Y, Shimomura I. Adiponectin, a unique adipocyte-derived factor beyond hormones. Atherosclerosis. (2020) 292:1–9. doi: 10.1016/j.atherosclerosis.2019.10.021
40. Lee S, Bacha F, Gungor N, Arslanian S. Racial differences in adiponectin in youth: relationship to visceral fat and insulin sensitivity. Diabetes Care. (2006) 29:51–6. doi: 10.2337/diacare.29.01.06.dc05-0952
41. Medina-Urrutia A, Posadas-Romero C, Posadas-Sánchez R, Jorge-Galarza E, Villarreal-Molina T, González-Salazar MC, et al. Role of adiponectin and free fatty acids on the association between abdominal visceral fat and insulin resistance. Cardiovasc Diabetol. (2015) 14:20. doi: 10.1186/s12933-015-0184-5
42. Hakim O, Bello O, Ladwa M, Shojaee-Moradie F, Jackson N, Peacock J, et al. Adiponectin is associated with insulin sensitivity in white European men but not black African men. Diabet Med. (2021) 38:e14571. doi: 10.1111/dme.14571
43. Kim B, Lee S, Ryu W, Kim C, Yoon B. Adipocytokines and ischemic stroke: differential associations between stroke subtypes. J Neurol Sci. (2012) 312:117–22. doi: 10.1016/j.jns.2011.08.007
44. Bang O, Saver J, Ovbiagele B, Choi Y, Yoon S, Lee K. Adiponectin levels in patients with intracranial atherosclerosis. Neurology. (2007) 68:1931–7. doi: 10.1212/01.wnl.0000263186.20988.9f
45. Wang Y, Lam K, Chan L, Chan K, Lam J, Lam M, et al. Post-translational modifications of the four conserved lysine residues within the collagenous domain of adiponectin are required for the formation of its high molecular weight oligomeric complex. J Biol Chem. (2006) 281:16391–400. doi: 10.1074/jbc.M513907200
46. Kizer J, Benkeser D, Arnold A, Djousse L, Zieman S, Mukamal K, et al. Total and high-molecular-weight adiponectin and risk of coronary heart disease and ischemic stroke in older adults. J Clin Endocrinol Metab. (2013) 98:255–63. doi: 10.1210/jc.2012-2103
47. Rizza S, Cardellini M, Farcomeni A, Morabito P, Romanello D, Di Cola G, et al. Low molecular weight adiponectin increases the mortality risk in very old patients. Aging Dis. (2018) 9:946–51. doi: 10.14336/AD.2017.1117
48. Tagawa N, Fujinami A, Natsume S, Mizuno S, Kato I. Relationship between adiponectin multimer levels and subtypes of cerebral infarction. PLoS ONE. (2022) 17:e0262542. doi: 10.1371/journal.pone.0262542
Keywords: intracranial atherosclerotic stenosis, abdominal obesity, visceral adipose, adiponectin, stroke
Citation: Wang F-H, Meng L-Y, Yu T-Y, Tan Y, Quan H, Hu J-Y, Bai Q-K, Xie J-C and Zhao Y-X (2022) Associations of Abdominal Visceral Fat Content and Plasma Adiponectin Level With Intracranial Atherosclerotic Stenosis: A Cross-Sectional Study. Front. Neurol. 13:893401. doi: 10.3389/fneur.2022.893401
Received: 10 March 2022; Accepted: 25 May 2022;
Published: 22 June 2022.
Edited by:
Wen-Jun Tu, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaReviewed by:
Sofya Pchelina, Petersburg Nuclear Physics Institute (RAS), RussiaCopyright © 2022 Wang, Meng, Yu, Tan, Quan, Hu, Bai, Xie and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qing-Ke Bai, emhhb3poZW5ndW8xQHNpbmEuY29t; Jun-Chao Xie, eGllanVuY2hhb19zaEAxMjYuY29t; Yan-Xin Zhao, emhhb195YW54aW5AMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.