- Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila, L'Aquila, Italy
Background: Migraine is a recurrent headache disorder that has a still unclear pathophysiology, involving several circuits of both the central and peripheral nervous system. Monoclonal antibodies acting on the calcitonin gene-related (CGRP) pathway (CGRP-MAbs) are the first drugs specifically designed for migraine; those drugs act peripherally on the trigeminal ganglion without entering the blood-brain barrier. Conversely, neuromodulation techniques such as transcranial direct current stimulation (tDCS) act centrally by increasing or decreasing the neuronal firing rate of brain cortical areas. The aim of the study will be to evaluate whether tDCS, in addition to CGRP-MAbs, is an effective add-on treatment in reducing headache frequency, intensity and acute medication use in patients with migraine. To demonstrate the biological effects of tDCS, the electroencephalographic (EEG) power changes after tDCS will be assessed.
Methods: We will include patients with migraine on treatment with CGRP-MAbs and reporting ≥8 monthly migraine days. During a prospective 28-day baseline period, patients will fill in a headache diary and questionnaires to evaluate migraine-related disability, anxiety and depressive symptoms, sleep quality, and health-related quality of life. Subjects will be randomly assigned in a 1:1 ratio to active or sham tDCS. The stimulation protocol will consist in five daily sessions, the cathodes will be applied bilaterally above the occipital areas, with the reference anode electrodes positioned above the primary motor areas. Before the first, and immediately after the last stimulation session, patients will perform a 10-min resting EEG recording. During a 28-day follow-up period following tDCS, patients will have to fill in a headache diary and questionnaires identical to those of the baseline period.
Discussion: This trial will evaluate the efficacy of an add-on treatment acting on the brain in patients with migraine, who are already treated with peripherally acting drugs, showing how tDCS acts in restoring the dysfunctional brain networks typical of the migraine patient.
Clinical Trial Registration: NCT05161871.
Introduction
Migraine is a recurrent headache disorder representing the second cause of disability under 50 years of age and the first in young women (1). It is a central nervous system disorder whose mechanisms are poorly understood. Electrophysiology (EEG) studies have revealed abnormalities in cortical responsivity to external stimuli, in the different phases of the migraine cycle. During the days between attacks—i.e., the interictal period—, patients with migraine lack of habituation to external stimuli which normalizes in the hours that precede an attack (2). In the premonitory phase of migraine, changes in thalamo-cortical connectivity were observed; the presence of the so-called “thalamocortical dysrhythmia” is supported by MRI studies, before and during migraine attacks (3–5). Those studies showed altered connectivity of the cortex, thalamus, hypothalamus, brainstem and amygdala, which may be involved in the modulation of pain and sensory function (6).
Cortical spreading depression (CSD) is an event occurring in the brain which is supposed to play an important role in the genesis of migraine and to directly generate migraine aura (7). CSD consists in the diffusion of a wave of depolarization in the cerebral cortex that spreads slowly from the posterior areas of the brain; a “second phase” of neurophysiological and vascular changes ensues, characterized by a prolonged direct current potential shift that is lower in amplitude than the initial CSD wave, along with sustained vasoconstriction and reduced blood oxygenation (8). All those events lead to the activation of nociceptive centers, including a peripheral neural structure, the trigeminal ganglion (TG), which releases pain-inducing peptides and mostly calcitonin gene-related peptide (CGRP) (9, 10).
Several drug classes can be used for the prevention of migraine; they can be classified into antidepressants, antiepileptics, antihypertensives, onabotulinumtoxin A, beta-blockers, calcium agonists, and drugs that act on the calcitonin gene-related peptide (CGRP) pathway (11). Preventive drug treatment is not always viable due to potential contraindications; besides, patients may report adverse events or unsatisfactory benefit. Due to non-optimal adherence and poor tolerability to drugs, pharmacological preventive treatment can be replaced or integrated with non-pharmacological methods. Neuromodulation techniques, such as transcranial direct current stimulation (tDCS), are already used as a treatment for migraine and other chronic pain conditions (12–14), and it can be used in patients who prefer non-pharmacological management, or who cannot be adequately managed with drugs.
tDCS is a non-invasive and painless technique of brain modulation, consisting of delivering a weak current (1–2 mA) through two sponge electrodes fixed on the scalp and connected to a battery-driven stimulator; the aim of tDCS is to modulate spontaneous neuronal firing rate by the polarization of resting membrane potential (15). After-effects of the stimulation rely on the modulation of NMDA receptors and synaptic GABAergic activity (15). Anodal stimulation increases cortical excitability by depolarizing neurons in the stimulated area, while cathodal stimulation hyperpolarizes neurons with inhibitory effects (16).
Monoclonal antibodies acting on the CGRP pathway (CGRP-MAbs) are the first preventive drugs specifically designed for migraine; these drugs act by blocking the CGRP pathway, thereby inhibiting vasodilation and the transmission of pain (10). Those drugs demonstrated high efficacy in randomized controlled trials (17–19) and even more effectiveness in real-world studies (20–25). In real-life, the reduction in monthly migraine days due to CGRP-MAbs was up to 12.2 days at 6 months compared with baseline, while monthly days of acute medication consumption decreased up to 8; 50% response rates ranged from 10 to 76.5% (20–26). However, both randomized controlled trials and real-life studies showed that up to one half of patients in clinical practice do not attain a 50% reduction in monthly migraine days from baseline even with those specific treatments and need further improvements in their migraine prevention. Besides, many patients, even if reporting a significant response to those drugs, may have a high number of residual monthly headache days resulting in a substantial impact on daily activities (27). The number of residual monthly migraine days after treatment, although clinically relevant, is not reported by the available studies (28).
Due to their huge molecular dimensions, CGRP-MAbs inhibit CGRP release from the TG without crossing the blood-brain barrier (10); hence, they are not expected to interfere with the mechanisms of migraine occurring within the brain. On the contrary, tDCS acts on the central nervous system, by modulating the electrical activity of areas implied in pain modulation (29). Therefore, tDCS with CGRP-MAbs have different targets located at different levels in the nervous system; hence, we speculate that their combined administration can have a synergistic or additive effect.
Objectives
The primary aim of the present study will be to assess whether tDCS as an add-on treatment to CGRP-MAbs is effective in reducing headache frequency, intensity, and acute medication use in patients with migraine. Secondarily, we will assess the effect of tDCS add-on on migraine-related disability, quality of life, sleep disturbance, and psychological symptoms. To demonstrate and quantify the biological effects of tDCS, we will assess the electroencephalographic (EEG) power changes after tDCS.
Ethical Issues
The study was approved by the Ethics Committee for the districts of L'Aquila and Teramo with Protocol Number 272/21. All patients will sign an informed consent to participate in the study.
Inclusion and Exclusion Criteria
Our trial will follow the guidelines issued by the International Headache Society for neuromodulation in headaches (30). The protocol follows the SPIRIT checklist (31, 32) (Supplementary Material 1). The inclusion criteria will be the following:
- male or female patients, aged between 40 and 70 years, referring to the Headache Center of the University of L'Aquila;
- a diagnosis of migraine with or without aura according to the International Classification of Headache Disorders, 3rd Edition (33);
- migraine must have been present for at least 12 months;
- treated with CGRP-MAbs (erenumab, fremanezumab or galcanezumab) for 90–180 days since the first subcutaneous administration (this time range was chosen to ensure a stable CGRP pathway inhibition);
- reporting ≥8 monthly migraine days in the last 30 days of observation despite treatment with CGRP-MAbs;
- able to discriminate between migraine and tension-type headaches;
- written informed consent to participate in the study.
Treatment with CGRP-MAbs will be prescribed according to Italian reimbursement criteria, i.e., in patients reporting ≥8 monthly migraine days with a Migraine Impact and Disability Assessment Scale score ≥11 and having failed at least three preventive medication classes among beta-blockers, tricyclic antidepressants, anticonvulsants, and onabotulinumtoxinA.
Patients with other concomitant primary headache types will be included if attacks are <1 day/month and <12 days/year.
Subjects with medication overuse headache and menstrually-related migraine will be not excluded from the study but will be included in exploratory subgroup analyses. According to the clinical practice of the recruiting center, patients with medication overuse will not undergo detoxication treatments.
The exclusion criteria will be the following:
- use of any concurrent migraine preventive medication other than CGRP-MAbs;
- secondary migraine-like headache;
- epilepsy or any other neurologic condition that may be worsened by transcranial electrical stimulation;
- metallic head implants, cardiac pacemaker or any other device that could malfunction or be displaced by electrical stimulation;
- pregnancy or lactation.
Acute migraine treatment will be allowed during the study. Migraine preventive treatments other than CGRP-MAbs must be withdrawn for at least 60 days before inclusion in the trial.
Visit Schedule and Assessment
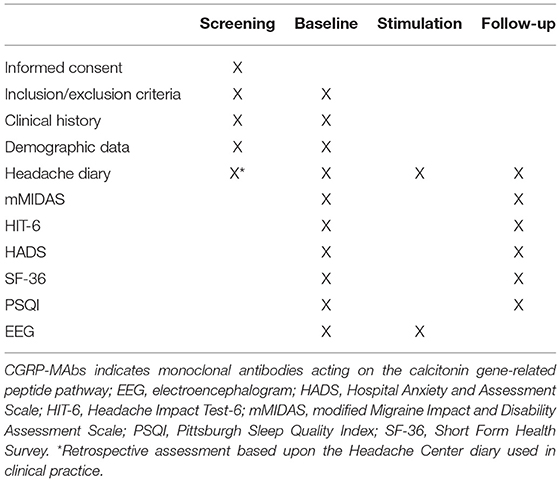
The study includes a 90- to 180-day retrospective screening period, a 28-day baseline period, a 5-day stimulation period, and a 28-day follow-up period. The planned inclusion period of the study will be 12 months. Assessment schedule is summarized in Table 1.
At the beginning of the study, all subjects will be thoroughly informed about all aspects of the study, including the study treatment, visit schedule, required evaluations, diary compliance, and all regulatory requirements for informed consent. Subjects who sign an informed consent but fail to be assigned to the study treatment for any reason will be considered a screen failure. The reason for not being started on treatment will be recorded.
Subject demographic and baseline characteristic data will be collected on all subjects. This will include age, race, ethnicity, and relevant physiological and medical history. Prior headache characteristics and previous headache medication history, including information on the suitability for migraine prophylactics and prior migraine prophylactic treatment failure history, will be collected as part of screening and baseline characteristics.
Randomization and Blinding
To control for placebo and nocebo effects, subjects will be randomly assigned in a 1:1 ratio to active or sham tDCS. Randomization will be performed by one of the investigators (AdA) unaware of personal data of study participants. A random allocation sequence will be generated in MATLAB environment; consecutive patients will then be allocated according to that sequence. The investigators who will administer the stimulation protocol (CR, RO), as well as the patient, will be blind as regards the type of stimulation applied (double blind). Finally, outcome assessment will be performed by an investigator (VC) blinded to the intervention performed.
Study Procedures
Baseline
Eligible subjects will undergo a 28-day baseline period to confirm their eligibility, by filling out a headache diary containing information about headache occurrence, its intensity on a 1–10 Numerical Rating Scale, its duration (in hours), associated symptoms (nausea, vomiting, photophobia, phonophobia), and consumption of drugs for the acute treatment. For each headache day, patients will have to rate their degree of headache-related disability as low-medium, or high (Supplementary Material 2).
At baseline, subjects will have to fill out questionnaires to assess migraine-related disability, quality of life, sleep disturbance and psychological aspects: the modified Migraine Disability Assessment (mMIDAS); the Headache Impact Test-6 (HIT-6); Short Form Health Survey (SF-36); Pittsburgh Sleep Quality Index (PSQI); Hospital Anxiety and Depression Scale (HADS).
Stimulation Period
tDCS will be administered by trained personnel; one of the investigators (AdA) has years of experience in the tDCS field and will train two other investigators (CR, RO). The stimulation protocol will consist in five daily sessions, each lasting 20 min. The stimulation montage will provide a bilateral cathodal stimulation on occipital areas, with the reference anodal electrodes positioned on the M1 areas. The stimulation will be applied via 4 conductive-rubber square electrodes (5 × 5 cm) placed in sponges saturated with high conductivity gel and connected to a battery-operated stimulator system (BrainSTIM, EMS medical). In the active tDCS group, a direct current with maximal intensity of 1.5 mA with be provided for 20 mins (30 s ramp-in/ramp-out); those parameters are within the range of the available randomized controlled trials (34). In the sham group, the current will be turned off after 10 s (30 s ramp-in/ramp-out) at the beginning and at the end of the 20-min interval, in order to maintain the same tingling sensation that subjects refer during the gradual increase/decrease of the current intensity at the beginning/end of the ‘real' stimulation procedure. Patients will fill out the headache diary during the 5 days of tDCS.
EEG Recording
Patients will perform a 10-min resting EEG recording (5 min eyes-open, 5-min eyes-closed), immediately before the first and immediately after the last tDCS session. EEG will be performed with a 64-channel apparel (BrainAmp, Brain Products GmbH) according to the 10–10 international system.
Follow-Up
Patients will undergo a 28-day follow-up assessment period starting from the day following the last tDCS session, filling out a diary identical to those of the baseline period. At the end of the follow-up period, patients will fill out the same questionnaires as during the baseline period. To verify blindness, patients will also be asked whether they received active or sham tDCS. The study procedures are summarized in Figure 1.
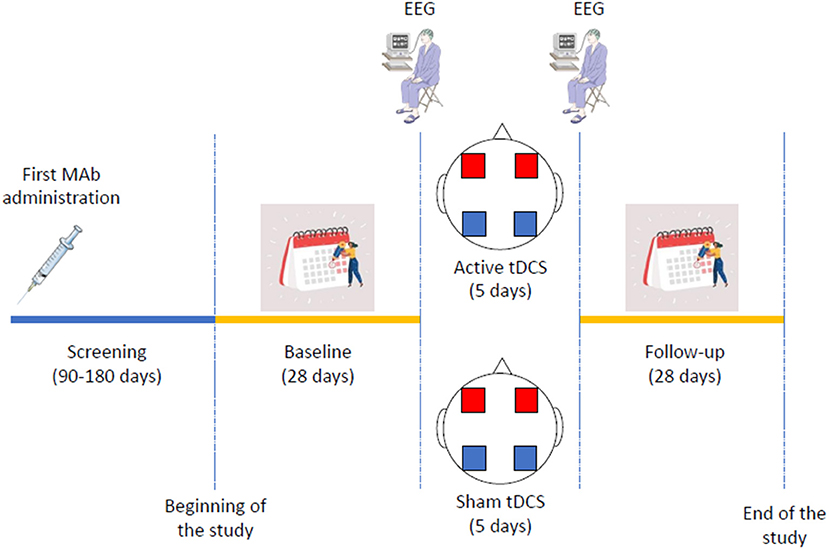
Figure 1. Schematic representation of trial procedures. Mab indicates monoclonal antibody; EEG, electroencephalogram; tDCS, transcranial direct current stimulation. In the transcranial direct current stimulation figure, blue squares indicate the cathodes, while red squares indicate the anodes.
Study Discontinuation
The study will be discontinued under the following circumstances:
- Subject decision;
- Pregnancy;
- Failed to meet the inclusion/exclusion criteria at any time during the study;
- Any situation in which study participation might result in a safety risk to the subject;
- Any change (initiation, withdrawal, or dosing change) in concurrent medication, including preventive and abortive treatment for migraine;
- New diagnosis of diseases that may be negatively affected by tDCS, such as epilepsy.
Study subjects will be consecutively recruited until the number of subjects completing the study reaches the number of 30 (15 treated with tDCS and 15 with sham stimulation). In case of screening failure or any of the conditions listed above and leading to study discontinuation, subjects will be replaced, provided that their inclusion falls within the 12-months inclusion period.
Patients discontinuing CGRP-MAbs due to non-response or lack of tolerance, as well as patients starting oral migraine preventive treatments as add-on, will be excluded from the study due to change in their medication. To ensure that treatment with CGRP-MAbs is stable and well-tolerated and to minimize the risk of including patients who will then withdraw treatment with CGRP-MAbs or change their medication, patient screening will be performed after 90–180 days from the first MAb administration. Patients lost to follow-up, unwilling to continue the trial, or developing a contraindication to continue the trial, will be excluded from efficacy analyses; their adverse events will be monitored and reported.
Study Outcomes and Data Analysis
Efficacy Outcomes
As we expect a short, 5-day course of tDCS to have a short-lasting effect on brain function, we will only assess short-term outcomes at 28 days after the end of tDCS.
The primary efficacy outcome will be the change in headache days from the 28-day baseline to the 28-day follow-up period.
The secondary outcomes will include the change in migraine days, headache hours, mean pain intensity (0–10 Visual Analog Scale), acute treatment consumption (doses), migraine-related disability (mMIDAS score) and impact (HIT-6 score), quality of life (SF-36 score), sleep quality (PSQI score), and anxious and depressive symptoms (HADS score) from the 28-day baseline to the 28-day follow-up period. The change in the number of days with low, medium, and high disability will also be assessed by using of a specifically designed headache diary (Supplementary Material 2).
The additional outcome will be the changes in spectral power and coherence in the delta (1–4 Hz), theta (5–7 Hz), alpha (8–12 Hz), and beta bands (13–30 Hz), both overall and over the occipital regions, at EEG recording between the two measurements (before vs. after tDCS). The EEG power changes will be correlated with the improvement in primary and secondary outcomes. The age limit of 40–70 years was chosen to limit the variability in EEG activity generated by the inclusion of too young or old subjects, which could act as a confounder in outcome assessment.
Each outcome will be assessed in the group of active and sham tDCS; additionally, between-group comparisons will be made.
A migraine day will be defined as a day with headache lasting at least 4 h if left untreated and accompanied by typical symptoms (nausea, vomiting, photo- and/or phonophobia) or preceded by aura. All days with headache not accompanied by any of those symptoms will be considered as non-migraine headache days. The total count of headache days will include both migraine and non-migraine headache days.
Safety Outcomes
Safety assessment will include adverse event reporting. Adverse event monitoring will be performed during the tDCS stimulation sessions and during the 28-day follow-up period after tDCS. Adverse events will be detected and collected by investigators with a standard questionnaire (35) and open-ended questions. Monitoring for serious adverse events (SAEs) will be performed according to common clinical practice.
Statistical Analysis
Continuous data will be summarized by mean, standard deviation (SD), median, first and third quartiles, minimum and maximum, Categorical data will be presented by absolute and relative frequencies (n and %). Bilateral 95% confidence limit will be presented as appropriate.
Comparison between groups (active/sham) for the variables under study (headache days, days of disabling headache, intensity of pain, consumption of acute treatments, headache-related disability, and scores on questionnaires) will be performed using parametric or non-parametric statistics, depending on the data distribution.
Primary analyses will be performed on primary and secondary outcomes. Exploratory subgroup analyses will be performed on patients with a history of menstrual migraine and on patients with chronic migraine with medication overuse.
To evaluate electrophysiological changes, the dependent variable will be the variations in EEG activity after vs. before tDCS. Specifically, we will compute the spectral power via Fast Fourier Transform (FFT) and the coherence in cortical activity among brain areas via magnitude-squared coherence (MSC) for the artifact-free epochs in each EEG frequency band. For each group of patients (tDCS vs. sham), the power change before vs. after tDCS will be compared for each electrode and each frequency band. Given the results of a previous study (36), particular attention will be given to power in the alpha band in occipital areas. The EEG index changes will be correlated with changes in migraine parameters (headache days, migraine days, pain intensity, acute medication consumption, questionnaires score) to directly link the modifications in brain physiology to the frequency and severity of migraine episodes. Source current density of cortical generators of relevant EEG indexes will be also assessed by low-resolution electromagnetic tomography (LORETA) (37), to confirm the cortical origin of the physiological changes induced by tDCS. Outcomes will be compared between the active and sham tDCS groups by chi-squared or t-test statistics as appropriate.
Sample Size
The sample size calculation was performed using GPower, version 3.1. According to previous literature (36), a between-groups mean difference of 3 ± 2 migraine days per month was considered significant. The computation was made with the following parameters: confidence interval (two-sided): 95%; power: 80%; ratio of sample size: 1:1; mean change in group 1: −4 days; mean change in group 2: −1 day; standard deviation: 2. The minimum sample size suggested was of 9 patients per group. In consideration of possible dropouts, we set our population size to 30 patients, 15 per group.
Discussion
CGRP-MAbs have significantly changed the landscape of migraine prevention. They are an effective and well tolerated class of drugs which can substantially improve the quality of life of patients with migraine. CGRP-MAbs were proved to be effective even in patients who had failures to other preventatives. The degree of benefit of CGRP-MAbs is highly variable; in up to 10% of patients they can lead to migraine freedom (100% responders) (17, 18, 38, 39), while in all the other patients there is a residual migraine impact despite the treatment. Some patients, even if meeting criteria to be considered as responders to CGRP-MAbs, continue to experience a significant burden of migraine. In fact, in real-life studies 14.4–57% of patients received add-on treatment (21, 22, 26). It is unclear which is the optimal treatment to be associated with CGRP-MAbs. We aim to evaluate if patients, with a significant migraine burden (>8 migraine days per month) despite the use of CGRP-MAbs, may achieve further benefit by adding a non-pharmacological (tDCS) treatment targeting central mechanisms involved in migraine. The rationale to choose tDCS is its non-pharmacological nature and the central mechanism of action which may be complementary to the peripheral mechanism of action of anti-CGRP-MAbs. We will randomize patients who are on treatment with CGRP-MAbs and who still experience a significant migraine burden (>8 migraine days per month) to tDCS or placebo.
So far, several studies have already evaluated tDCS for migraine prevention proving that it is a promising treatment to prevent migraine (36, 40–50). Available RCTs included a variable number of patients (from 15 to 135 patients) with highly heterogeneous patient populations, outcomes, time schedules, and tDCS montages (34). Most of the available RCTs performed either cathodal occipital stimulation with anterior reference (40, 43, 44, 46) or anodal frontal stimulation with supraorbital reference (36, 41, 42, 45). Those montages are both justified by neurophysiology, as studies on migraine showed a hyperresponsivity of the visual cortex, while frontal stimulation reduces the excitability of the thalamus, which is responsible for pain generation (29). Results of those RCTs were overall positive in the short term, while being more controversial 12 months after tDCS (36, 48). The available RCTs are limited by the underuse of neurophysiological tests, which would improve our understanding of the effect of tDCS and how to improve it (34).
With respect to the available RCTs, our study has several differences. Firstly, we will test tDCS as an add-on to a class of drugs specifically designed to prevent migraine. Besides, our montage will be bilateral with 4 electrodes (2 anodes and 2 cathodes), while the other trials all performed unilateral stimulation. The bilateral stimulation is justified by the supposed bilateral alterations of the migraine brain (4) and will likely optimize current flow through the brain. Moreover, our montage will merge cathodal occipital stimulation and anodal frontal stimulation, by positioning the cathode over both occipital regions and the anode over both frontal regions. Those procedures are intended to maximize neuromodulation of circuits involved in migraine and pain (29). Additionally, our study will follow as closely as possible the recently issued guidelines for trials of neuromodulation in patients with migraine (30).
We will also study the cortical effect of neuromodulation by electrophysiology (EEG). Previous studies have shown that EEG activity is different between subjects with and without migraine. In detail, migraineurs showed increased slow activity between attacks compared with non-migraineurs (51, 52) and the degree of EEG slowing on the occipital areas showed a correlation with the burden of migraine (53); the increase in slow activity is coupled with decreased power of the alpha frequency. Interestingly, a trial of anodal tDCS over the frontal motor areas showed that active treatment was associated with increased alpha power over the occipital regions (36), suggesting that tDCS can mitigate the neurophysiological abnormalities of the migraineurs' brain. Quantifying EEG activity in our trial will provide a neurophysiological correlate to clinical findings and will help explaining the effect of tDCS on neural structures. The use of high-density EEG will provide accurate information on the sites of tDCS action.
The present trial has a robust double-blind, randomized approach with blinded outcome assessment. The trial complies with the most recent guidelines for neuromodulation in migraine. Besides, the tDCS montage was designed specifically for migraine prevention by reflection on the most plausible neuroanatomical targets. However, the study also has limitations. The study is single-center; besides, its sample will be sufficient to calculate the primary outcome, while subgroup analyses will be only exploratory. We will correct for low numbers by assessing the normality of variable distributions and perform conservative, non-parametric tests for non-normally distributed variables. As an add-on to highly effective migraine preventatives, we cannot exclude that the clinical effect of tDCS in the present RCT will be negative. Nevertheless, we will include patients with a high burden of migraine (≥8 monthly migraine days) to correct for this effect. Besides, a previous trial showed that active tDCS is more effective than sham even on top of topiramate (47), suggesting that tDCS could be an effective add-on migraine preventative. Besides, we will assess not only the possible clinical efficacy of tDCS, but also its effect on brain circuitry; therefore, even results that are clinically neutral will be interesting to discuss with respect to the functional effects of tDCS. Our trial will contribute to assess the possible central, indirect effects of CGRP-MAbs by verifying whether the central circuits of migraine generation can be inhibited in addition to the action of those drugs.
Conclusion
In conclusion, our trial will assess the efficacy of an add-on non-pharmacological treatment acting on the brain in patients with migraine who are already treated with peripherally acting CGRP-MAbs. The trial will also allow us to better understand the pathophysiology of migraine, and to evaluate how tDCS acts in restoring the dysfunctional brain networks typical of the migraine patient.
Author Contributions
RO, AD'A, and SS conceived the study and wrote the protocol draft. All other authors provided critical revision to the draft. All authors read and approved the final protocol.
Funding
This study will be partly funded by intramural DISCAB GRANT 2021 awarded by the Department of Biotechnological and Applied Clinical Sciences, University of L'Aquila.
Conflict of Interest
RO declares personal fees from Novartis, Teva, and Eli Lilly, and non-financial support from Novartis, Allergan/AbbVie, and Teva. SS declares personal fees and non-financial support from Allergan, Abbott, Eli Lilly, Novartis, and Teva, personal fees from Medscape; and other from Bayer, Pfizer, Medtronic, Starmed, Bristol-Myers Squibb, and Daiichi Sankyo.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Figure 1 was created with pictures from Servier Medical Art (https://smart.servier.com) and Shutterstock (https://www.shutterstock.com).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.890364/full#supplementary-material
Supplementary Material 1. SPIRIT checklist.
Supplementary Material 2. Headache diary.
References
1. Steiner TJ, Stovner LJ, Jensen R, Uluduz D, Katsarava Z. Lifting the Burden: The Global Campaign Against Migraine remains second among the world's causes of disability, and first among young women: findings from GBD2019. J Headache Pain. (2020) 21:137. doi: 10.1186/s10194-020-01208-0
2. Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev. (2017) 97:553–622. doi: 10.1152/physrev.00034.2015
3. Coppola G, Di Renzo A, Tinelli E, Di Lorenzo C, Di Lorenzo G, Parisi V, et al. Thalamo-cortical network activity during spontaneous migraine attacks. Neurology. (2016) 87:2154–60. doi: 10.1212/WNL.0000000000003327
4. Coppola G, Di Renzo A, Tinelli E, Lepre C, Di Lorenzo C, Di Lorenzo G, et al. Thalamo-cortical network activity between migraine attacks: Insights from MRI-based microstructural and functional resting-state network correlation analysis. J Headache Pain. (2016) 17:100. doi: 10.1186/s10194-016-0693-y
5. De Tommaso M, Vecchio E, Quitadamo SG, Coppola G, Di Renzo A, Parisi V, et al. Pain-related brain connectivity changes in migraine: a narrative review and proof of concept about possible novel treatments interference. Brain Sci. (2021) 11:234. doi: 10.3390/brainsci11020234
6. Charles A. The pathophysiology of migraine: implications for clinical management. Lancet Neurol. (2018) 17:174–82. doi: 10.1016/S1474-4422(17)30435-0
7. Harriott AM, Takizawa T, Chung DY, Chen SP. Spreading depression as a preclinical model of migraine. J Headache Pain. (2019) 20:45. doi: 10.1186/s10194-019-1001-4
8. Charles AC, Baca SM. Cortical spreading depression and migraine. Nat Rev Neurol. (2013) 9:637–44. doi: 10.1038/nrneurol.2013.192
9. Noseda R, Burstein R. Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, CSD, sensitization and modulation of pain. Pain. (2013) 154 Suppl 1. doi: 10.1016/j.pain.2013.07.021
10. Edvinsson L, Haanes KA, Warfvinge K, Krause DN. CGRP as the target of new migraine therapies—successful translation from bench to clinic. Nat Rev Neurol. (2018) 14:338–50. doi: 10.1038/s41582-018-0003-1
11. Sacco S, Braschinsky M, Ducros A, Lampl C, Little P, Van Den Brink AM, et al. European headache federation consensus on the definition of resistant and refractory migraine: developed with the endorsement of the European Migraine and Headache Alliance (EMHA). J Headache Pain. (2020) 21:76. doi: 10.1186/s10194-020-01130-5
12. Knotkova H, Cruciani RA. Non-invasive transcranial direct current stimulation for the study and treatment of neuropathic pain. Methods Mol Biol. (2010) 617:505–15. doi: 10.1007/978-1-60327-323-7_37
13. Stilling JM, Monchi O, Amoozegar F, Debert CT. Transcranial magnetic and direct current stimulation (TMS/tDCS) for the treatment of headache: a systematic review. Headache. (2019) 59:339–57. doi: 10.1111/head.13479
14. Fregni F, El-Hagrassy MM, Pacheco-Barrios K, Carvalho S, Leite J, Simis M, et al. Evidence-based guidelines and secondary meta-analysis for the use of transcranial direct current stimulation in neurological and psychiatric disorders. Int J Neuropsychopharmacol. (2021) 24:256–313. doi: 10.1093/ijnp/pyaa051
15. Medeiros LF, De Souza IC, Vidor LP, De Souza A, Deitos A, Volz MS, et al. Neurobiological effects of transcranial direct current stimulation: a review. Front Psychiatry. (2012) 3:110. doi: 10.3389/fpsyt.2012.00110
16. Lefaucheur JP, Antal A, Ayache SS, Benninger DH, Brunelin J, Cogiamanian F, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. (2017) 128:56–92. doi: 10.1016/j.clinph.2016.10.087
17. Reuter U, Goadsby PJ, Lanteri-Minet M, Wen S, Hours-Zesiger P, Ferrari MD, et al. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. Lancet. (2018) 392:2280–7. doi: 10.1016/S0140-6736(18)32534-0
18. Ferrari MD, Diener HC, Ning X, Galic M, Cohen JM, Yang R, et al. Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double-blind, placebo-controlled, phase 3b trial. Lancet. (2019) 394:1030–40. doi: 10.1016/S0140-6736(19)31946-4
19. Mulleners WM, Kim BK, Lainez MJA, Lanteri-Minet M, Pozo-Rosich P, Wang S, et al. Safety and efficacy of galcanezumab in patients for whom previous migraine preventive medication from two to four categories had failed (CONQUER): a multicentre, randomised, double-blind, placebo-controlled, phase 3b trial. Lancet Neurol. (2020) 19:814–25. doi: 10.1016/S1474-4422(20)30279-9
20. Lambru G, Hill B, Murphy M, Tylova I, Andreou AP. A prospective real-world analysis of erenumab in refractory chronic migraine. J Headache Pain. (2020) 21:61. doi: 10.1186/s10194-020-01127-0
21. Ornello R, Casalena A, Frattale I, Gabriele A, Affaitati G, Giamberardino MA, et al. Real-life data on the efficacy and safety of erenumab in the Abruzzo region, central Italy. J Headache Pain. (2020) 21:32. doi: 10.1186/s10194-020-01102-9
22. Russo A, Silvestro M, Scotto Di Clemente F, Trojsi F, Bisecco A, Bonavita S, et al. Multidimensional assessment of the effects of erenumab in chronic migraine patients with previous unsuccessful preventive treatments: a comprehensive real-world experience. J Headache Pain. (2020) 21:69. doi: 10.1186/s10194-020-01143-0
23. De Vries Lentsch S, Verhagen IE, Van Den Hoek TC, Maassenvandenbrink A, Terwindt GM. Treatment with the monoclonal calcitonin gene-related peptide receptor antibody erenumab: a real-life study. Eur J Neurol. (2021) 28:4194–203. doi: 10.1111/ene.15075
24. Pensato U, Baraldi C, Favoni V, Cainazzo MM, Torelli P, Querzani P, et al. Real-life assessment of erenumab in refractory chronic migraine with medication overuse headache. Neurol Sci. (2021) 43:1273–80. doi: 10.1007/s10072-021-05426-5
25. Vernieri F, Altamura C, Brunelli N, Costa CM, Aurilia C, Egeo G, et al. Galcanezumab for the prevention of high frequency episodic and chronic migraine in real life in Italy: a multicenter prospective cohort study (the GARLIT study). J Headache Pain. (2021) 22:35. doi: 10.1186/s10194-021-01247-1
26. Raffaelli B, Kalantzis R, Mecklenburg J, Overeem LH, Neeb L, Gendolla A, et al. Erenumab in chronic migraine patients who previously failed five first-line oral prophylactics and onabotulinumtoxina: a dual-center retrospective observational study. Front Neurol. (2020) 11:417. doi: 10.3389/fneur.2020.00417
27. Ornello R, Baraldi C, Guerzoni S, Lambru G, Andreou AP, Raffaelli B, et al. Comparing the relative and absolute effect of erenumab: is a 50% response enough? Results from the ESTEEMen study. J Headache Pain. (2022) 23:1–8. doi: 10.1186/s10194-022-01408-w
28. Tfelt-Hansen P, Diener HC, Steiner TJ. Problematic presentation and use of efficacy measures in current trials of CGRP monoclonal antibodies for episodic migraine prevention: a mini-review. Cephalalgia. (2020) 40:122–6. doi: 10.1177/0333102419877663
29. Dasilva AF, Truong DQ, Dossantos MF, Toback RL, Datta A, Bikson M. State-of-art neuroanatomical target analysis of high-definition and conventional tDCS montages used for migraine and pain control. Front Neuroanat. (2015) 9:89. doi: 10.3389/fnana.2015.00089
30. Tassorelli C, Diener HC, Silberstein SD, Dodick DW, Goadsby PJ, Jensen RH, et al. Guidelines of the International Headache Society for clinical trials with neuromodulation devices for the treatment of migraine. Cephalalgia. (2021) 41:1135–51. doi: 10.1177/03331024211010413
31. Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gotzsche PC, Krleza-Jeric K, et al. SPIRIT 2013 statement: defining standard protocol items for clinical trials. Ann Intern Med. (2013) 158:200–7. doi: 10.7326/0003-4819-158-3-201302050-00583
32. Chan AW, Tetzlaff JM, Gotzsche PC, Altman DG, Mann H, Berlin JA, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. (2013) 346:e7586. doi: 10.1136/bmj.e7586
33. Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia. (2018) 38:1–211. doi: 10.1177/0333102417738202
34. Ornello R, Caponnetto V, Ratti S, D'aurizio G, Rosignoli C, Pistoia F, et al. Which is the best transcranial direct current stimulation protocol for migraine prevention? A systematic review and critical appraisal of randomized controlled trials. J Headache Pain. (2021) 22:144. doi: 10.1186/s10194-021-01361-0
35. Fertonani A, Rosini S, Cotelli M, Rossini PM, Miniussi C. Naming facilitation induced by transcranial direct current stimulation. Behav Brain Res. (2010) 208:311–8. doi: 10.1016/j.bbr.2009.10.030
36. De Icco R, Putorti A, De Paoli I, Ferrara E, Cremascoli R, Terzaghi M, et al. Anodal transcranial direct current stimulation in chronic migraine and medication overuse headache: A pilot double-blind randomized sham-controlled trial. Clin Neurophysiol. (2021) 132:126–36. doi: 10.1016/j.clinph.2020.10.014
37. Pascual-Marqui RD, Michel CM, Lehmann D. Low resolution electromagnetic tomography: a new method for localizing electrical activity in the brain. Int J Psychophysiol. (1994) 18:49–65. doi: 10.1016/0167-8760(84)90014-X
38. Detke HC, Goadsby PJ, Wang S, Friedman DI, Selzler KJ, Aurora SK. Galcanezumab in chronic migraine: the randomized, double-blind, placebo-controlled REGAIN study. Neurology. (2018) 91:e2211–21. doi: 10.1212/WNL.0000000000006640
39. Brandes JL, Diener HC, Dolezil D, Freeman MC, Mcallister PJ, Winner P, et al. The spectrum of response to erenumab in patients with chronic migraine and subgroup analysis of patients achieving >/=50%, >/=75%, and 100% response. Cephalalgia. (2020) 40:28–38. doi: 10.1177/0333102419894559
40. Antal A, Kriener N, Lang N, Boros K, Paulus W. Cathodal transcranial direct current stimulation of the visual cortex in the prophylactic treatment of migraine. Cephalalgia. (2011) 31:820–8. doi: 10.1177/0333102411399349
41. Auvichayapat P, Janyacharoen T, Rotenberg A, Tiamkao S, Krisanaprakornkit T, Sinawat S, et al. Migraine prophylaxis by anodal transcranial direct current stimulation, a randomized, placebo-controlled trial. J Med Assoc Thai. (2012) 95:1003–12.
42. Dasilva AF, Mendonca ME, Zaghi S, Lopes M, Dossantos MF, Spierings EL, et al. tDCS-induced analgesia and electrical fields in pain-related neural networks in chronic migraine. Headache. (2012) 52:1283–95. doi: 10.1111/j.1526-4610.2012.02141.x
43. Rocha S, Melo L, Boudoux C, Foerster A, Araujo D, Monte-Silva K. Transcranial direct current stimulation in the prophylactic treatment of migraine based on interictal visual cortex excitability abnormalities: a pilot randomized controlled trial. J Neurol Sci. (2015) 349:33–9. doi: 10.1016/j.jns.2014.12.018
44. Wickmann F, Stephani C, Czesnik D, Klinker F, Timaus C, Chaieb L, et al. Prophylactic treatment in menstrual migraine: a proof-of-concept study. J Neurol Sci. (2015) 354:103–9. doi: 10.1016/j.jns.2015.05.009
45. Andrade SM, De Brito Aranha REL, De Oliveira EA, De Mendonca C, Martins WKN, Alves NT, et al. Transcranial direct current stimulation over the primary motor vs prefrontal cortex in refractory chronic migraine: a pilot randomized controlled trial. J Neurol Sci. (2017) 378:225–32. doi: 10.1016/j.jns.2017.05.007
46. Ahdab R, Mansour AG, Khazen G, El-Khoury C, Sabbouh TM, Salem M, et al. Cathodal transcranial direct current stimulation of the occipital cortex in episodic migraine: a randomized sham-controlled crossover study. J Clin Med. (2019) 9:60. doi: 10.3390/jcm9010060
47. Dalla Volta G, Marceglia S, Zavarise P, Antonaci F. Cathodal tDCS guided by thermography as adjunctive therapy in chronic migraine patients: a sham-controlled pilot study. Front Neurol. (2020) 11:121. doi: 10.3389/fneur.2020.00121
48. Grazzi L, Usai S, Bolognini N, Grignani E, Sansone E, Tramacere I, et al. No efficacy of transcranial direct current stimulation on chronic migraine with medication overuse: a double blind, randomised clinical trial. Cephalalgia. (2020) 40:1202–11. doi: 10.1177/0333102420931050
49. Rahimi MD, Fadardi JS, Saeidi M, Bigdeli I, Kashiri R. Effectiveness of cathodal tDCS of the primary motor or sensory cortex in migraine: a randomized controlled trial. Brain Stimul. (2020) 13:675–82. doi: 10.1016/j.brs.2020.02.012
50. Pohl H, Moisa M, Jung HH, Brenner K, Aschmann J, Riederer F, et al. Long-term effects of self-administered transcranial direct current stimulation in episodic migraine prevention: results of a randomized controlled trial. Neuromodulation. (2021) 24:890–8. doi: 10.1111/ner.13292
51. Bjork MH, Stovner LJ, Engstrom M, Stjern M, Hagen K, Sand T. Interictal quantitative EEG in migraine: a blinded controlled study. J Headache Pain. (2009) 10:331–9. doi: 10.1007/s10194-009-0140-4
52. Bjork M, Stovner LJ, Hagen K, Sand T. What initiates a migraine attack? Conclusions from four longitudinal studies of quantitative EEG and steady-state visual-evoked potentials in migraineurs. Acta Neurol Scand Suppl. (2011) 124:56–63. doi: 10.1111/j.1600-0404.2011.01545.x
Keywords: migraine treatment, transcranial direct current stimulation, electroencephalogram, monoclonal antibodies, calcitonin gene-related peptide, randomized controlled trials
Citation: Ornello R, Rosignoli C, Caponnetto V, Pistoia F, Ferrara M, D'Atri A and Sacco S (2022) Effectiveness of Transcranial Direct Current Stimulation and Monoclonal Antibodies Acting on the CGRP as a Combined Treatment for Migraine (TACTIC): Protocol for a Randomized, Double-Blind, Sham-Controlled Trial. Front. Neurol. 13:890364. doi: 10.3389/fneur.2022.890364
Received: 05 March 2022; Accepted: 14 April 2022;
Published: 10 May 2022.
Edited by:
Lei Lan, Chengdu University of Traditional Chinese Medicine, ChinaReviewed by:
Andrea Negro, Sapienza University of Rome, ItalyChristoph Schankin, Bern University Hospital, Switzerland
Copyright © 2022 Ornello, Rosignoli, Caponnetto, Pistoia, Ferrara, D'Atri and Sacco. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Simona Sacco, c2ltb25hLnNhY2NvQHVuaXZhcS5pdA==
 Raffaele Ornello
Raffaele Ornello Chiara Rosignoli
Chiara Rosignoli Valeria Caponnetto
Valeria Caponnetto Francesca Pistoia
Francesca Pistoia Michele Ferrara
Michele Ferrara Aurora D'Atri
Aurora D'Atri Simona Sacco
Simona Sacco