- 1Department of Neurology, Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, China
- 2Department of Outpatient, People's Liberation Army the General Hospital of Western Theater Command, Chengdu, China
Background: This study aims to assess the efficacy and safety of different doses of intravenous tissue-type plasminogen activator (tPA) for acute ischemic stroke (AIS) by adopting a network meta-analysis (NMA).
Methods: Studies comparing different doses of tPA in AIS were identified by retrieving electronic databases. NMAs of outcome measures included favorable functional outcome with a modified Rankin scale score (mRS) of 0 or 1 at 3 months after treatment (3M-FF), the functional independence with a mRS of 0, 1, or 2 at 3 months (3M-FI), symptomatic intracranial hemorrhage (sICH) and 3-month all-cause mortality (3M-M). Symptomatic intracranial hemorrhage (sICH) and 3-month all-cause mortality (3M-M) were assessed. Probability-based ranking and surface under cumulative ranking (SUCRA) were performed to identify the best dose of tPA. Inconsistency was evaluated by node-splitting analysis and a loop-specific approach. Publication bias was analyzed by funnel plots.
Results: A total of 14 studies were included in the quantitative synthesis. The NMA results revealed no difference among low (<0.7 mg/kg), moderate (0.8 mg/kg), and standard (0.9 mg/kg) doses of tPA with regard to efficacy and safety. The SUCRAs of 3M-FF and 3M-FI showed that the standard dose ranked first, the moderate dose ranked second, and the low dose ranked third. The SUCRA of sICH showed that the standard dose ranked first (78.1%), the low dose ranked second (61.0%), and the moderate dose ranked third (11.0%). The SUCRAs of 3-month mortality showed that the standard dose ranked first (73.2%), the moderate dose ranked second (40.8%), and the low dose ranked third (36.1%). No significant inconsistency was shown by node-splitting analysis and no publication bias was shown in funnel plots.
Conclusion: Lower dose tPA was comparable to the standard dose with regard to efficacy and safety. Based on the SUCRA results and American Heart Association/American Stroke Association (AHA/ASA) guidelines, the standard dose was still the optimal selection for AIS.
Introduction
Globally, stroke remains the second-leading cause of death. Prevalent cases of stroke were estimated to be 101 million in 2019, 62.4% of which were ischemic stroke (1). Until now, tissue plasminogen activator (tPA) is the only treatment approved by the US Food and Drug Administration (FDA) serving as the first line of treatment for acute ischemic stroke (AIS) (2). However, due to the high cost, narrow therapeutic time window, and risk of intracranial hemorrhage, tPA is clinically limited. Therefore, it is urgent to find ways to reduce the medical burden and risk of intracranial hemorrhage. Under this context, several studies (3–5) were performed to explore the efficacy and safety of a lower dose of tPA for AIS in Japan, which demonstrated that the efficacy and safety of low dose (0.6 mg/kg) tPA was comparable to a dose of 0.9 mg/kg from published data.
Thereafter, numerous studies compared the efficacy and safety of different doses of tPA for AIS, which remain inconclusive. A previous meta-analysis aimed at analyzing whether low dose tPA can effectively reduce symptomatic intracranial hemorrhage (sICH) and has the same efficacy as standard dose tPA showed that low dose tPA was comparable to standard-dose tPA in terms of efficacy and safety in Asian patients with AIS (6). However, in this meta-analysis, the low dose was defined as <0.75 mg/kg, and the standard dose was defined as >0.75 mg/kg. In addition, five new studies (7–11) were reported after this meta-analysis. Moreover, traditional meta-analysis is difficult to use to assess the effects of two or more interventions. By contrast, network meta-analysis (NMA) can make comparisons of all the interventions and can also provide information on which is the best treatment by ranking analysis. Thus, in this study, we will perform an NMA by combining all studies concerning different doses of tPA for AIS to compare their efficacy and safety.
Methods
Literature Search
The presentation of this study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline (12). A systematic search of PubMed, Embase, and Web of Science (last search was updated on 4 January 2022) was performed with a combination of the following keywords: (stroke/cerebral infarction/cerebral ischemia) with (alteplase OR tissue plasminogen activator OR intravenous thrombolysis OR rtPA) and low dose (Supplementary Table 1). The language was restricted to English. We manually searched the references of articles retrieved. When the same patient population was used in several publications, only the complete or largest study was included. Two investigators screened each of the titles, abstracts, and full texts to determine inclusion independently. The results were compared and disagreements were resolved by consensus.
Selection Criteria
The inclusion criteria were as follows: (1) studies with retrospective and prospective cohort design; (2) studies should report functional outcome (with modified Rankin scale score [mRS] assessment) at 3 months after symptom onset, incidence of mortality, and sICH; (3) studies with full-text articles; and (4) studies comparing the effect of different doses of tPA in AIS.
The exclusion criteria were as follows: (1) non-control study or placebo-control studies; (2) studies sharing the same patient population; (3) studies with data that could not be extracted or converted into valid data.
Data Extraction
Information was carefully extracted from all included publications independently by the two authors according to the inclusion criteria listed above. Disagreement was resolved by consensus or discussion with a third reviewer. The following data were collected from each study: first author's name, publication date, country, number of patients, the dose of tPA, the incidence rate of favorable functional outcome at 3 months after treatment (3M-FF), functional independence at 3 months (3M-FI), sICH, and 3-month all cause-mortality (3M-M), respectively.
Data Synthesis
The doses of tPA were classified as low (<0.7 mg/kg), moderate (0.8 mg/kg), and standard (0.9 mg/kg).
Outcome Measures
The efficacy outcomes included the proportion of patients achieving an mRS of 0 or 1 at 3M-FF and an mRS of 0, 1, or 2 at 3M-FI. The safety outcome included the incidence rate of sICH and 3M-M. The sICH is defined by the European Cooperative Acute Stroke Study (ECASS) criteria (13). If the sICH data defined by ECASS criteria were not available, the incidence rate of sICH defined by National Institute of Neurological Disorders and Stroke (NINDS) (14) was applied.
Assessment of Risk of Bias
Risk of bias was assessed using the Cochrane Collaboration Tool. Judgment as “low,” “unclear,” or “high” risk of bias was provided in each of the domains for each study.
Statistical Analyses
A network meta-analysis was carried out using STATA version 15.0 based on the Bayesian framework model. The corresponding odds ratios (ORs) with 95% CIs were calculated. Rank plots based on probabilities and the surface under cumulative ranking (SUCRA) for different outcomes were performed to identify the best treatment. Inconsistency was evaluated by node-splitting analysis and a loop-specific approach. Publication bias was analyzed by funnel plots.
Results
Characteristics of the Study
The study selection process is detailed in Figure 1. There were 1,568 potentially relevant articles identified after the search. After screening titles and abstracts, a total of 34 studies were included for full-text article assessment. Five studies were non-control studies (3–5, 15, 16). One study was a placebo-control study (17). Two studies reported the same cohort (18, 19), and the larger study (19) was included. Four studies reported the same cohort (20–23), and the largest study (20) was included. One study was an individual patient data pooling study from six Asian countries (China, Japan, Philippines, Singapore, South Korea, and Taiwan) (24). One study was a letter to the editor (25). Four studies provided no data on indexed outcomes (26–29).
Finally, 18 studies were included in the qualitative synthesis (7–11, 19, 20, 30–40). The doses of three studies (30, 34, 39) did not meet the design of the present study and one study (40) without specific data was excluded from the final quantitative analysis. Fourteen studies were included in the quantitative synthesis (7–11, 19, 20, 31–33, 35–38). Table 1 summarizes the characteristics of the 14 included studies. In brief, these studies were reported between 2010 and 2021. The majority (10/14) were two-arm studies; four studies had three arms. Seven studies were retrospective cohort studies, one study was a randomized controlled trial, and six studies were prospective cohort studies. Eight studies were from China, one study was from Singapore, one study was from Korea, one study was from the Czech Republic, one study was from Srpska, one study was from Egypt, and one study was worldwide. The risk of bias of included studies in this NMA was generally low to unclear. Details about the risk of bias assessment are graphically summarized in Supplementary Figure 1.
Results of Network Meta-Analysis
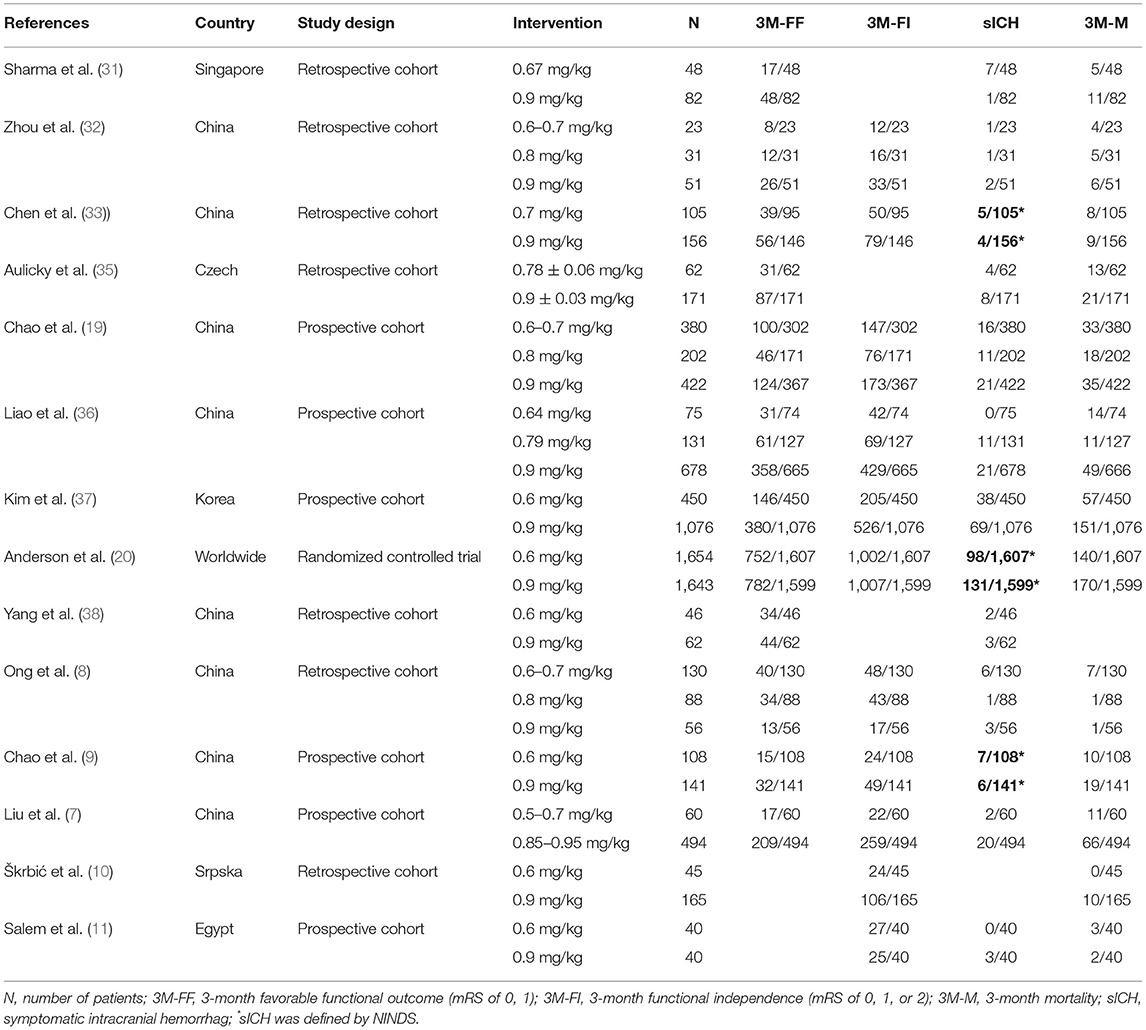
Twelve studies with 8,332 patients reported 3M-FF (Figure 2A). The pooled meta-analysis results (Figure 2B) showed no significant difference between different doses of tPA. The probability-based ranking result is shown in Figures 2C,D. Results of SUCRA showed that the standard dose ranked first (90.2%), the moderate dose ranked second (31.3%), and the low dose ranked third (28.5%). In this result, the first rank had the highest proportion of 3M-FF.
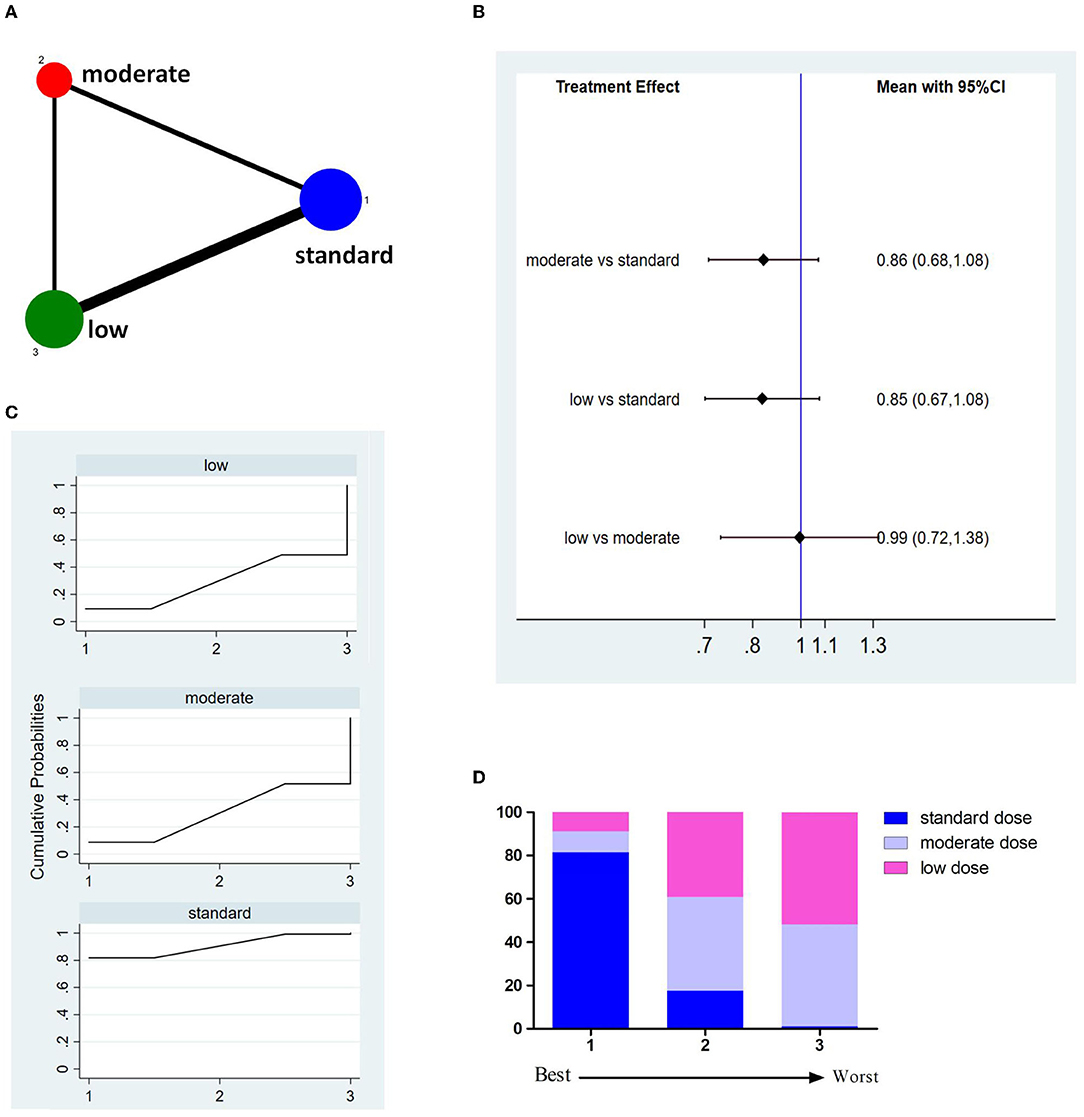
Figure 2. Results of favorable functional outcome at 3 months after treatment (3M-FF). (A) Network plots of eligible comparisons. The width of the lines represents the number of studies being compared, and the node size reflects the sample size. (B) The forest plot of network results. The black diamonds represent the combined ORs; OR > 1 indicates that the proportion of 3M-FF in the former group is greater than that in the latter group. (C) The cumulative ranking curve of 3M-FF. (D) The ranking of different doses of tPA is based on the cumulative probability plots. Ranking first means having the highest proportion of 3M-FF.
Eleven studies involving 8,151 patients reported 3M-FI (Figure 3A). The pooled results showed no significant difference between different doses of tPA (Figure 3B). The probability-based ranking result is shown in Figures 3C,D. Results of SUCRA showed that the standard dose ranked first (90.2%), the moderate dose ranked second (36.9%), and the low dose ranked third (22.9%). In this result, the first rank had the highest proportion of 3M-FI.
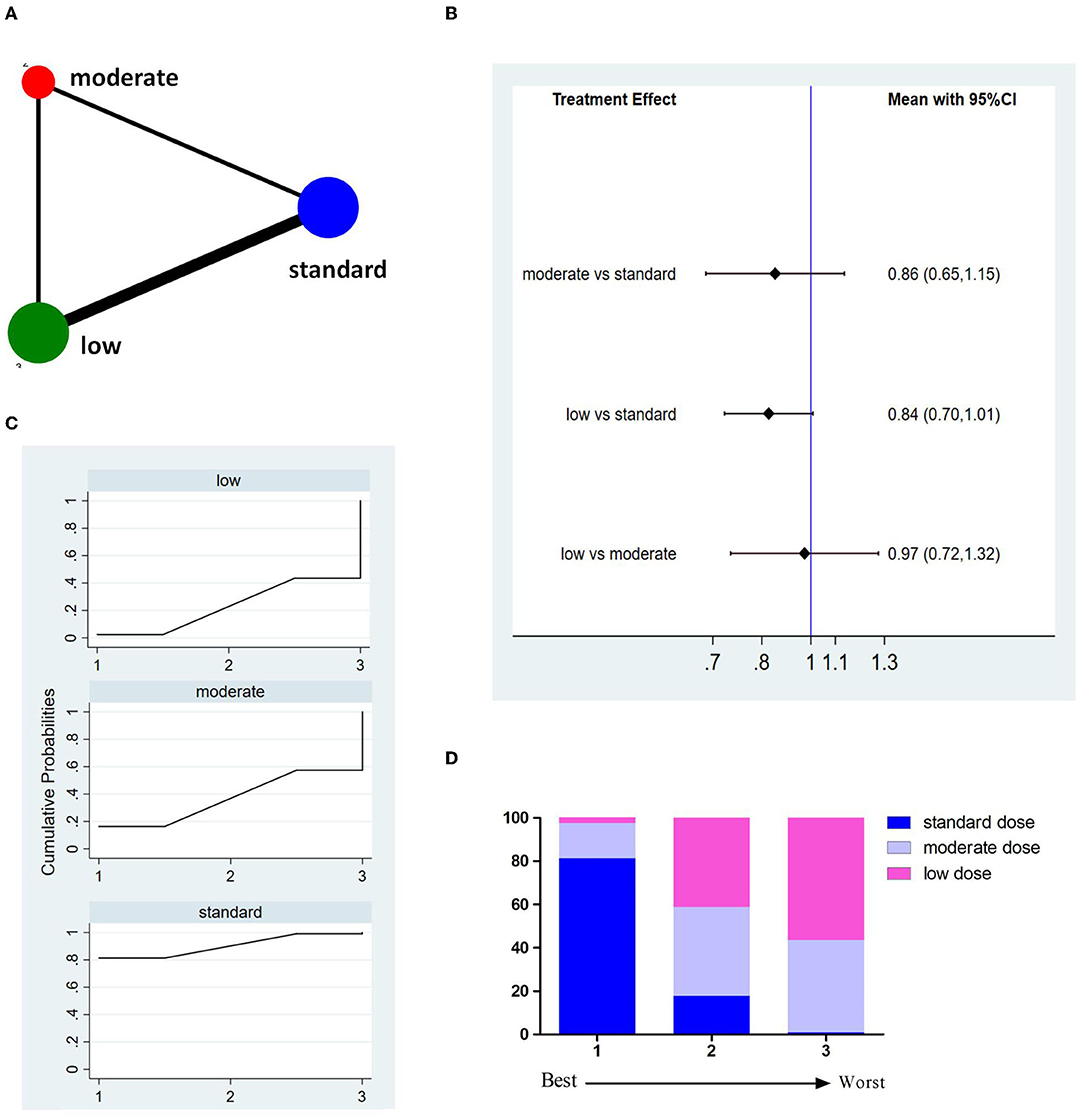
Figure 3. Results of functional independence at 3 months (3M-FI). (A) Network plots of eligible comparisons. The width of the lines represents the number of studies being compared, and the node size reflects the sample size. (B) The forest plot of network results. The black diamonds represent the combined ORs; OR > 1 indicates that the proportion of 3M-FI in the former group is greater than that in the latter group. (C) The cumulative ranking curve of 3M-FI. (D) The ranking of different doses of tPA is based on the cumulative probability plots. Ranking first means having the highest proportion of 3M-FI.
Fourteen studies involving 8,614 patients reported incidences of sICH (Figure 4A). The pooled results showed no significant difference between different doses of tPA (Figure 4B). The probability-based ranking result is shown in Figures 4C,D. Results of SUCRA showed that the standard dose ranked first (78.1%), the low dose ranked second (61.0%), and the moderate dose ranked third (11.0%). In this result, the first rank had the lowest incidence of sICH.
Figure 4. Results of symptomatic intracranial hemorrhage (sICH). (A) Network plots of eligible comparisons. The width of the lines represents the number of studies being compared, and the node size reflects the sample size. (B) The forest plot of network results. The black diamonds represent the combined ORs; OR > 1 indicates that the incidence rate of sICH in the former group is higher than in the latter group. (C) The cumulative ranking curve of sICH. (D) The ranking of different doses of tPA is based on the cumulative probability plots. Ranking first means having the lowest incidence of sICH.
Thirteen studies involving 8,699 patients reported incidences of 3M-M (Figure 5A). The pooled results showed no significant difference between different doses of tPA (Figure 5B). The probability-based ranking result is shown in Figures 5C,D. Results of SUCRA showed that the standard dose ranked first (73.2%), the moderate dose ranked second (40.8%), and the low dose ranked third (36.1%). In this result, the first rank had the lowest incidence of 3M-M.
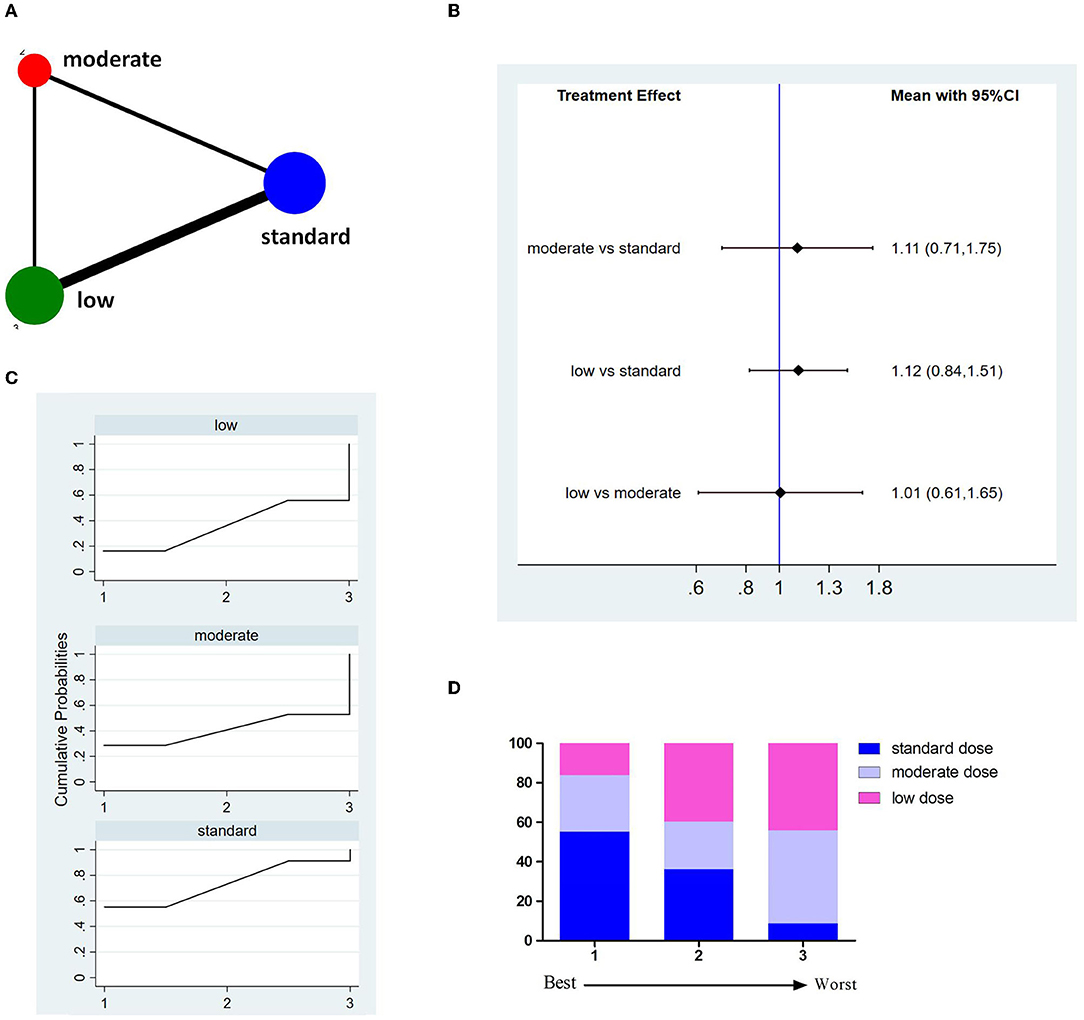
Figure 5. Results of 3-month all cause-mortality (3M-M). (A) Network plots of eligible comparisons. The width of the lines represents the number of studies being compared, and the node size reflects the sample size. (B) The forest plot of network results. The black diamonds represent the combined ORs; OR > 1 indicates that the incidence rate of 3M-M in the former group is higher than in the latter group. (C) The cumulative ranking curve of 3M-M. (D) The ranking of different doses of tPA is based on the cumulative probability plots. Ranking first means having the lowest incidence of 3M-M.
Consistency Analysis and Publication Bias
The node-splitting analysis was applied to evaluate the inconsistency by comparing the differences between direct and indirect evidence. No significant inconsistency was shown indicating that the results were reliable. With regard to publication bias, no asymmetry evidence was shown in funnel plots (Figure 6).
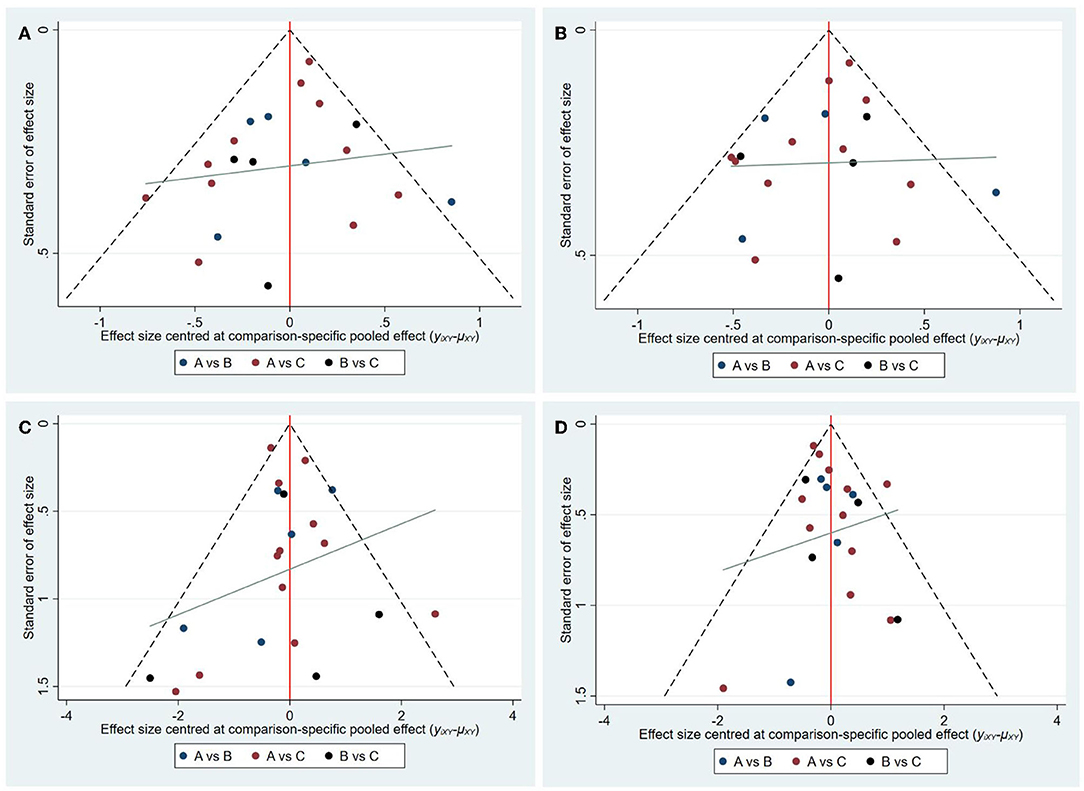
Figure 6. Funnel plots of each outcome. (A) Funnel plots of 3M-FF; (B) Funnel plots of 3M-FI; (C) Funnel plots of sICH; (D) Funnel plots of 3M-M. (A: standard dose, B: moderate dose, C: low dose).
Discussion
To date, different doses of tPA have been applied in AIS, but it is not determined which one is the best. Traditional meta-analysis only allows the direct comparison of two doses that have been evaluated head-to-head, while NMA can compare all doses of tPA simultaneously within a single framework and rank available doses of tPA according to efficacy and safety. NMA is helpful for clinical decision-making by providing information on which is the best treatment.
After the studies (3–5) performed in Japan demonstrated that the efficacy and safety of low dose (0.6 mg/kg) tPA was comparable to the standard dose, many studies have evaluated the efficacy and safety of different doses of tPA in AIS, which varied from 0.5 mg/kg to 0.9 mg/kg. In terms of efficacy, four studies showed that the standard dose was better than lower doses (7, 9, 31, 36) and an opposite conclusion was reported in two studies (19, 30). In terms of safety, two studies showed that sICH occurred more frequently with a low dose (14.5%) (31, 36); two studies showed that there were significantly fewer sICH with a low dose of tPA (20, 39); one study showed that the 3-month mortality rate was higher in the standard dose group (30). In our study, we did not find any difference between low (<0.7 mg/kg), moderate (0.8 mg/kg), and standard (0.9 mg/kg) doses of tPA with regard to efficacy and safety.
Although there was no difference in the efficiency and safety of different doses, results of SUCRAs demonstrated that the standard dose ranked as the most effective dose. Namely, the standard dose was still the best option for patients with AIS. With respect to an individual patient, who may be suitable for a lower dose? One study including 3,479 patients with AIS reported that in patients who had moderate stroke (NIHSS 5–14), lower doses of tPA are associated with reduced sICH and non-inferior performance in efficacy (29). Another study demonstrated that a low dose (0.6 mg/kg) of tPA may be preferable in patients with AIS with younger age, lower systolic blood pressure, and mild neurological impairment (28). Moreover, in patients aged 71–80 years, there was a significant trend of increasing sICH (P = 0.013) and fewer good functional outcomes (P = 0.0179) with increasing doses of tPA (19). Therefore, future studies should observe the efficacy and safety of different doses of tPA in different patient populations with AIS as discussed above. In addition, considering the high medical burden, lower doses of tPA may be suitable for those patients with poor financial ability as it was reported that the total cost during hospitalization for the 0.6 mg/kg dose group was significantly less than that for the standard-dose group (3,401.7 USD vs. 4,157.4 USD) (38).
To better interpret the results, some limitations of this NMA should be acknowledged. First, the present study was not based on individual patient data, which limited the evaluation of other clinical outcomes and the influence of other confounders, such as the combination of characteristics as discussed above. Secondly, most of the included studies focused on the Asian population, which cannot exclude racial differences. Third, most of the studies were observational and only one was a large international RCT, which may affect the validity of our results. Fourth, baseline characteristics of included studies have not been analyzed; thus, the comparability of results between studies may be debatable.
Conclusion
In summary, the results from this NMA suggest that lower dose tPA is comparable to the standard dose with regard to efficacy and safety. Based on the SUCRA results and AHA/ASA guidelines (2), the standard dose was still the optimal selection for AIS. Due to the limitations of the present study, further studies of high quality are needed.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
B-HL, J-HW, and N-WY conceived and designed the study and were involved in literature search and data collection. B-HL, HW, and D-ZW analyzed the data. B-HL, SY, and F-QG wrote the paper. J-HW and N-WY reviewed and edited the manuscript. All authors have read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.884267/full#supplementary-material
Supplementary Figure 1. Results of the bias risk assessment.
Supplementary Table 1. Search strategy.
References
1. Global regional and and national burden of stroke and its risk factors 1990-2019: 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Neurol. (2021) 20:795–820. doi: 10.1016/S1474-4422(21)00252-0
2. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. (2019) 50:e344–418. doi: 10.1161/STR.0000000000000211
3. Yamaguchi T, Mori E, Minematsu K, Nakagawara J, Hashi K, Saito I, et al. Alteplase at 06 mg/kg for acute ischemic stroke within 3 hours of onset: Japan Alteplase Clinical Trial (J-ACT). Stroke. (2006) 37:1810–5. doi: 10.1161/01.STR.0000227191.01792.e3
4. Toyoda K, Koga M, Naganuma M, Shiokawa Y, Nakagawara J, Furui E, et al. Routine use of intravenous low-dose recombinant tissue plasminogen activator in Japanese patients: general outcomes and prognostic factors from the SAMURAI register. Stroke. (2009) 40:3591–5. doi: 10.1161/STROKEAHA.109.562991
5. Mori E, Minematsu K, Nakagawara J, Yamaguchi T, Sasaki M. Hirano T. Effects of 06 mg/kg intravenous alteplase on vascular and clinical outcomes in middle cerebral artery occlusion: Japan Alteplase Clinical Trial II (J-ACT II). Stroke. (2010) 41:461–5. doi: 10.1161/STROKEAHA.109.573477
6. Cheng JW, Zhang XJ, Cheng LS, Li GY, Zhang LJ, Ji KX, et al. Low-dose tissue plasminogen activator in acute ischemic stroke: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. (2018) 27:381–90. doi: 10.1016/j.jstrokecerebrovasdis.2017.09.014
7. Liu M, Pan Y, Zhou L, Wang Y. Low-dose rt-PA may not decrease the incidence of symptomatic intracranial haemorrhage in patients with high risk of symptomatic intracranial haemorrhage. Neurol Res. (2019) 41:473–9. doi: 10.1080/01616412.2019.1580454
8. Ong CT, Wong YS, Wu CS, Su YH. Outcome of stroke patients receiving different doses of recombinant tissue plasminogen activator. Drug Des Devel Ther. (2017) 11:1559–66. doi: 10.2147/DDDT.S133759
9. Chao AC, Han K, Lin SF, Lin RT, Chen CH, Chan L, et al. Low-dose versus standard-dose intravenous alteplase for octogenerian acute ischemic stroke patients: a multicenter prospective cohort study. J Neurol Sci. (2019) 399:76–81. doi: 10.1016/j.jns.2019.01.047
10. Škrbić R, Vujković Z, Stojiljković MP, Gajanin R, Bokonjić D, Komić J. Efficacy and safety of low-dose versus standard-dose alteplase regimens in patients with acute ischaemic stroke. Psychiatr Danub. (2019) 31:32–8.
11. Salem GM, El-Sheik WM, El-Shanawany BG, Afifi KH. Low versus standard dose intravenous alteplase in the treatment of acute ischemic stroke in Egyptian patients. Neurosciences. (2021) 26:179–85. doi: 10.17712/nsj.2021.2.20200148
12. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
13. Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian acute stroke study investigators. Lancet. (1998) 352:1245–51. doi: 10.1016/S0140-6736(98)08020-9
14. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. (1995) 333:1581–7. doi: 10.1056/NEJM199512143332401
15. Nakagawara J, Minematsu K, Okada Y, Tanahashi N, Nagahiro S, Mori E, et al. Thrombolysis with 06 mg/kg intravenous alteplase for acute ischemic stroke in routine clinical practice: the Japan post-marketing alteplase registration study (J-MARS). Stroke. (2010) 41:1984–9. doi: 10.1161/STROKEAHA.110.589606
16. Zaman Babar MU, Khan AZ, Hakim H, Gilani J, Wasay M. Utilization and outcomes with low dose tissue plasminogen activator as intravenous thrombolytic therapy for ischaemic stroke at Aga Khan University Hospital, Karachi: a retrospective analysis. J Pak Med Assoc. (2019) 69:1705–10. doi: 10.5455/JPMA.282873
17. Ohta T, Okada K, Fukuda M, Masahira N, Matsuoka T, Tsuno T, et al. Safety and efficacy of intravenous low-dose alteplase in relative contraindication patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. (2018) 27:1844–51. doi: 10.1016/j.jstrokecerebrovasdis.2018.02.026
18. Chao AC, Hsu HY, Chung CP, Liu CH, Chen CH, Teng MM, et al. Outcomes of thrombolytic therapy for acute ischemic stroke in Chinese patients: the Taiwan thrombolytic therapy for acute ischemic stroke (TTT-AIS) study. Stroke. (2010) 41:885–90. doi: 10.1161/STROKEAHA.109.575605
19. Chao AC, Liu CK, Chen CH, Lin HJ, Liu CH, Jeng JS, et al. Different doses of recombinant tissue-type plasminogen activator for acute stroke in Chinese patients. Stroke. (2014) 45:2359–65. doi: 10.1161/STROKEAHA.114.005245
20. Anderson CS, Robinson T, Lindley RI, Arima H, Lavados PM, Lee TH, et al. Low-dose versus standard-dose intravenous alteplase in acute ischemic stroke. N Engl J Med. (2016) 374:2313–23. doi: 10.1056/NEJMoa1515510
21. Wang X, Robinson TG, Lee TH, Li Q, Arima H, Bath PM, et al. Low-dose vs standard-dose alteplase for patients with acute ischemic stroke: secondary analysis of the ENCHANTED randomized clinical trial. JAMA Neurol. (2017) 74:1328–35. doi: 10.1001/jamaneurol.2017.2286
22. Chen G, Wang X, Robinson TG, Pikkemaat M, Lindley RI, Zhou S, et al. Comparative effects of low-dose versus standard-dose alteplase in ischemic patients with prior stroke and/or diabetes mellitus: The ENCHANTED trial. J Neurol Sci. (2018) 387:1–5. doi: 10.1016/j.jns.2018.01.014
23. Zhou Z, Delcourt C, Xia C, Yoshimura S, Carcel C, Torii-Yoshimura T, et al. Low-dose vs standard-dose alteplase in acute lacunar ischemic stroke: the ENCHANTED trial. Neurology. (2021) 96:e1512–26. doi: 10.1212/WNL.0000000000011598
24. Wang X, Li J, Moullaali TJ, Lee KJ, Kim BJ, Bae HJ, et al. Low-dose versus standard-dose alteplase in acute ischemic stroke in Asian stroke registries: an individual patient data pooling study. Int J Stroke. (2019) 14:670–7. doi: 10.1177/1747493019858777
25. den Hertog J, de Laat SW, Schlessinger J, Kruijer W. Neuronal differentiation in response to epidermal growth factor of transfected murine P19 embryonal carcinoma cells expressing human epidermal growth factor receptors. Cell Growth Differ. (1991) 2:155–64.
26. Xu H. Efficacy of different doses of alteplase thrombolysis on acute ischemic stroke in patients. Pak J Pharm Sci. (2019) 32:2465–9.
27. Chen H, Zhu G, Liu N, Zhang W. Low-dose tissue plasminogen activator is as effective as standard tissue plasminogen activator administration for the treatment of acute ischemic stroke. Curr Neurovasc Res. (2014) 11:62–7. doi: 10.2174/1567202610666131126150043
28. Wang X, Lee KJ, Moullaali TJ, Kim BJ, Li Q, Bae HJ„ et al. Who will benefit more from low-dose alteplase in acute ischemic stroke. Int J Stroke. (2020) 15:39–45. doi: 10.1177/1747493019858775
29. Dong Y, Han Y, Shen H, Wang Y, Ma F, Li H, et al. Who may benefit from lower dosages of intravenous tissue plasminogen activator? results from a cluster data analysis. Stroke Vasc Neurol. (2020) 5:348–52. doi: 10.1136/svn-2020-000388
30. Nguyen TH, Truong A, Ngo MB, Bui CTQ, Dinh QV, Doan TC, et al. Patients with thrombolysed stroke in Vietnam have an excellent outcome: results from the vietnam thrombolysis registry. Eur J Neurol. (2010) 17:1188–92. doi: 10.1111/j.1468-1331.2010.02995.x
31. Sharma VK, Tsivgoulis G, Tan JH, Wong LYH, Ong BKC, Chan BPL, et al. Feasibility and safety of intravenous thrombolysis in multiethnic Asian stroke patients in Singapore. J Stroke Cerebrovasc Dis. (2010) 19:424–30. doi: 10.1016/j.jstrokecerebrovasdis.2009.07.015
32. Zhou XY, Wang SS, Collins ML, Davis SM, Yan B. Efficacy and safety of different doses of intravenous tissue plasminogen activator in Chinese patients with ischemic stroke. J Clin Neurosci. (2010) 17:988–92. doi: 10.1016/j.jocn.2009.12.005
33. Chen CH, Hsieh CY, Lai TB, Chuang MT, Chen WL, Sun MC. Optimal dose for stroke thrombolysis in Asians: low dose may have similar safety and efficacy as standard dose. J Thromb Haemost. (2012) 10:1270–5. doi: 10.1111/j.1538-7836.2012.04761.x
34. Pan SM, Liu JF, Liu M, Shen S, Li HJ, Dai LH, et al. Efficacy and safety of a modified intravenous recombinant tissue plasminogen activator regimen in Chinese patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. (2013) 22:690–3. doi: 10.1016/j.jstrokecerebrovasdis.2012.08.018
35. Aulicky P, Rabinstein A, Seet RC, Neumann J, Mikulik R. Dosing of tissue plasminogen activator often differs from 09 mg/kg, but does not affect the outcome. J Stroke Cerebrovasc Dis. (2013) 22:1293–7. doi: 10.1016/j.jstrokecerebrovasdis.2012.10.010
36. Liao X, Wang Y, Pan Y, Wang C, Zhao X, Wang DZ, et al. Standard-dose intravenous tissue-type plasminogen activator for stroke is better than low doses. Stroke. (2014) 45:2354–8. doi: 10.1161/STROKEAHA.114.005989
37. Kim BJ, Han MK, Park TH, Park SS, Lee KB, Lee BC, et al. Low-versus standard-dose alteplase for ischemic strokes within 45 hours: a comparative effectiveness and safety study. Stroke. (2015) 46:2541–8. doi: 10.1161/STROKEAHA.115.010180
38. Yang J, Yu F, Liu H, An H, Xiong R, Huang D. A retrospective study of thrombolysis with 06 mg/kg recombinant tissue plasminogen activator (rt-PA) in mild stroke. Sci Rep. (2016) 6:31344. doi: 10.1038/srep31344
39. Zhao G, Huang T, Zheng M, Cui Y, Liu Y, Cheng Z, et al. Comparative analysis on low- and standard-dose regimes of alteplase thrombolytic therapy for acute ischemic stroke: efficacy and safety. Eur Neurol. (2018) 79:68–73. doi: 10.1159/000485460
Keywords: tissue-type plasminogen activator, ischemic stroke, network meta-analysis, intravenous thrombolysis, symptomatic intracranial hemorrhage
Citation: Li B-H, Wang J-H, Wang H, Wang D-Z, Yang S, Guo F-Q and Yu N-W (2022) Different Doses of Intravenous Tissue-Type Plasminogen Activator for Acute Ischemic Stroke: A Network Meta-Analysis. Front. Neurol. 13:884267. doi: 10.3389/fneur.2022.884267
Received: 26 February 2022; Accepted: 19 May 2022;
Published: 23 June 2022.
Edited by:
Aristeidis H. Katsanos, McMaster University, CanadaReviewed by:
Craig S. Anderson, University of New South Wales, AustraliaLina Palaiodimou, University General Hospital Attikon, Greece
Copyright © 2022 Li, Wang, Wang, Wang, Yang, Guo and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Neng-Wei Yu, MTg5ODE4Mzg2NTJAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Bing-Hu Li
Bing-Hu Li Jian-Hong Wang
Jian-Hong Wang Han Wang2
Han Wang2 Duo-Zi Wang
Duo-Zi Wang Fu-Qiang Guo
Fu-Qiang Guo Neng-Wei Yu
Neng-Wei Yu