- 1Evidence-Based and Clinical Research Laboratory, Department of Health, Social and Clinical Pharmacy, College of Pharmacy, Chung-Ang University, Seoul, South Korea
- 2The Graduate School for Food and Drug Administration, The Graduate School for Pharmaceutical Industry Management, College of Pharmacy, Chung-Ang University, Seoul, South Korea
Objectives: To identify neurological aspects of Coronavirus disease 2019 (COVID-19) and to investigate COVID-19 infected patients with and without olfactory dysfunction in relation to polymerase chain reaction (PCR) assay results for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in the cerebrospinal fluid (CSF).
Methods: PubMed and EMBASE databases were searched until March 26, 2021, for observational studies with COVID-19 patients that had performed CSF PCR assay due to the neurologic symptom and reported anosmia status.
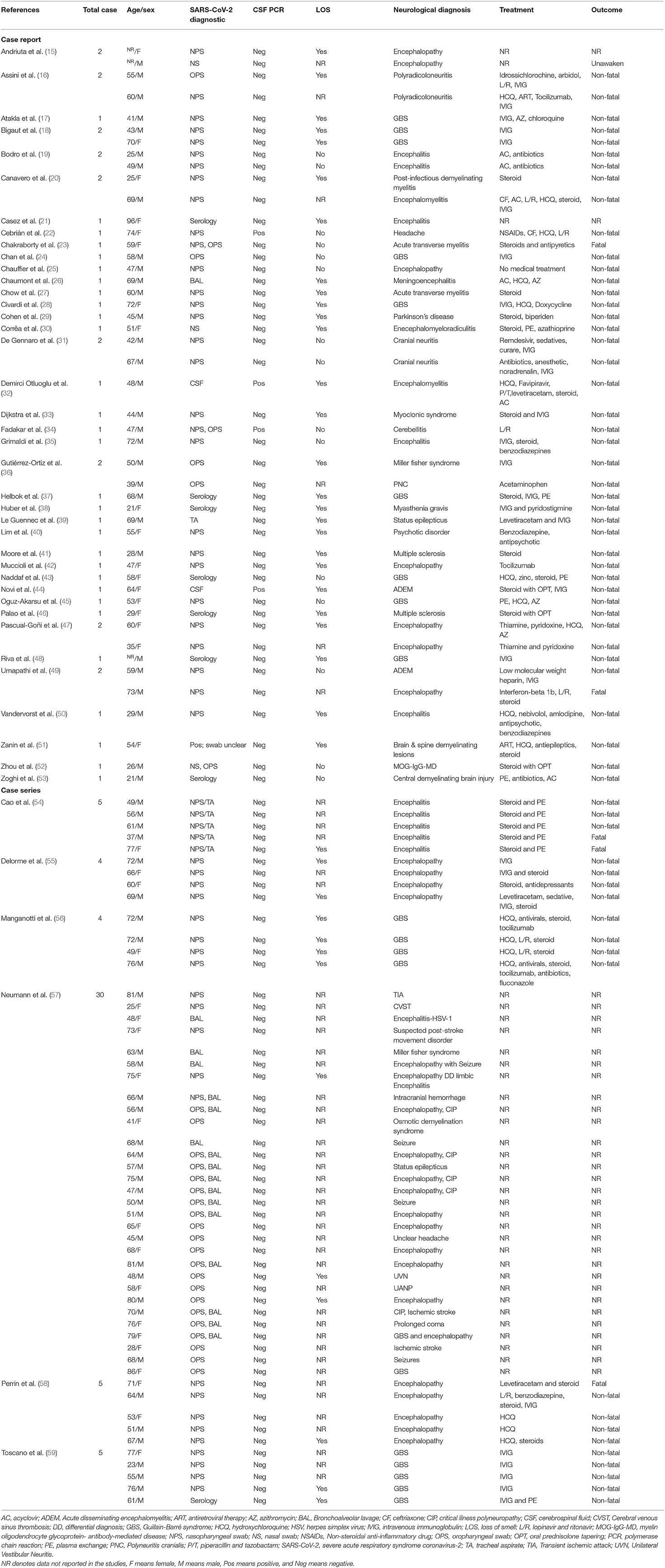
Results: Initially, 2,387 studies were identified;167 studies performed SARS-CoV-2 CSF PCR assay, of which our review comprised 45 observational studies that conducted CSF PCR assay for SARS-CoV-2 in 101 patients and reported anosmia status in 55 of 101 patients. Central and peripheral neurological manifestations observed in COVID-19 patients were diverse. The most common neurological diagnoses were Guillain-Barré syndrome (GBS) and its variants (24%), followed by encephalopathy (21%). The SARS-CoV-2 PCR assay was positive in only four CSF samples, of which two patients had olfactory dysfunction while the others did not.
Conclusions: The neurological spectrum of COVID-19 is diverse, and direct neuroinvasion of SARS-CoV-2 is rare. The neuroprotection against SARS-CoV-2 in COVID-19 patients with anosmia is controversial, as an equal number of patients with and without olfactory dysfunction had positive CSF PCR results for SARS-CoV-2 in our study, and further studies are required to provide more insight into this topic.
Introduction
The olfactory nerve connects the nasal cavity to the central nervous system (CNS) and provides a neuroinvasive shortcut to respiratory neurotropic viruses (1). The detection of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in the olfactory nerve and CNS of patients with coronavirus disease 2019 (COVID-19) suggests that SARS-CoV-2 has neuroinvasive potential via the olfactory pathway (2). Although SARS-CoV-2 neuroinvasion is uncommon, CNS viral transmission poses a significant threat to life (3).
Previous animal studies have demonstrated that respiratory neurotropic viral invasion induces apoptosis of olfactory receptor neurons (ORNs), preventing the viral transmission to the olfactory bulb and the CNS (4, 5). Although the exact mechanism underlying COVID-19 related anosmia is unclear, human and animal studies have shown that anosmia is a consequence of a host defense mechanism against viral invasion involving the damage of olfactory epithelium might provide neuroprotection (2, 5–9). Furthermore, anosmia is frequently seen in milder forms of COVID-19 with a lower mortality rate (10, 11). Therefore, neuroprotection is anticipated in COVID-19 patients with anosmia.
Understanding the underlying mechanism and prognostic value of COVID-19-related anosmia will aid better patient management since olfactory dysfunction is often associated with several neurological disorders (12). This systematic review aimed to compile studies involving COVID-19 patients with neurological manifestations who have undergone polymerase chain reaction (PCR) testing for SARS-CoV-2 in cerebrospinal fluid (CSF) and reported the patient's anosmia status for identifying neurological aspects of COVID-19 and exploring the COVID-19 infected patients with or without anosmia in relation to their CSF PCR assay results.
Methods
Eligibility Criteria
The observational studies related to CSF analysis of COVID-19 patients with neurological symptoms were included. Target patients were COVID-19 patients diagnosed based on either positive SARS-CoV-2 PCR or serologic testing who had a neurological manifestation and have undergone SARS-CoV-2 CSF PCR testing to identify COVID-19-related neurological disorders. Studies that conducted CSF PCR assay for SARS-CoV-2 but did not report information on the status of anosmia were excluded. The study covered primary, retrievable scientific literature available in English. Collected data were each patient's sex and age distribution, SAR-CoV-2 CSF PCR assay, neurological presentation, treatment, and outcome. Therefore, studies that did not report these data properly were also excluded.
Search Strategy
We conducted a broad literature search of databases such as EMBASE and PubMed until March 26, 2021, following preferred reporting items for systematic reviews and meta-analysis (PRISMA) checklist (13) for studies that performed CSF PCR assay for SARS-CoV-2 in COVID-19 patients using population search terms “SARS-CoV-2” or “COVID-19” and intervention search terms “brain” or “cerebrospinal fluid” or “anosmia”.
Study Selection
Two independent authors screened studies based on the titles and abstracts. Any studies relevant to the CSF analysis of patients with COVID-19 were advanced to the second stage of the review. Full texts were reviewed using the eligibility criteria mentioned above in the second screening. Any disagreement between the authors was resolved by discussion.
Risk of Bias Assessment
The Joanna Briggs Institute (JBI) critical appraisal checklist was used to assess the risk of bias in each included study (14).
Data Extraction and Analysis
Two authors independently collected the data items included in the study design for each eligible study. For evaluating neurological aspects of COVID-19, individual patient data on neurological presentation, treatment, and outcomes were collected. The data items included individual's age and sex distribution, CSF PCR assay result, anosmia status, COVID-19-related neurological symptoms, neurological diagnosis, treatment, and outcomes. Each COVID-19 patient's data with neurological manifestations who had undergone CSF PCR testing for SARS-CoV-2 to identify COVID-19-related neurological disorders was summarized to characteristics, clinical presentation, SARS-CoV-2 PCR assay results, neurological diagnosis, treatment, and outcomes.
Results
Study Selection
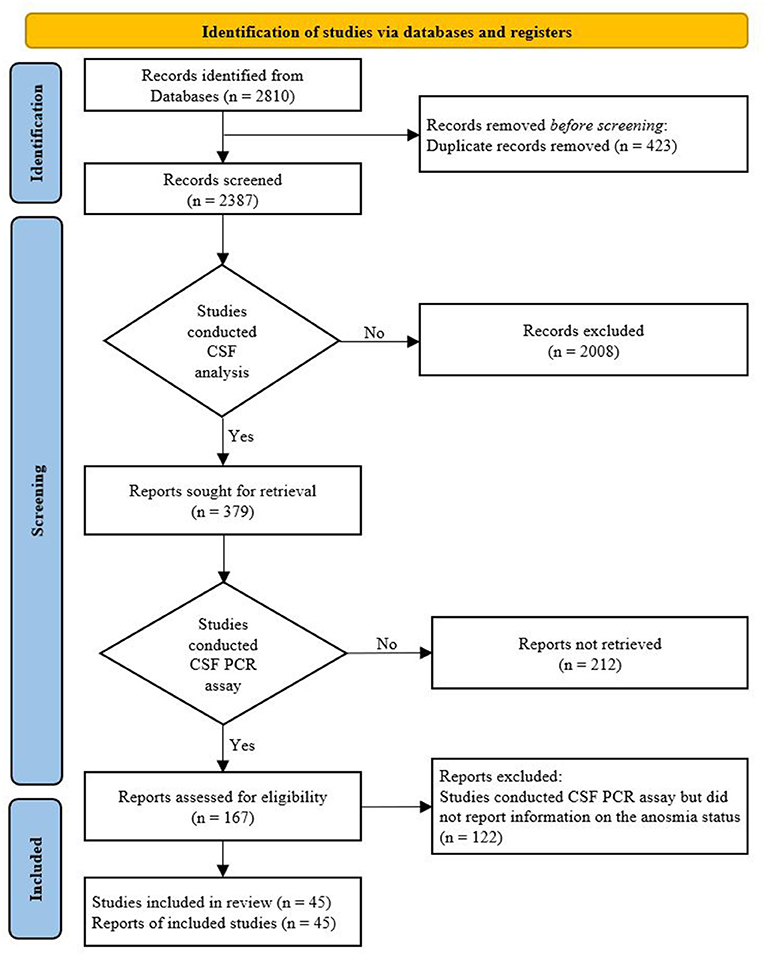
In total, 2,387 studies were identified through a literature search after removing duplicates. After preliminary screening based on the titles and abstracts, a total of 379 studies related to CSF analysis of COVID-19 patients with neurological symptoms were included; among them, 167 studies (44%) that conducted PCR tests for SARS-CoV-2 in CSF were selected for full-text review. A total of 122 studies that conducted CSF PCR assay for SARS-CoV-2 but did not report information on the status of anosmia were excluded. Thus, only 45 articles that met the inclusion criteria were included in our study (15–59). A flow diagram of the study selection process is shown in Figure 1.
Risk of Bias
Overall, the risk of bias in the included studies was low except for three studies (15, 21, 57). The summary of JBI critical appraisal results for case reports and case series can be seen in Supplementary Tables 1, 2.
Participants and Characteristics of Studies
The total number of participants was 104, while the SARS-CoV-2 CSF PCR testing was performed on only 101 patients. Table 1 shows the characteristics of the 101 participants included in the review. More than 63.4% (64/101) were men. The mean age was 57 ± 16.37 years. The number of men and women infected with COVID-19 increased with age. COVID-19 infected patients of both sexes, predominantly in the 60–79 age group (Figure 2).
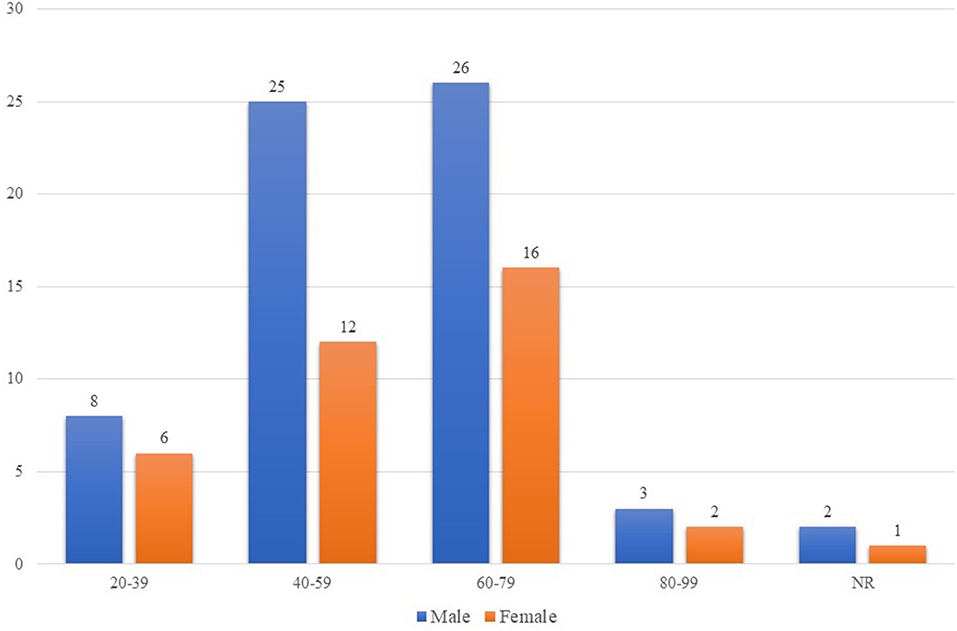
Figure 2. Age and sex distribution of COVID-19 patients who underwent CSF PCR assay for SARS-CoV-2. The number of men and women infected with COVID-19 who developed severe neurological manifestations and underwent CSF PCR assay for SARS-CoV-2 increased as the age of the individuals increased. The impact of COVID-19 was higher in patients aged 60-70 years old of both sexes. In addition, there were more COVID-19 infected men than COVID-19 infected women of all ages. COVID-19, coronavirus disease 2019; CSF, cerebrospinal fluid; PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2. NR denotes that the ages of two males and one female were not reported.
Neurological Aspects of COVID-19
Clinical Presentation
The neurological symptoms observed in COVID-19 individuals were diverse. The most common COVID-19-related neurological symptoms were smell disorder, taste disorder, headache, myalgia, altered consciousness, related paresis, and related cognitive and behavioral disturbances.
Neurological Diagnosis
The neurological diagnosis made after the neurological and radiological examination was localized to CNS (61.39%), peripheral nervous system (32.67%), or both (5.94%). In a study comprising 30 participants, six patients were diagnosed with two neurological disorders (57). The most common neurological diagnosis included Guillain-Barré syndrome and its variant (24%), followed by encephalopathy (21%) (Table 1).
Treatment and Outcomes
Information on the therapeutic management of COVID-19 was available for only 69 patients, including one patient who did not require medical treatment. In most cases, therapeutic management of COVID-19 patients involved combinational therapies. Common treatments included steroids administration (n = 32/69), intravenous immunoglobulin infusion (n = 28/69), hydroxychloroquine (n = 18/69), and plasma exchange (n = 11/69). Other medications used in the management of COVID-19 patients are shown in Table 1. The administration of medications resulted in neurological improvement in most patients. There were 63 non-fatal cases, five fatal cases, and one patient did not regain consciousness even after sedation was discontinued.
SARS-CoV-2 PCR Assay Results
Patients were confirmed to be COVID-19 positive when tested positive in PCR assay from nasopharyngeal swab or nasal swab (50/101), oropharyngeal swab (14/101), bronchoalveolar lavage (5/101), tracheal aspirate (1/101), or a combination of them (20/101). The PCR assay was positive in one study, but the swab used was not specified (51). Two patients were confirmed COVID-19 positive with the presence of SARS-CoV-2 in CSF (32, 44), and in eight patients with negative PCR test, COVID-19 infection was diagnosed with the presence of anti-SARS-CoV-2 in serum (Table 1) (21, 37, 38, 43, 46, 48, 53, 59).
CSF PCR assay for SARS-CoV-2 was positive for only four (3.96%) patients (22, 32, 34, 44) and negative in 97 (96.04%) patients (15–21, 23–31, 33, 35–43, 45–59). Of the 101 patients, information on the status of anosmia was available in 55 patients (51 patients had negative CSF PCR results, while four had positive CSF PCR results). Out of 51 patients with negative CSF PCR results, 38 had smell disorder, while 13 had no nasal symptoms. Meanwhile, two of the four patients with positive CSF PCR results for SARS-CoV-2 had olfactory dysfunction, while the other two did not (Table 1).
Discussion
This systematic review identified studies that performed CSF PCR assay for SARS-CoV-2 in COVID-19 positive patients and reported anosmia status to identify the common neurological manifestations associated with COVID-19 and to analyze the interrelation between CSF PCR results and anosmia. The neurological manifestations of COVID-19 are diverse. There was an equal number of patients with and without olfactory disorders who had positive CSF PCR results for SARS-CoV-2.
COVID-19 can trigger other autoimmune neurological complications such as neuromyelitis optica spectrum disorders or multiple sclerosis (30, 41, 46), which should be identified and treated promptly (44). In addition, COVID-19 patients with olfactory disorders and other severe neurological symptoms should be examined for possible neurodegenerative disease when suspected of having one (29).
In our study, ~4% of the participants had positive CSF PCR assay for SARS-CoV-2, similar to the finding of one study, which showed positive results in 6% of the participants, indicating SARS-CoV-2 neuroinvasion is a rare occurrence (3). However, negative CSF PCR results for SARS-CoV-2 may be due to delayed immune-mediated neurological damage after viral clearance (51, 53). Furthermore, the sensitivity decreases if samples are tested after a long period of symptom onset, giving negative results (43, 58). In addition, according to our review, only 44% of the published articles on CSF studies performed CSF PCR assay for SARS-CoV-2 in COVID-19 infected patients who experienced neurological symptoms. Therefore, despite the procedural and logistical complexity, the authors suggest an early collection of CSF samples, performing CSF PCR assay for SARS-CoV-2, detecting anti-neuronal autoantibodies, and using 18 F-fluorodeoxyglucose positron emission tomography in suspected cases could aid in the diagnosis and management of the patients, notably in magnetic resonance imaging negative cases (35, 51). Although the additional financial concern associated with the CSF PCR assay cannot be avoided, there were cases of testing positive in CSF PCR assay despite being negative in a nasal PCR or rapid COVID-19 test (32, 44). In addition, cost-effective studies in other neurotropic viruses have shown that the CSF PCR assay is cost-effective; similar studies in COVID-19 are required (60). Furthermore, a negative CSF PCR assay does not rule out the presence of the virus in the CNS; therefore, further studies of SARS-CoV-2 antibodies are required (57). Moreover, a recent study has shown that SARS-CoV-2 retrograde neuroinvasion via the olfactory route causes neuroinflammation (9). The detection of SARS-CoV-2 in the olfactory epithelium and various radiological findings in patients with COVID-19 suggests that despite the rarity of SARS-CoV-2 neuroinvasion via the olfactory system, it should not be overlooked (9, 21, 39, 61).
Similar to other respiratory neurotropic viruses, the direct neuroinvasion of SARS-CoV-2 in COVID-19 patients could occur mainly in two ways: damage to the olfactory epithelium or diffusion through the olfactory ensheathing cell (OEC) (1, 2) (Figure 3). Although ORNs of humans do not express SARS-CoV-2 entry proteins, factors other than angiotensin-converting enzyme-2 may be involved in a viral entry, such as neurolipin-1, which is highly expressed in ORNs (62–64) or SARS-CoV-2 can have non-neuronal mechanism (6, 9, 63). The neuronal and non-neuronal damage of the olfactory epithelium are responsible for the mechanism of loss of smell observed in COVID-19 patients (6, 7, 9). Nevertheless, viruses that are rapidly transported to the olfactory bulb before being affected by ORN apoptotic actions may invade the CNS (5).
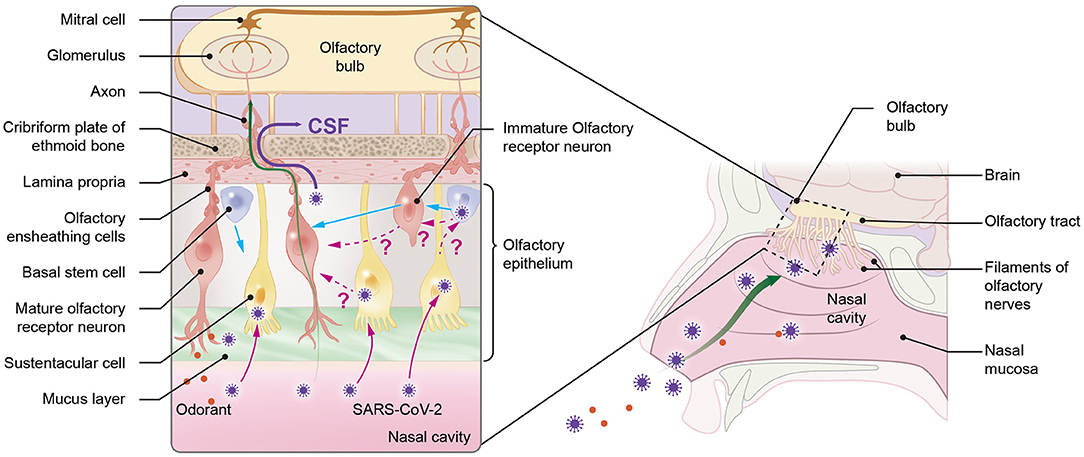
Figure 3. Possible mechanisms of neuroinvasion of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) via the olfactory pathway. SARS-CoV-2 can enter the central nervous system (CNS) through the olfactory system in one of two ways: directly through the cerebrospinal fluid (CSF) by crossing the channels created by olfactory ensheathing cells (OECs) (purple straight line) or through olfactory receptor neurons (ORNs). Since ORNs lack angiotensin-converting enzyme-2 (ACE-2), viruses are hypothesized to be transferred from sustentacular (SUS) cells, which contain ACE-2, either directly to mature ORNs (mORNs) or to stem cells (also containing ACE-2), which can then transfer the virus to immature ORNs (iORNs) during ORN regeneration process, where infected iORNs could grow into infected mORNs. The pink dotted line represents the hypotheses. Viruses can directly enter the CNS from ORN through ACE-2-independent mechanisms (green straight line) using factors such as neurolipin-1. The blue straight line represents the regeneration of ORNs and SUS cells from stem cells.
In addition, viruses as small as 100nm can also diffuse via the channels formed by OEC gaining direct access to the CSF (1, 65). The size of SARS-CoV-2 ranges from 60 to 140 nm (66). Additionally, direct infection of the OEC can release viruses into these channels and subsequently transport the virus to the olfactory bulb (1). Thus, SARS-CoV-2 with a smaller size can utilize this mode of viral transmission.
Studies analyzing the olfactory mucosa of COVID-19 patients with and without anosmia are required to acknowledge that apoptosis of ORNs is the cause of COVID-19-related anosmia. Future studies with a larger sample size involving nasal brush sampling method and CSF PCR assay can be performed on COVID-19 patients to determine whether apoptosis of ORNs could provide neuroprotection in COVID-19 patients with anosmia (9).
This study has several limitations. Olfactory mucosa biopsy is required to effectively analyze the association between apoptosis of ORNs with anosmia and neuroprotection. However, few studies were included in this analysis. Because the biopsy is an invasive procedure, it is rarely done in patients with COVID-19 only for research purposes (9), unlike animal studies. Additionally, studies that determine whether apoptosis of ORNs occurs in COVID-19 patients experiencing anosmia and SARS-CoV-2 CSF PCR assays are not available. For these reasons, the study design for analyzing the hypothesis was only feasible with observational studies. Though the risk of bias assessment showed an overall low risk, fundamental bias from the study design cannot be fully excluded. The findings from this review are not directly comparable with the results from other neurotropic viruses till these unanswered issues are solved. The number of patients with positive CSF PCR results did not differ by anosmia status, which may be related to the limited sample size and non-standard CSF PCR assay procedures. The CSF PCR assay is not commonly performed in COVID-19 patients with neurological manifestation. In this study, among COVID-19 patients with neurological manifestation, only 44% of patients underwent PCR assay for SARS-CoV-2 in the CSF to identify COVID-19 related neurological disorders. Though anosmia is common in COVID-19 patients, underreporting issues cannot be ignored, and because of limitation to our methodology, the neurological manifestations observed in individuals with COVID-19 cannot be generalized. Similarly, the possibility of an indirect mechanism of neuroinvasion of SARS-CoV-2 should not be overlooked. We could not investigate the neurological aspects of different strains of SARS-CoV-2 in COVID-19 infected patients and geographical and temporal relationships, particularly those concerning olfactory alteration, because information about the SARS-CoV-2 strain along with geographical and temporal information was not available in the included studies. Future studies with proper sample sizes involving definitive methods such as the nasal mucosa sampling method could provide a clear answer to the association between apoptosis of ORNs with anosmia and neuroprotection.
Conclusion
The neurological spectrum of COVID-19 is wide. Direct neuroinvasion of SARS-CoV-2 via the olfactory route is uncommon. Although previous experimental models of respiratory neurotropic viruses have demonstrated that apoptosis of the olfactory nerve blocks its neuroinvasive ability, this remains controversial in the case of SARS-CoV-2, since at present, human evidence is too scare limiting any conclusion to be drawn about the protection role of virus' olfactory mucosa invasion toward CNS invasion. More research with definitive methods is required to study the neuroprotective potential of ORN apoptosis in COVID-19 patients.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
EK had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. SP and EK: conceptualization, writing original draft, and formal analysis. SP, SO, and EK: data acquisition and writing review and editing. EK: funding and supervision. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by a grant from the Korean government, South Korea (Ministry of Science and ICT, MICT; NRF-2021R1F1A1062044) and by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, South Korea (Grant Number 2021R1A6A1A03044296). The funder had no role in the trial design, data collection, data interpretation, or report preparation.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.887164/full#supplementary-material
References
1. Van Riel D, Verdijk R, Kuiken T. The olfactory nerve: a shortcut for influenza other viral diseases into the central nervous system. J Pathol. (2015) 235:277–87. doi: 10.1002/path.4461
2. Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. (2021) 24:168–175. doi: 10.1101/2020.06.04.135012
3. Lewis A, Frontera J, Placantonakis DG, Lighter J, Galetta S, Balcer L, et al. Cerebrospinal fluid in COVID-19: a systematic review of the literature. J Neurol Sci. (2021) 421:117316. doi: 10.1016/j.jns.2021.117316
4. Mori I, Goshima F, Imai Y, Kohsaka S, Sugiyama T, Yoshida T, et al. Olfactory receptor neurons prevent dissemination of neurovirulent influenza A virus into the brain by undergoing virus-induced apoptosis. J Gen Virol. (2002) 83:2109–2116. doi: 10.1099/0022-1317-83-9-2109
5. Mori I, Nishiyama Y, Yokochi T, Kimura Y. Virus-induced neuronal apoptosis as pathological and protective responses of the host. Rev Med Virol. (2004) 14:209–16. doi: 10.1002/rmv.426
6. Butowt R. and von Bartheld CS, Anosmia in COVID-19: underlying mechanisms and assessment of an olfactory route to brain infection. Neuroscientist. (2020) 2020:1073858420956905. doi: 10.1177/1073858420956905
7. Bryche B, St Albin A, Murri S, Lacôte S, Pulido C, Ar Gouilh M, et al. Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain Behav Immun. (2020) 89:579–86. doi: 10.1016/j.bbi.2020.06.032
8. Le Bon SD, Horoi M Is anosmia the price to pay in an immune-induced scorched-earth policy against COVID-19? Med Hypotheses. (2020) 143:109881. doi: 10.1016/j.mehy.2020.109881
9. de Melo GD, Lazarini F, Levallois S, Hautefort C, Michel V, Larrous F, et al. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med. (2021) 13:eabf8396. doi: 10.1126/scitranslmed.abf8396
10. Talavera B, García-Azorín D, Martínez-Pías E, Trigo J, Hernández-Pérez I, Valle-Peñacoba G, et al. Anosmia is associated with lower in-hospital mortality in COVID-19. J Neurol Sci. (2020) 419:117163. doi: 10.1016/j.jns.2020.117163
11. Purja S, Shin H, Lee JY, Kim E. Is loss of smell an early predictor of COVID-19 severity: a systematic review and meta-analysis. Arch Pharm Res. (2021) 44:725–40. doi: 10.1007/s12272-021-01344-4
12. Ciurleo R, De Salvo S, Bonanno L, Marino S, Bramanti P, Caminiti F. Parosmia and neurological disorders: a neglected association. Front Neurol. (2020) 11:543275. doi: 10.3389/fneur.2020.543275
13. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
14. Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. Chapter 7: systematic reviews of etiology and risk. in JBI Manual for Evidence Synthesis JBI, editors Aromataris E, Munn Z (2020). Available online at: https://synthesismanual.jbi.global (accessed October 21, 2021).
15. Andriuta D, Roger PA, Thibault W, Toublanc B, Sauzay C, Castelain S, et al. COVID-19 encephalopathy: detection of antibodies against SARS-CoV-2 in CSF. J Neurol. (2020) 267:2810–1. doi: 10.1007/s00415-020-09975-1
16. Assini A, Benedetti L, Di Maio S, Schirinzi E, Del Sette M. New clinical manifestation of COVID-19 related Guillain-Barrè syndrome highly responsive to intravenous immunoglobulins: two Italian cases. Neurol Sci. (2020) 41:1657–8. doi: 10.1007/s10072-020-04484-5
17. Atakla HG, Noudohounsi M, Sacca H, Tassiou NRA, Noudohounsi WC, Houinato DS. Acute Guillain-Barré polyradiculoneuritis indicative of COVID-19 infection: a case report. Pan Afr Med J. (2020) 35:150. doi: 10.11604/pamj.supp.2020.35.2.25745
18. Bigaut K, Mallaret M, Baloglu S, Nemoz B, Morand P, Baicry F, et al. Guillain-Barré syndrome related to SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm. (2020) 7:e785. doi: 10.1212/NXI.0000000000000785
19. Bodro M, Compta Y, Llansó L, Esteller D, Doncel-Moriano A, Mesa A, et al. Increased CSF levels of IL-1β, IL-6, and ACE in SARS-CoV-2-associated encephalitis. Neurol Neuroimmunol Neuroinflamm. (2020) 7:e821. doi: 10.1212/NXI.0000000000000821
20. Canavero I, Valentino F, Colombo E, Franciotta D, Ferrandi D, Mussa M, et al. Acute myelopathies associated to SARS-CoV-2 infection: viral or immune-mediated damage? Travel Med Infect Dis. (2021) 40:102000. doi: 10.1016/j.tmaid.2021.102000
21. Casez O, Willaume G, Grand S, Nemoz B, Lupo J, Kahane P, et al. Teaching neuroimages: SARS-CoV-2–related encephalitis. MRI Pattern Olfact Tract Involvement Neurol. (2021) 96:e645–6. doi: 10.1212/WNL.0000000000011150
22. Cebrián J, Gonzalez-Martinez A, García-Blanco MJ, Celdrán-Vivancos D, Palacios EL, Reig-Roselló G, et al. Headache and impaired consciousness level associated with SARS-CoV-2 in CSF: a case report. Neurology. (2020) 95:266–8. doi: 10.1212/WNL.0000000000010213
23. Chakraborty U, Chandra A, Ray AK, Biswas P. COVID-19-associated acute transverse myelitis: a rare entity. BMJ Case Rep. (2020) 13:e238668. doi: 10.1136/bcr-2020-238668
24. Chan JL, Ebadi H, Sarna JR. Guillain-Barré Syndrome with facial diplegia related to SARS-CoV-2 infection. Can J Neurol Sci. (2020) 47:852–4. doi: 10.1017/cjn.2020.106
25. Chauffier J, Poey N, Husain M, de Broucker T, Khalil A, Lariven S, et al. First case of mild encephalopathy with reversible splenial lesion in SARS-CoV-2 infection. Infect Dis Now. (2021) 51:99–101. doi: 10.1016/j.medmal.2020.09.018
26. Chaumont H, Etienne P, Roze E, Couratier C, Roger PM, Lannuzel A. Acute meningoencephalitis in a patient with COVID-19. Rev Neurol. (2020) 176:519–21. doi: 10.1016/j.neurol.2020.04.014
27. Chow CCN, Magnussen J, Ip J, Su Y. Acute transverse myelitis in COVID-19 infection. BMJ Case Rep. (2020) 13:e236720. doi: 10.1136/bcr-2020-236720
28. Civardi C, Collini A, Geda DJ, Geda C. Antiganglioside antibodies in Guillain-Barré syndrome associated with SARS-CoV-2 infection. J Neurol Neurosurg Psychiatry. (2020) 91:1361–2. doi: 10.1136/jnnp-2020-324279
29. Cohen ME, Eichel R, Steiner-Birmanns B, Janah A, Ioshpa M, Bar-Shalom R, et al. A case of probable Parkinson's disease after SARS-CoV-2 infection. Lancet Neurol. (2020) 19:804–5. doi: 10.1016/S1474-4422(20)30305-7
30. Corrêa DG, de Souza Lima FC, da Cruz Bezerra D, Coutinho AC, Hygino da. Cruz LC. COVID-19 associated with encephalomyeloradiculitis and positive anti-aquaporin-4 antibodies: cause or coincidence? Mult Scler. (2021) 27:973–6. doi: 10.1177/1352458520949988
31. De Gennaro R, Gastaldo E, Tamborino C, Baraldo M, Casula N, Pedrali M, et al. Selective cranial multineuritis in severe COVID-19 pneumonia: two cases and literature review. Neurol Sci. (2021) 2021:1–6. doi: 10.1007/s10072-021-05087-4
32. Demirci Otluoglu G, Yener U, Demir MK, Yilmaz B. Encephalomyelitis associated with Covid-19 infection: case report. Br J Neurosurg. (2020) 2020:1–3. doi: 10.1080/02688697.2020.1787342
33. Dijkstra F, Van den Bossche T, Willekens B, Cras P, Crosiers D. Myoclonus and cerebellar ataxia following Coronavirus Disease (2019). (COVID-19) Mov Disord Clin Pract. (2020) 7:974–6. doi: 10.1002/mdc3.13049
34. Fadakar N, Ghaemmaghami S, Masoompour SM, Shirazi Yeganeh B, Akbari A, Hooshmandi S, et al. A first case of acute cerebellitis associated with coronavirus disease (COVID-19): a case report and literature review. Cerebellum. (2020) 19:911–4. doi: 10.1007/s12311-020-01177-9
35. Grimaldi S, Lagarde S, Harlé JR, Boucraut J, Guedj E. Autoimmune Encephalitis Concomitant with SARS-CoV-2 infection: insight from (18)F-FDG PET imaging and neuronal autoantibodies. J Nucl Med. (2020) 61:1726–9. doi: 10.2967/jnumed.120.249292
36. Gutiérrez-Ortiz C, Méndez-Guerrero A, Rodrigo-Rey S, San Pedro-Murillo E, Bermejo-Guerrero L, Gordo-Mañas R, et al. Miller fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. (2020) 95:e601–5. doi: 10.1212/WNL.0000000000009619
37. Helbok R, Beer R, Löscher W, Boesch S, Reindl M, Hornung R, et al. Guillain-Barré syndrome in a patient with antibodies against SARS-CoV-2. Eur J Neurol. (2020) 27:1754–6. doi: 10.1111/ene.14388
38. Huber M, Rogozinski S, Puppe W, Framme C, Höglinger G, Hufendiek K, et al. Postinfectious onset of myasthenia gravis in a COVID-19 patient. Front Neurol. (2020) 11:576153. doi: 10.3389/fneur.2020.576153
39. Le Guennec L, Devianne J, Jalin L, Cao A, Galanaud D, Navarro V, et al. Orbitofrontal involvement in a neuroCOVID-19 patient. Epilepsia. (2020) 61:e90–4. doi: 10.1111/epi.16612
40. Lim ST, Janaway B, Costello H, Trip A, Price G. Persistent psychotic symptoms following COVID-19 infection. BJPsych Open. (2020) 6:e105. doi: 10.1192/bjo.2020.76
41. Moore L, Ghannam M, Manousakis G. first presentation of multiple sclerosis with concurrent COVID-19 infection. eNeurologicalSci. (2021) 22:100299. doi: 10.1016/j.ensci.2020.100299
42. Muccioli L, Pensato U, Cani I, Guerra L, Provini F, Bordin G, et al. COVID-19-related encephalopathy presenting with aphasia resolving following tocilizumab treatment. J Neuroimmunol. (2020) 349:577400. doi: 10.1016/j.jneuroim.2020.577400
43. Naddaf E, Laughlin RS, Klein CJ, Toledano M, Theel ES, Binnicker MJ, et al. Guillain-barré syndrome in a patient with evidence of Recent SARS-CoV-2 Infection. Mayo Clin Proc. (2020) 95:1799–801. doi: 10.1016/j.mayocp.2020.05.029
44. Novi G, Rossi T, Pedemonte E, Saitta L, Rolla C, Roccatagliata L, et al. Acute disseminated encephalomyelitis after SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm. (2020) 7:e797. doi: 10.1212/NXI.0000000000000797
45. Oguz-Akarsu E, Ozpar R, Mirzayev H, Acet-Ozturk NA, Hakyemez B, Ediger D, et al. Guillain-Barré syndrome in a patient with minimal symptoms of COVID-19 infection. Muscle Nerve. (2020) 62:e54–7. doi: 10.1002/mus.26992
46. Palao M, Fernández-Díaz E, Gracia-Gil J, Romero-Sánchez CM, Díaz-Maroto I, Segura T. Multiple sclerosis following SARS-CoV-2 infection. Mult Scler Relat Disord. (2020) 45:102377. doi: 10.1016/j.msard.2020.102377
47. Pascual-Goñi E, Fortea J, Martínez-Domeño A, Rabella N, Tecame M, Gómez-Oliva C, et al. COVID-19-associated ophthalmoparesis and hypothalamic involvement. Neurol Neuroimmunol Neuroinflamm. (2020) 7:e823. doi: 10.1212/NXI.0000000000000823
48. Riva N, Russo T, Falzone YM, Strollo M, Amadio S, Del Carro U, et al. Post-infectious Guillain-Barré syndrome related to SARS-CoV-2 infection: a case report. J Neurol. (2020) 267:2492–4. doi: 10.1007/s00415-020-09907-z
49. Umapathi T, Quek WMJ, Yen JM, Khin HSW, Mah YY, Chan CYJ, et al. Encephalopathy in COVID-19 patients; viral, parainfectious, or both? eNeurologicalSci. (2020) 21:100275. doi: 10.1016/j.ensci.2020.100275
50. Vandervorst F, Guldolf K, Peeters I, Vanderhasselt T, Michiels K, Berends KJ, et al. Encephalitis associated with the SARS-CoV-2 virus: a case report. Interdiscip Neurosurg. (2020) 22:100821. doi: 10.1016/j.inat.2020.100821
51. Zanin L, Saraceno G, Panciani PP, Renisi G, Signorini L, Migliorati K, et al. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir (Wien). (2020) 162:1491–4. doi: 10.1007/s00701-020-04374-x
52. Zhou S, Jones-Lopez EC, Soneji DJ, Azevedo CJ, Patel VR. Myelin oligodendrocyte glycoprotein antibody-associated optic neuritis and myelitis in COVID-19. J Neuroophthalmol. (2020) 40:398–402. doi: 10.1097/WNO.0000000000001049
53. Zoghi A, Ramezani M, Roozbeh M, Darazam IA, Sahraian MA. case of possible atypical demyelinating event of the central nervous system following COVID-19. Mult Scler Relat Disord. (2020) 44:102324. doi: 10.1016/j.msard.2020.102324
54. Cao A, Rohaut B, Le Guennec L, Saheb S, Marois C, Altmayer V, et al. Severe COVID-19-related encephalitis can respond to immunotherapy. Brain. (2020) 143:e102–e102. doi: 10.1093/brain/awaa337
55. Delorme C, Paccoud O, Kas A, Hesters A, Bombois S, Shambrook P, et al. COVID-19-related encephalopathy: a case series with brain FDG-positron-emission tomography/computed tomography findings. Eur J Neurol. (2020) 27:2651–7. doi: 10.1111/ene.14478
56. Manganotti P, Bellavita G, D'Acunto L, Tommasini V, Fabris M, Sartori A, et al. Clinical neurophysiology and cerebrospinal liquor analysis to detect Guillain-Barré syndrome and polyneuritis cranialis in COVID-19 patients: a case series. J Med Virol. (2021) 93:766–74. doi: 10.1002/jmv.26289
57. Neumann B, Schmidbauer ML, Dimitriadis K, Otto S, Knier B, Niesen WD, et al. Cerebrospinal fluid findings in COVID-19 patients with neurological symptoms. J Neurol Sci. (2020) 418:117090. doi: 10.1016/j.jns.2020.117090
58. Perrin P, Collongues N, Baloglu S, Bedo D, Bassand X, Lavaux T, et al. Cytokine release syndrome-associated encephalopathy in patients with COVID-19. Eur J Neurol. (2021) 28:248–58. doi: 10.1111/ene.14491
59. Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, Cuzzoni MG, et al. Guillain-Barré syndrome associated with SARS-CoV-2. N Engl J Med. (2020) 382:2574–6. doi: 10.1056/NEJMc2009191
60. Hauser RG, Campbell SM, Brandt CA, Wang S. Cost-effectiveness study of criteria for screening cerebrospinal fluid to determine the need for herpes simplex virus PCR testing. J Clin Microbiol. (2017) 55:1566–75. doi: 10.1128/JCM.00119-17
61. Guedj E, Million M, Dudouet P, Tissot-Dupont H, Bregeon F, Cammilleri S, et al. (18)F-FDG brain PET hypometabolism in post-SARS-CoV-2 infection: substrate for persistent/delayed disorders? Eur J Nucl Med Mol Imaging. (2021) 48:592–5. doi: 10.1007/s00259-020-04973-x
62. Daly JL, Simonetti B, Klein K, Chen KE, Williamson MK, Antón-Plágaro C, et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. (2020) 370:861–5. doi: 10.1126/science.abd3072
63. Brann DH, Tsukahara T, Weinreb C, Lipovsek M, Van den Berge K, Gong B, et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv. (2020) 6:eabc5801. doi: 10.1126/sciadv.abc5801
64. Cantuti-Castelvetri L, Ojha R, Pedro LD, Djannatian M, Franz J, Kuivanen S, et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. (2020) 370:856–60. doi: 10.1126/science.abd2985
65. Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, et al. Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol. (2004) 16:437–45. doi: 10.1080/08958370490439597
Keywords: COVID-19, SARS-CoV-2, anosmia, cerebrospinal fluid, neuroinvasion
Citation: Purja S, Oh S and Kim E (2022) A Systematic Review on Neurological Aspects of COVID-19: Exploring the Relationship Between COVID-19-Related Olfactory Dysfunction and Neuroinvasion. Front. Neurol. 13:887164. doi: 10.3389/fneur.2022.887164
Received: 01 March 2022; Accepted: 21 June 2022;
Published: 15 July 2022.
Edited by:
Anastasios Mpotsaris, München Hospital, GermanyReviewed by:
Jorge Matias-Guiu, Complutense University of Madrid, SpainAmjad Maher Elmashala, Al-Quds Cognitive Neuroscience Lab, Al-Quds University, Palestine
Copyright © 2022 Purja, Oh and Kim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: EunYoung Kim, ZXlraW1qY2I3NzdAY2F1LmFjLmty
†ORCID: Sujata Purja orcid.org/0000-0001-6507-915X
SuA Oh orcid.org/0000-0001-5682-4066
EunYoung Kim orcid.org/0000-0003-3525-8805
 Sujata Purja
Sujata Purja SuA Oh
SuA Oh EunYoung Kim
EunYoung Kim