
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 26 July 2022
Sec. Stroke
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.891622
Objective: Previous studies have reported that hypo-high-density lipoproteinemia (HHDL) was an independent risk factor for the cerebrovascular event. However, the risk of HHDL for stroke recurrence in moyamoya disease (MMD) during long-term follow-up after revascularization remains poorly understood. We aim to investigate the association between HHDL and stroke recurrence in adult patients with MMD.
Methods: A total of 138 adult patients with MMD were prospectively recruited from 1 July to 31 December 2019. After excluding 15 patients who did not meet the inclusion criteria, all the 123 patients were enrolled. Participants were grouped according to the stroke recurrence and HHDL presentation, respectively. Clinical data and laboratory examinations were compared by the statistical analysis. The Kaplan–Meier survival analysis was conducted to compare the stroke-free survival rates between participants with HHDL and those without. Univariate and multivariate logistic regression analyses were performed to identify independent factors of the neurological status. Univariate and multivariate Cox regression analyses were conducted to identify the predictors for the recurrent stroke.
Results: Participants with recurrent stroke group showed a lower level of high-density lipoprotein (HDL) (p = 0.030). More participants in the recurrent stroke group had HHDL (p = 0.045). What is more, there was statistical significance in the Kaplan–Meier curve of stroke incidence between the normal HDL group and the HHDL group (log-rank test, p = 0.034). Univariate logistic analysis results showed that HHDL (OR 0.916, 95% CI 0.237–3.543; p = 0.899) and HDL (OR 0.729, 95% CI 0.094–5.648; p = 0.763) were not predictive factors for the neurological status. In the multivariate Cox regression analysis, diabetes (HR 4.195, 95% CI 1.041–16.899; p = 0.044), HDL (HR 0.061, 95% CI 0.006–0.626; p = 0.019), and HHDL (HR 3.341, 95% CI 1.110–10.051; p = 0.032) were independent risk factors for the recurrent stroke.
Conclusions: Hypo-high-density lipoproteinemia might be a predictor or the potential therapeutic target for recurrent stroke during the long-term follow-up after revascularization in adult patients with MMD.
Moyamoya disease (MMD) is an unusual type of cerebrovascular disorder, associated with progressive stenosis of the intracranial internal carotid arteries and the aberrant vascular network in brain (1, 2). Also, MMD is the most common cause of stroke in children in China, Japan, and South Korea (3, 4).
Though stroke recurrence during long-term follow-up after revascularization is a rare situation, which may cause severe neurological dysfunction. It is necessary to investigate the relationship between stroke recurrence and MMD. Many studies have explored the risk factors for the postoperative stroke of MMD at the early phase (5–7). Nevertheless, there are few studies focusing on the risk factors of the stroke recurrence after surgical therapy during the long-term follow-up. One of our previous studies with 3–6 months of follow-up showed that hypertension and Suzuki stage were risk factors for the recurrent stroke in MMD (8). In addition, another retrospective study indicated that diabetes and posterior cerebral artery stenosis were predictors for stroke recurrence in patients with MMD aged 18–45 years (9). However, there is no consensus on the cause of stroke recurrence during the long-term follow-up in patients with MMD.
High-density lipoprotein (HDL), a common laboratory indicator, is composed of the lipids and proteins and the regulatory factors they carry. HDL exerts its anti-atherosclerotic effect by reversing cholesterol transport (10, 11). Previous studies have reported that hypo-high density lipoproteinemia (HHDL) was an independent risk factor for the cardiovascular disorders or cerebrovascular accidents (12, 13). A prospective cohort study among 267,500 participants indicated that the risks of ischemic and hemorrhagic stroke were higher when HDL was lower than 50 mg/dl during the long-term follow-up (14). Another prospective study based on the community cohorts also suggested that HHDL was associated with the risk of ischemic stroke over the follow-up of several years (15). In addition, a recent prospective cohort study found that HHDL was related to higher risks of total, ischemic, and hemorrhagic stroke during a median follow-up of 10 years (16). Nonetheless, the risk of HHDL for stroke recurrence in MMD during the long-term follow-up after revascularization remains elusive. In this research, we aimed to explore the association between HHDL and stroke recurrence by gathering the clinical and follow-up data of adult patients with MMD.
During 1 July to 31 December 2019, 138 adult participants with MMD were enrolled prospectively. Pediatric patients were not included in this study. In this study, we excluded 9 and 3 participants who did not accept surgical therapy or died due to comorbidities unrelated to MMD progression, respectively. Furthermore, three participants were lost to follow-up. Ultimately, 123 patients with MMD were enrolled in this research. Digital subtraction angiography (DSA) was used to diagnose MMD, according to the Japanese guidelines published in 2012 (17). The detailed study design and procedures have been described previously (18). The processes of this study were conducted on the basis of the guidelines of the Declaration of Helsinki. Moreover, this research was approved by the Ethics Committees of Beijing Tiantan Hospital, Capital Medical University. All the participants received and signed the informed consents.
The following clinical data were included: demographic information (age and sex), comorbidities, personal history (smoking and drinking), clinical characteristics (primary symptoms, heart rate, and blood pressure), laboratory examinations, and manifestations of the neuroimaging. Comorbidities were summarized into hypertension, diabetes mellitus, and thyroid disease. Suzuki stage and posterior cerebral artery involvement on the operative side was measured blindly through DSA by two neurosurgeons. Any divergence on the radiological manifestations was re-assessed by a third neurosurgeon. We evaluated the preoperative cerebral hemodynamic status of the participants by CT perfusion. Our previous study divided the hemodynamic status in the pre-stroke period into the following four stages (19): Stage I, time to peak (TTP) was delayed, mean transit time (MTT), regional cerebral blood flow (rCBF), and regional cerebral blood volume (rCBV) were normal; Stage II, TTP, and MTT were delayed, rCBF was normal, and rCBV was normal or slightly increased; Stage III, TTP, and MTT were delayed, cCBF was decreased, and rCBV was normal or slightly decreased; Stage IV, TTP, and MTT were delayed, rCBF and rCBV were decreased. The laboratory examinations concerning peripheral blood samples included glucose, creatinine (Cr), uric acid (UA), albumin (ALB), triglyceride (TG), total cholesterol (TC), HDL, low-density lipoprotein (LDL), apolipoprotein A (ApoA), apolipoprotein B (ApoB), and homocysteine (Hcy). Peripheral blood samples were gathered at 8:00 a.m. when patients with MMD had fasted for over 12 h. Serum Hcy levels above 15 μmol/L were defined as hyperhomocysteinemia (HHcy) (20). Serum HDL levels below 0.9 mmol/L were described as HHDL (21).
As described previously, three types of surgical treatment were conducted by neurosurgeons, namely, direct bypass, indirect bypass, and combined bypass. Direct bypass and combined bypass were performed for the most participants in our institution. When the superficial temporal artery or middle cerebral artery were too fragile to conduct artery anastomosis, we performed the indirect surgical bypass (7). As for the indication of revascularization, the clinical manifestation of patients should be considered first. The symptomatic hemisphere was given priority for the revascularization surgery. CT perfusion was taken into account for the patients who had no obvious symptom. The hemisphere with lower perfusion was treated first (22). The long-term outcome was determined through clinic visits and telephone interviews 24–35 months after discharge. Follow-up accidents concluded transient ischemia attack (TIA), ischemic stroke, hemorrhagic stroke, and loss of life. Recurrent stroke was regarded as a newly neurological deficiency that was persistent over 24 h, which was related to a new infarct or hemorrhage by using MRI or CT. The site and characteristics of the ischemic lesion were measured using the Oxfordshire Community Stroke Project (OCSP) system (23). OCSP subtypes included total anterior circulation infarcts (TACIs), partial anterior circulation infarcts (PACIs), posterior circulation infarcts (POCIs), and lacunar infarcts (LACIs). TACI refers to large anterior circulation infarcts with both the cortical and subcortical involvement, PACI refers to more restricted and predominantly cortical infarcts, POCI refers to infarcts associated with vertebrobasilar arterial territory, and LACI refers to infarcts confirmed to the territory of deep perforating arteries (24). The modified Rankin Scale (mRS) was to assess the neurological status at follow-up, with scores of 0–2 indicating a favorable neurological status and 3–6 representing an unfavorable neurological status. Assessment of the mRS score and stroke-free rate was performed by the two neurosurgical residents.
All the statistical analysis was executed by using the IBM SPSS Statistics (version 22.0; IBM Corp.). The counts (percentages) were evaluated by the Pearson Chi-square test or the Fisher exact test. The continuous variables were assessed by t-test and the Mann–Whitney U test. The Kaplan–Meier (KM) survival analysis was conducted to compare the stroke-free survival rates between participants with HHDL or diabetes and those without. The identification of independent factors of the neurological status were evaluated by univariate and multivariate logistic regression analyses. Univariate and multivariate Cox regression analyses were performed to identify the predictors of the recurrent stroke. Statistical significance was regarded as p < 0.05 in a two-tailed test. Variables with p < 0.05 in univariate analysis were included in the multivariate analysis. Multivariate Cox regression analyses were adjusted for age, gender, glucose, Suzuki stage, primary symptom, and LDL.
In total, 123 eligible patients with MMD, aged 19–61 years, were enrolled in the present study (39.02% males). The subjects were aged 39.11 ± 9.69 years (mean ± SD). The clinical and laboratory characteristics of the participants with and without stroke recurrence are shown in Table 1. Concerning the comorbidities, the incidence of diabetes was remarkably higher in the recurrent stroke group than in the non-recurrent stroke group (p = 0.022). No statistical differences in the primary symptom or personal history were presented between the two groups. Heart rate, blood pressure, BMI, surgical option, and the Suzuki stage also showed similarities between the recurrent group and the non-recurrent stroke group. Regarding laboratory examination, participants with the recurrent stroke group showed a lower level of HDL (p = 0.030). Moreover, more participants in the recurrent stroke group had HHDL (p = 0.045).
The clinical features of participants with and without HHDL are presented in Table 2. HHDL was observed in 28 participants (19 men and 9 women). The mean age of these participants was 42.18 ± 9.53 years. Compared with the normal HDL group, participants with HHDL were more likely to be men (p = 0.000). In the terms of primary symptom, 20 (71.43 %) participants were infarction and 8 (28.57 %) participants were non-infarction in HHDL group. As for comorbidities and personal history, participants with HHDL had higher incidences of diabetes (p = 0.000), smoking (p = 0.003), and drinking (p = 0.003) compared with the normal HDL group. Comparing to the normal HDL group, participants with HHDL showed a higher level of BMI (p = 0.050). In the terms of laboratory examinations, the incidences of UA (p = 0.013) and TG (p = 0.000) were significantly higher in participants with HHDL than those with normal HDL. Contrastingly, the incidences of TC (p = 0.002), LDL (p = 0.026), and ApoA (p = 0.000) were remarkably higher in the normal HDL group than in the HHDL group. Suzuki stage between the two groups also exhibited differences (p = 0.018).
The results of the long-term clinical outcomes analysis according to HHDL are shown in Table 3. After discharge, follow-up events during 28 months occurred more frequently in the HHDL group than in the normal HDL group (p = 0.025). However, no significant difference was displayed in the ischemic stroke, hemorrhagic stroke, and TIA (p > 0.05 for all). Functional outcomes (mRS score) did not show statistical differences between the two groups. What's more, there was statistical significance in the KM curve of stroke incidence between the normal HDL group and the HHDL group (log-rank test, p = 0.034) (Figure 1). As illustrated in Figure 2, the KM survival curve showed significant difference in the stroke incidence between the participants with diabetes and those without.
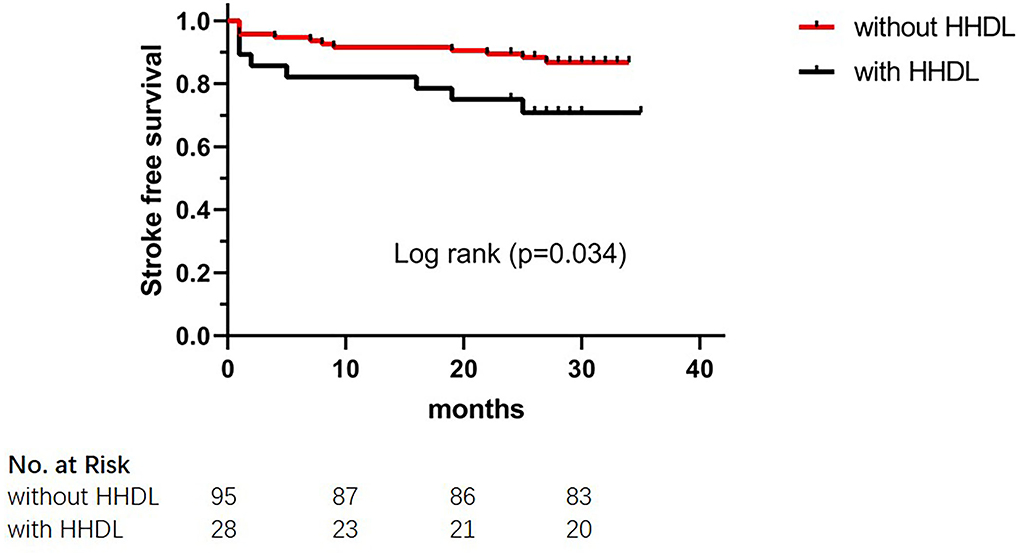
Figure 1. The Kaplan–Meier cumulative hazard curve for stroke recurrence comparing participants with HHDL and participants without HHDL.
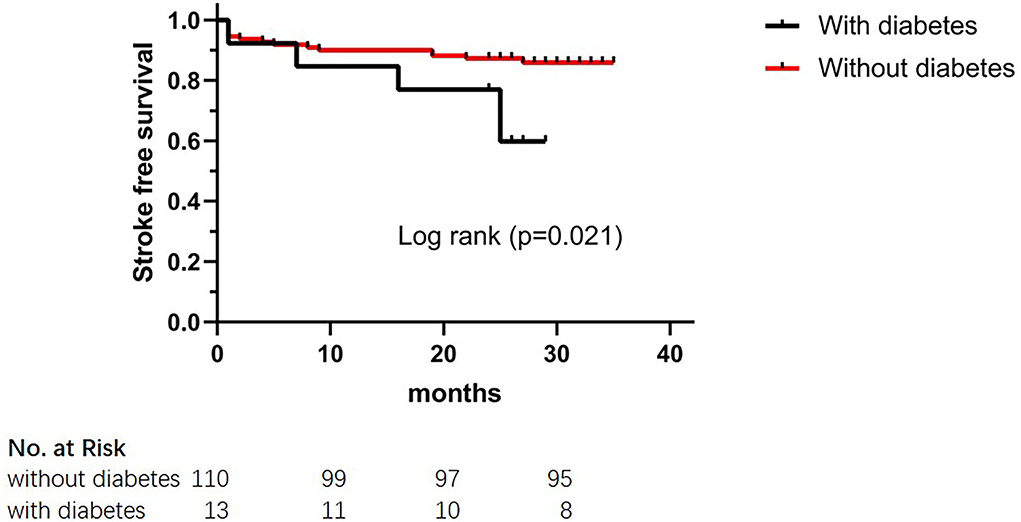
Figure 2. The Kaplan–Meier cumulative hazard curve for stroke recurrence comparing participants with diabetes and participants without diabetes.
Predictive factors of the neurological status were also evaluated in this study. As demonstrated in Table 4, univariate logistic regression indicated that diabetes (OR 4.444, 95% CI 1.158–17.063; p = 0.030) and Suzuki stage (OR 1.853, 95% CI 1.134–3.028; p = 0.014) were related to the neurological status. It also could be found from the univariate logistic regression that HHDL (OR 0.916, 95% CI 0.237–3.543; p = 0.899) and HDL (OR 0.729, 95% CI 0.094–5.648; p = 0.763) were not related to the neurological status. Multivariate logistic regression analysis showed that the Suzuki stage (OR 1.779, 95% CI 1.085–2.917; p = 0.022) was significantly related to the neurological status. Diabetes (OR 4.036, 95% CI 0.974–16.732; p = 0.054) was not a predictive factor of the neurological status in the multivariate regression analysis.
As shown in Table 5, we identified diabetes (HR 3.078, 95% CI 1.116–8.492; p = 0.030), HDL (HR 0.121, 95% CI 0.018–0.815; p = 0.030), HHDL (HR 2.526, 95% CI 1.031–6.187; p = 0.043) were predictive for the recurrent stroke in the univariate Cox regression analysis. In the multivariate analysis, diabetes (HR 4.195, 95% CI 1.041–16.899; p = 0.044), HDL (HR 0.061, 95% CI 0.006–0.626; p = 0.019), HHDL (HR 3.341, 95% CI 1.110–10.051; p = 0.032) were independent risk factors for the recurrent stroke. Multivariate analyses were adjusted for age, gender, glucose, Suzuki stage, primary symptom, and LDL.
We utilized the OCSP classification to classify 17 participants who developed the recurrent ischemic stroke. As illustrated in Table 6, 6 cases in the HHDL group and 4 cases in the diabetes group were classified as LACI among the 17 participants with the recurrent ischemic stroke. Only one participant in each of the two groups was classified as PACI.
The purpose of this prospective study involving adult patients with MMD was to explore the relationship between HHDL and the stroke recurrence. This study showed that that HHDL was significantly related to an increased risk of the recurrent stroke. Contrastingly, HHDL was not a predictive factor of an unfavorable neurological status over a follow-up of 24–35 months. The results of our prospective study may reveal a potential predictor of stroke recurrence during the long-term follow-up after revascularization in patients with MMD.
Hypo-high-density lipoproteinemia is a risk factor for the cardiovascular and cerebrovascular events (12, 14). Increasing evidences have indicated that an association between HHDL and increased risk of stroke. Several studies have reported that HHDL might increase the risk of ischemic stroke during the long-term follow-up (14, 15). In addition, HHDL was found to be a risk factor for the hemorrhagic stroke over the follow-up of several years (14). In this study, we also found that HHDL was remarkably associated with a higher risk of recurrent stroke during the long-term follow-up in patients with MMD. Although how HHDL leads to the recurrent stroke of MMD is still unclear, some hypothesis may help us to understand this process. HDL, or “good” cholesterol, is a heterogeneous group of lipid-protein complexes. The major biological function of HDL is to transport cholesterol from the extrahepatic tissues to the liver, thus, exerting an anti-atherosclerotic effect (25). What is more, another crucial biological role of HDL is protection against inflammation (26). Inflammation plays a crucial role in the initiation and progress of atherosclerosis, which could cause stroke (27). Understandably, the anti-atherosclerotic effect of the HHDL group was weakened, leading to the recurrent stroke. Furthermore, several preclinical and clinical studies have demonstrated that HDL could promote angiogenesis (28–30). We speculated that participants in the normal HDL group had better postoperative collateral formation than participants with HHDL. Thus, according to this theory, the HHDL group was more likely to have recurrent stroke than the normal HDL group. Arregui et al. illustrated the relationship between HDL and ischemic stroke from a genetic perspective. Their finding suggested that both plasma levels of HDL and ischemic stroke were associated with rs2943634 (2q36.3) genetic polymorphism (31). Therefore, it is expected to further investigate the association between recurrent stroke and HDL in patients with MMD from the genetic perspective in the future. To sum up, HHDL might be a promising predictor of the recurrent stroke during the long-term follow-up after revascularization in patients with MMD. Physicians should pay appropriate attention to plasma levels of HDL during the long-term follow-up of patients with MMD.
Several previous studies have indicated an association between the diabetes and recurrent stroke (32–34). In addition, a recent retrospective study identified diabetes as the independent risk factor of stroke recurrence after revascularization in adult patients with MMD (9). The same conclusion was reached in this study. In addition, a large number of epidemiological evidences have suggested that lower HDL level was correlated with the higher risk of diabetes (35–37). We also noted a higher proportion of diabetes in the HHDL group in this study. The association between low HDL levels and diabetes mellitus is complicated and has not been elucidated fully. Thus, further studies are needed to confirm the relationship among HHDL, diabetes and recurrent stroke. Contrastingly, HHDL was not related to a higher risk of an unfavorable neurological status. So, HHDL cannot be used to evaluate the long-term functional outcomes of patients with MMD. Moreover, neither functional outcomes nor recurrent stroke was associated with the Suzuki stage in a retrospective study (9). Suzuki stage was a predictor for an unfavorable neurological status but was not associated with the recurrent stroke in this prospective study. In conclusion, Suzuki stage could predict the long-term functional outcomes to some extent, but further clinical evidence is needed to confirm this point. Furthermore, our data suggested that preoperative hemodynamic status was not associated with the long-term outcomes or stroke recurrence. The Berlin grading system, proposed by Czabaka et al., consists of DSA, MRI, and cerebrovascular reserve capacity (38). Some studies have shown that the Berlin grading system could stratify preoperative clinical severity (39, 40). In addition, perioperative complications and long-term functional outcomes were associated with the Berlin grading system (39, 40). However, acetazolamide challenge test was not performed on the enrolled patients in this study. We will confirm this hypothesis in the future studies. We utilized the OCSP classification to classify 17 participants who developed the recurrent ischemic stroke. The vast majority of participants in both the HHDL group and diabetes groups were classified as LACI. Therefore, we have reason to believe that recurrent ischemic stroke in this study was mainly related to moyamoya disease rather than atherosclerosis.
To sum, this study has illustrated the significant role of HHDL in stroke recurrence during the long-term follow-up after revascularization in adult patients with MMD. HHDL could be a potential therapeutic target, which indicated that recurrent stroke might be prevented by monitoring HHDL. Furthermore, diabetes could also be regarded as a risk factor for the recurrent stroke in adult patients with MMD.
This study had several limitations. First, the sample size of this prospective study was relatively limited; second, our clinical data from a single-center, the results need to be validated in the multiple centers; third, recurrent stroke during the long-term follow-up after revascularization is a rare situation. Our results may be biased to some extent due to the low incidence; fourth, the reproducibility of this study was poor; fifth, only the Chinese adult patients with MMD included, these findings could not be generalized to other ethnicities.
In general, HHDL was significantly associated with the recurrent stroke during the long-term follow-up after revascularization in adult patients with MMD. Thus, HHDL maybe a predictor or target of drug intervention for the recurrent stroke during the long-term follow-up after revascularization. The monitoring of HDL levels and the mechanism of HHDL could be the direction of the future research.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by IRB of Beijing Tiantan Hospital. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Conceptualization and methodology: PG and DZ. Data curation and writing—original draft: XY. Visualization, investigation, software, and validation: YZ. Supervision: RW. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med. (2009) 360:1226–37. doi: 10.1056/NEJMra0804622
2. Suzuki J, Takaku A. Cerebrovascular “moyamoya” disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol. (1969) 20:288–99. doi: 10.1001/archneur.1969.00480090076012
3. Kim T, Oh CW, Bang JS, Kim JE, Cho WS. Moyamoya disease: treatment and outcomes. J Stroke. (2016) 18:21–30. doi: 10.5853/jos.2015.01739
4. Kuriyama S, Kusaka Y, Fujimura M, Wakai K, Tamakoshi A, Hashimoto S, et al. Prevalence and clinicoepidemiological features of moyamoya disease in Japan: findings from a nationwide epidemiological survey. Stroke. (2008) 39:42–7. doi: 10.1161/STROKEAHA.107.490714
5. Chen Y, Ma L, Lu J, Chen X, Ye X, Zhang D, et al. Postoperative hemorrhage during the acute phase after direct or combined revascularization for moyamoya disease: risk factors, prognosis, and literature review. J Neurosurg. (2019) 133:1–10. doi: 10.3171/2019.7.JNS19885
6. Araki Y, Yokoyama K, Uda K, Kanamori F, Kurimoto M, Shiba Y, et al. Postoperative stroke and neurological outcomes in the early phase after revascularization surgeries for moyamoya disease: an age-stratified comparative analysis. Neurosurg Rev. (2021) 44:2785–95. doi: 10.1007/s10143-020-01459-0
7. Li J, Ge P, Zhang Q, Lin F, Wang R, Zhang Y, et al. Hyperhomocysteinemia is a risk factor for postoperative ischemia in adult patients with moyamoya disease. Neurosurg Rev. (2021) 44:2913–21. doi: 10.1007/s10143-021-01482-9
8. Ge P, Ye X, Liu X, Deng X, Wang R, Zhang Y, et al. Association between p.R4810K variant and long-term clinical outcome in patients with moyamoya disease. Front Neurol. (2019) 10:662. doi: 10.3389/fneur.2019.00662
9. Zhao M, Deng X, Gao F, Zhang D, Wang S, Zhang Y, et al. Ischemic stroke in young adults with moyamoya disease: prognostic factors for stroke recurrence and functional outcome after revascularization. World Neurosurg. (2017) 103:161–7. doi: 10.1016/j.wneu.2017.03.146
10. Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis The New England. J Med. (2011) 364:127–35. doi: 10.1056/NEJMoa1001689
11. Lewis GF, Rader DJ. New insights into the regulation of HDL metabolism and reverse cholesterol transport. Circ Res. (2005) 96:1221–32. doi: 10.1161/01.RES.0000170946.56981.5c
12. Ebtehaj S, Gruppen EG, Bakker SJL, Dullaart RPF, Tietge UJF. HDL (High-Density Lipoprotein) cholesterol efflux capacity is associated with incident cardiovascular disease in the general population. Arterioscler Thromb Vasc Biol. (2019) 39:1874–83. doi: 10.1161/ATVBAHA.119.312645
13. Shen Y, Shi L, Nauman E, Katzmarzyk PT, Price-Haywood EG, Bazzano AN, et al. Inverse association between HDL (high-density lipoprotein) cholesterol and stroke risk among patients with type 2 diabetes mellitus. Stroke. (2019) 50:291–7. doi: 10.1161/STROKEAHA.118.023682
14. Gu X, Li Y, Chen S, Yang X, Liu F, Li Y, et al. Association of lipids with ischemic and hemorrhagic stroke: a prospective cohort study among 267 500 Chinese. Stroke. (2019) 50:3376–84. doi: 10.1161/STROKEAHA.119.026402
15. Pikula A, Beiser AS, Wang J, Himali JJ, Kelly-Hayes M, Kase CS, et al. Lipid and lipoprotein measurements and the risk of ischemic vascular events: Framingham Study. Neurology. (2015) 84:472–9. doi: 10.1212/WNL.0000000000001202
16. Li H, Qian F, Zuo Y, Yuan J, Chen S, Wu S, et al. U-shaped relationship of high-density lipoprotein cholesterol and incidence of total, ischemic and hemorrhagic stroke: a prospective cohort study. Stroke. (2022) 53:1624–32. doi: 10.1161/STROKEAHA.121.034393
17. Guidelines for diagnosis and treatment of moyamoya disease (spontaneous occlusion of the circle of Willis). Neurologia medico-chirurgica. (2012) 52:245–66. doi: 10.2176/nmc.52.245
18. Ge P, Zhang Q, Ye X, Liu X, Deng X, Wang J, et al. Modifiable risk factors associated with moyamoya disease: a case-control study. Stroke. (2020) 51:2472–9. doi: 10.1161/STROKEAHA.120.030027
19. Yin H, Liu X, Zhang D, Zhang Y, Wang R, Zhao M, et al. A novel staging system to evaluate cerebral hypoperfusion in patients with moyamoya disease. Stroke. (2018) 49:2837–43. doi: 10.1161/STROKEAHA.118.022628
20. Kim J, Kim H, Roh H, Kwon Y. Causes of hyperhomocysteinemia and its pathological significance. Arch Pharm Res. (2018) 41:372–83. doi: 10.1007/s12272-018-1016-4
21. Qian Y, Wang Y, Liu G, Pan Y. Effect of low HDL-C on one-year outcome in patients with acute ischemic stroke. Chin J Geriatr Heart Brain Vessel Dis. (2014) 16:676–81. doi: 10.3969/j.issn.1009-0126.2014.07.002
22. Deng X, Gao F, Zhang D, Zhang Y, Wang R, Wang S, et al. Effects of different surgical modalities on the clinical outcome of patients with moyamoya disease: a prospective cohort study. J Neurosurg. (2018) 128:1327–37. doi: 10.3171/2016.12.JNS162626
23. Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. (1991) 337:1521–6. doi: 10.1016/0140-6736(91)93206-O
24. Civelek GM, Atalay A, Turhan N. Association of ideomotor apraxia with lesion site, etiology, neglect, and functional independence in patients with first ever stroke. Top Stroke Rehabil. (2015) 22:94–101. doi: 10.1179/1074935714Z.0000000027
25. Plubell DL, Fenton AM, Rosario S, Bergstrom P, Wilmarth PA, Clark WM, et al. High-density lipoprotein carries markers that track with recovery from stroke. Circ Res. (2020) 127:1274–87. doi: 10.1161/CIRCRESAHA.120.316526
26. Madsen CM, Varbo A, Tybjærg-Hansen A, Frikke-Schmidt R, Nordestgaard BG. U-shaped relationship of HDL and risk of infectious disease: two prospective population-based cohort studies. Eur Heart J. (2018) 39:1181–90. doi: 10.1093/eurheartj/ehx665
27. Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. Atherosclerosis. Nat Rev Dis Primers. (2019) 5:56. doi: 10.1038/s41572-019-0106-z
28. Li HM, Mo ZW, Peng YM, Li Y, Dai WP, Yuan HY, et al. Angiogenic and Antiangiogenic mechanisms of high density lipoprotein from healthy subjects and coronary artery diseases patients. Redox Biol. (2020) 36:101642. doi: 10.1016/j.redox.2020.101642
29. Miura S, Fujino M, Matsuo Y, Kawamura A, Tanigawa H, Nishikawa H, et al. High density lipoprotein-induced angiogenesis requires the activation of Ras/MAP kinase in human coronary artery endothelial cells. Arterioscler Thromb Vasc Biol. (2003) 23:802–8. doi: 10.1161/01.ATV.0000066134.79956.58
30. Seetharam D, Mineo C, Gormley AK, Gibson LL, Vongpatanasin W, Chambliss KL, et al. High-density lipoprotein promotes endothelial cell migration and reendothelialization via scavenger receptor-B type I. Circ Res. (2006) 98:63–72. doi: 10.1161/01.RES.0000199272.59432.5b
31. Arregui M, Fisher E, Knüppel S, Buijsse B, di Giuseppe R, Fritsche A, et al. Significant associations of the rs2943634 (2q36.3) genetic polymorphism with adiponectin high density lipoprotein cholesterol and ischemic stroke. Gene. (2012) 494:190–5. doi: 10.1016/j.gene.2011.12.009
32. Alter M, Lai SM, Friday G, Singh V, Kumar VM, Sobel E. Stroke recurrence in diabetics. Does control of blood glucose reduce risk? Stroke. (1997) 28:1153–7. doi: 10.1161/01.STR.28.6.1153
33. Sacco RL, Foulkes MA, Mohr JP, Wolf PA, Hier DB, Price TR. Determinants of early recurrence of cerebral infarction. The stroke data bank. Stroke. (1989) 20:983–9. doi: 10.1161/01.STR.20.8.983
34. Pennlert J, Eriksson M, Carlberg B, Wiklund PG. Long-term risk and predictors of recurrent stroke beyond the acute phase. Stroke. (2014) 45:1839–41. doi: 10.1161/STROKEAHA.114.005060
35. Abbasi A, Corpeleijn E, Gansevoort RT, Gans RO, Hillege HL, Stolk RP, et al. Role of HDL cholesterol and estimates of HDL particle composition in future development of type 2 diabetes in the general population: the PREVEND study. J Clin Endocrinol Metab. (2013) 98:E1352–9. doi: 10.1210/jc.2013-1680
36. Ahmed HM, Miller M, Nasir K, McEvoy JW, Herrington D, Blumenthal RS, et al. Primary low level of high-density lipoprotein cholesterol and risks of coronary heart disease, cardiovascular disease, and death: results from the multi-ethnic study of atherosclerosis. Am J Epidemiol. (2016) 183:875–83. doi: 10.1093/aje/kwv305
37. Hayashi T, Kawashima S, Itoh H, Yamada N, Sone H, Watanabe H, et al. Low HDL cholesterol is associated with the risk of stroke in elderly diabetic individuals: changes in the risk for atherosclerotic diseases at various ages. Diabetes Care. (2009) 32:1221–3. doi: 10.2337/dc08-1677
38. Czabanka M, Peña-Tapia P, Schubert GA, Heppner FL, Martus P, Horn P, et al. Proposal for a new grading of Moyamoya disease in adult patients. Cerebrovasc. Dis. (2011) 32:41–50. doi: 10.1159/000326077
39. Teo M, Furtado S, Kaneko OF, Azad TD, Madhugiri V, Do HM, et al. Validation and application for the berlin grading system of moyamoya disease in adult patients. Neurosurgery. (2020) 86:203–12. doi: 10.1093/neuros/nyz025
Keywords: moyamoya disease, high density lipoprotein, stroke recurrence, hypo-high density lipoproteinemia, follow-up
Citation: Yu X, Ge P, Zhai Y, Wang R, Zhang Y and Zhang D (2022) Hypo-high density lipoproteinemia is a predictor for recurrent stroke during the long-term follow-up after revascularization in adult moyamoya disease. Front. Neurol. 13:891622. doi: 10.3389/fneur.2022.891622
Received: 08 March 2022; Accepted: 06 July 2022;
Published: 26 July 2022.
Edited by:
Jean-Claude Baron, University of Cambridge, United KingdomReviewed by:
Elisa Francesca Ciceri, IRCCS Carlo Besta Neurological Institute Foundation, ItalyCopyright © 2022 Yu, Ge, Zhai, Wang, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peicong Ge, Z2VwZWljb25nQDE2My5jb20=; Dong Zhang, emhhbmdkb25nMDY2MEBhbGl5dW4uY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.