
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol., 29 April 2022
Sec. Multiple Sclerosis and Neuroimmunology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.874388
This article is part of the Research TopicMeasuring Progression in Multiple Sclerosis: Progressing Beyond the OrdinaryView all 7 articles
Multiple sclerosis is a serious neurological disease that affects millions of people worldwide. Cerebellar and brainstem symptoms are common in the course of multiple sclerosis, but their prognostic value is unclear. This systematic review aimed to determine the relationship between the location of lesions in the cerebellum and/or brainstem and the prognosis in multiple sclerosis. In this systematic review, we searched and comprehensively read articles related to this research topic in Chinese and English electronic databases (PubMed, Embase, Cochrane Library, CNKI, and CBM) using search terms “multiple sclerosis,” “cerebellum,” “brainstem,” “prognosis,” and others. Cerebellar and brainstem clinically isolated syndromes and clinically definite multiple sclerosis were important predictors of transformation (hazard ratio, 2.58; 95% confidence interval, 1.58–4.22). Cerebellar and/or brainstem lesions indicate a poor overall prognosis in multiple sclerosis, but because of inconsistency, more clinical data are needed.
Multiple sclerosis (MS) is a chronic progressive disease of the central nervous system. In patients with MS, approximately 10% to 15% have primary progressive MS, which has a slow disease progression with no remission or recurrence, and 85% to 90% have relapsing-remitting MS (RRMS), which has a marked course of relapse and remission. Of those with RRMS, 75% will progress to secondary progressive MS (SPMS), which has no remission process but has slow progressive exacerbation (1). With the progression of the disease, most patients with MS will develop physical and psychological dysfunction, such as disabilities, cognitive impairment, bladder dysfunction, intestinal dysfunction, anxiety, depression, fatigue, pain, poor sleep quality, and others, which seriously affect the quality of life of patients.
Studies have shown that 81.6% of patients with MS have cerebellar or brainstem clinical manifestations, and 22.5% of the first demyelination events have these two initial clinical manifestations (2). In addition, 10.1% of MS recurrences occur in the cerebellum and 16.6% occur in the brainstem (3). The pons is the most common site of distribution of infratentorial lesions, accounting for 46%, followed by the midbrain, medulla, and cerebellar hemispheres (4).
The cerebellum is responsible for coordination tasks and fine movement. It is believed that the cerebellum plays a key role not only in sensory-motor networks but also in cognitive-behavioral systems. The three predominant cerebellar symptoms (tremor, nystagmus, and scanning speech) were described by the French neurologist Jean-Martin Charcot in 1,877. With the deepening of understanding of MS, cerebellar symptoms are not limited to the three aforementioned symptoms, and gait and truncal ataxia, incoordination of voluntary movements, hypotonia, slurred speech, different types of nystagmus, and ocular dysmetria were also included (5). Studies have shown that cerebellar damage in patients with MS not only leads to motor and cognitive impairment, which hinders daily activities, but is also a marker of poor prognosis (6, 7). Brainstem involvement in MS has different clinical manifestations such as diplopia, facial sensory symptoms, unstable gait, vertigo, facial weakness/hemispasm, oscillopsia, and so forth (8). In addition to these sensory-motor disorders, sleep disorders, restless leg syndrome, and periodic leg movements were also found to be associated with damage to different parts of the brainstem (9). The relationship between brainstem symptoms and the progression of clinically isolated syndromes (CIS) to MS (10–12), which may be 50% to 60%, is also of concern (13). We systematically reviewed studies on cerebellar and brainstem injury and the prognosis of MS, with the aim of identifying patients who may benefit from early, targeted, and positive treatment.
Related articles were systematically reviewed according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (14) guidelines (Figure 1).We searched electronic databases of Chinese and English literature, including PubMed, Embase, the Cochrane Library, CNKI, and CBM. Using a combination of Medical Subject Headings and free text words, a search strategy with both specificity and sensitivity was developed for each database. English keywords included “multiple sclerosis,” “clinical isolation syndrome,” “CIS,” “PPMS” (for primary progressive MS), “SPMS,” “RRMS,” “cerebellum,” “brainstem,” “incidence,” “mortality,” “follow-up study,” “prognosis,” “prediction,” and “course of the disease.” Boolean operators were reasonably used to combine search terms to find relevant research.
The titles and abstracts that may be included in the study were initially screened by two researchers; then, the full text of the preliminary screening literature was read for further in-depth screening. The inclusion criteria were as follows: studies that included patients with MS; the objective of the study is clear and provides prognostic outcome indicators of cerebellar and/or brainstem lesions in MS; articles are in Chinese or English. The exclusion criteria were the following: repeated studies; conference abstracts, case reports, reviews, review abstracts, or quality evaluation of literature research that failed to extract valid information. At the end of the retrieval process, 14 articles (Table 1) were identified and systematically reviewed, including the objectives, methods, results, and limitations of the study. The quality of the included studies was evaluated by two investigators using the Newcastle-Ottawa Scale as an evaluation tool for observational studies on prognostic factors. Stata version 15.0 (Stata Corp., College Station, TX, USA) was used for the statistical analysis of the combined data. The hazard ratio (HR) was first reversed into beta coefficients with standard error, and then the combined HR and 95% confidence interval (CI) were calculated. A comparison of these findings provides a prognostic perspective on the damage to the cerebellum and brainstem in MS and thus aids in understanding the issue.
CIS refers to an single acute or subacute episode of isolated demyelinating events in the central nervous system excluding other diseases (25). The duration of the attack should last at least 24 h, without fever, infection, or clinical features of encephalopathy (26, 27). CIS should not meet the diagnostic criteria of MS in terms of time and space evolution. Studies have found that as many as 80% to 90% of patients with MS had CIS as their first manifestation, and 30% to 70% of patients with CIS progressed into having MS (28). According to the common symptoms and sites of potential involvement, CIS was divided into optic neuritis, myelitis, cerebellar, and brainstem CIS. Cerebellar and brainstem clinical features are often diverse or complex, and they are difficult to locate quickly and accurately. In a retrospective analysis of CIS with brainstem-cerebellar symptoms (assessed within 3 months of onset), the most common symptoms were diplopia (68%), facial sensory symptoms (32%), and gait impairment (31%) (8). Distinguishing brainstem and cerebellar symptoms purely based on clinical presentation is often difficult; thus, they are often discussed together. Studies have found that early drug intervention in CIS can delay the progression to MS (25). Therefore, understanding and identifying the risk factors of clinically definite MS (CDMS) and persistent disease activity (29) can assist in the study of the pathogenesis of demyelination and other diseases and provide a basis for the diagnosis, prognosis, and early intervention of such diseases.
Brainstem-cerebellar CIS is closely related to CDMS and can be used as one of the important predictors of transformation. Two studies (16, 17)have standardized reports and extracted this indicator, with combined HR of 2.58 (95% CI, 1.58–4.22) (as shown in Figure 2). A retrospective multicenter study (16) of 137 patients with a median follow-up duration of 3.1 years found that a clinical onset with brainstem-cerebellar CIS has a higher risk of early conversion to MS (HR, 2.0; 95% CI, 1.3–3.0). In addition, another study indicated that the presence of brainstem-cerebellar lesions not only increases the risk of transformation but also indicates a higher chance of developing a disability (17). A small study of 42 patients with CIS who were followed up for 8.7 years reported that two or more infratentorial diseases were associated with long-term disability (15), which also verified this view.
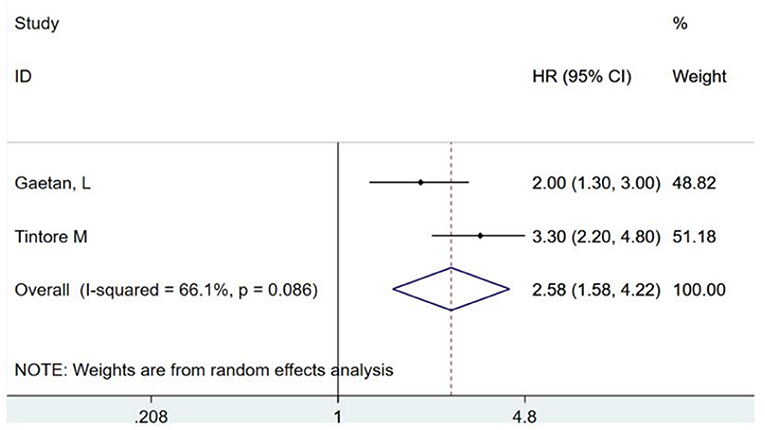
Figure 2. Brainstem and cerebellar lesions predict the risk ratio of conversion of clinically isolated syndromes to clinically definite multiple sclerosis. HR, hazards ratio; CI, confidence interval.
However, Phadke (19) proposed in 1,990 that patients with primary isolated brainstem lesions had significantly better prognosis (HR, 0.60; 95% CI, 0.38–0.95). Although the study is old, there are still negative outcome reports in recent years, and it is believed that cerebellar or brainstem clinical manifestations at onset have no statistical significance for predicting prognosis (18, 30). These differences can be explained as follows: first, the sample size of each study is small and not sufficiently representative to form persuasive evidence; second, the conversion rate differences may be due to geographic differences in the natural history of MS, length of follow-up, and the use of specific diagnostic criteria. Further prospective and large cohort longitudinal studies are needed to clarify the relationship between the cerebellum/brainstem and CIS conversion to MS.
Most patients with MS have a moderate disability (Extended Disability Status Scale [EDSS] ≤ 3) in the relapsing-remitting phase (31, 32), and some patients show little or no disability. After an indefinite period of onset (33, 34), the secondary progressive stage leads to severe disability, inability to live independently, or paralysis and being bedbound. This is due to the accumulation of persistent disability resulting from severe and irreversible nerve damage (with pathological manifestations of axon loss, cortical demyelination, and meningeal inflammatory aggregation) (35). At present, determining the beginning of the secondary progressive course remains difficult; thus, the timing for initiation of effective treatment is unknown. What is clear is that the transition from RRMS to SPMS is a key determinant of prognosis and a primary therapeutic target for the prevention of long-term disability.
The following studies may provide hints for the transformation of RRMS to SPMS. The study of Amato et al. showed that cerebellar manifestations at the onset of MS were associated with a high disability rate and rapid progression to secondary disease (36). Meanwhile, a prospective study of 1,903 patients showed that brainstem dysfunction was one of the important prognostic factors for RRMS conversion to SPMS (20), and brainstem dysfunction at onset was one of the predictors of treatment failure in the second year (21). However, other studies have shown that brainstem symptoms during the onset of MS have no statistical significance in the rate of conversion to SPMS and disability prognosis (22). Scalfari et al. believed that although the type of clinical onset was unrelated to the incubation period from the onset of MS to SPMS, patients with cerebellar and brainstem symptoms at the onset of the disease would have a faster rate of cumulative disability once they reached the secondary progressive stage, and the time from onset to EDSS 8 was significantly shorter (23).
Pediatric-onset MS (POMS), defined as MS in persons younger than 18 years, accounts for 2% to 10% of all MS cases and is the most common neuroimmune disorder in children and adolescents (37). The clinical characteristics of patients with POMS are mainly relapse and remission, and its symptoms mostly manifest as brainstem-cerebellar dysfunction (28.6%), pyramidal symptoms (18.4%), and optic neuritis (14.3%). Although POMS have similar a pathogenesis to MS in adults, they have different phenotypic characteristics and disease courses.
Compared with the course of the disease in adults, the early stage of POMS during childhood is characterized by a severe inflammatory process, but the initial recovery of children is better. In addition, POMS has slower disease progression and lower EDSS scores than MS in adults, but the number of relapses is not significantly different (38). In POMS, the cerebellum and brainstem (38) are the common sites of clinical and radiological involvements (39, 40). Children have a higher volume of infratentorial lesions than adult patients, which means that a higher volume of infratentorial lesions significantly promotes the development of disability due to MS-related cerebellar tissue damage; thus, the association between infratentorial lesions in childhood and disease progression is of particular interest (41).
A Kuwaiti study of prognostic indicators in patients with SPMS who were younger than 18 years highlighted the importance of infratentorial symptoms for prognosis. This finding suggests that brainstem-cerebellar involvement (adjusted HR, 5.71; P = 0.010) and onset time of MS (adjusted HR, 1.38; P = 0.042) were significantly correlated with the risk of SPMS (24). A retrospective analysis by De Meo et al. showed that baseline evidence of brainstem involvement in patients with POMS was a manifestation of poor prognosis (42). Therefore, patients with brainstem-cerebellar manifestations of POMS are often prone to SPMS, and active treatment is recommended (24).
This systematic review analyzed the relationship between lesion location in the cerebellum and brainstem and disease progression from POMS, CIS, and RRMS to SPMS as well as prognosis. With the continuous development of diagnostic criteria and in-depth understanding of the disease, this time-honored research topic has been refined. Children with MS have a higher incidence of brainstem-cerebellar involvement, which is often a symptom of poor prognosis. In CIS, patients with infratentorial lesions are more likely to be develop CDMS; thus, close attention and timely intervention are needed. In addition to the conversion rate from RRMS to SPMS, infratentorial lesions are also important in disease progression and disability prognosis. One possible explanation is that patients with infratentorial lesions with gait disorders as the main manifestation have higher EDSS scores than other symptoms. Hence, the overall prognosis of MS with cerebellar and brainstem lesions is poor, but more clinical data are needed to support it.
In addition to the typical infratentorial clinical manifestations and signs, imaging examination provides a number of information and plays an important role in the diagnosis and treatment of MS. For example, common cerebellar T2-hyperintense lesions, which were previously believed to have no significant correlation with the extent of lesions and disability (43), have recently been found to be associated with recurrent falls in patients with MS (44). Infratentorial T1-hypointense lesions are associated with disability severity, which is a sign of severe tissue damage and permanent axonal loss (45). Brainstem injury has a good correlation with T2 lesion load (46), but only approximately 60% of the patients with cranial nerve involvement had brainstem lesions identified by magnetic resonance imaging (4), which is called the clinicoradiological paradox. This is a reminder that imaging cannot be viewed in isolation, but must be combined with clinical signs.
With the development of imaging, the advent of diffusion tensor imaging has allowed the use of non-invasive visualization and analysis of white matter (WM) fiber bundles in detecting tissue changes at the micrometer level. In patients with MS, normal WM microstructural changes may precede macro-pathological changes and WM tissue atrophy. The most significant diffusion tensor imaging measurement index for quantitative evaluation of normal WM microstructure changes based on normal distribution is fractional anisotropy (FA) (47). A study in 2016 showed that, during the early MS stage, a normal cerebellum structure in T1- or T2-weighted magnetic resonance imaging showed microstructural changes under high-resolution magnetic resonance imaging and diffusion tensor imaging. Furthermore, cerebellar FA reduction was related to lower EDSS score, disease course, and ratio of cerebellar WM and gray matter volumes, suggesting that the change in the cerebellar microstructure is closely related to disease severity (48). A later study from another perspective showed that the microstructural changes in the cerebellum may be related to the tendency of CIS to convert to MS. If the initial cerebellar FA is lower than FAcrit = 0.352 (where FAcrit is the mean cerebellar FA of patients with early MS), CIS is likely to progress to RRMS within 1 year. This suggests that reduced cerebellar FA in patients with CIS may indicate that the disease is in an active and unstable stage, leading to a shorter transformation time to MS. This series of studies provides us with a more precise perspective in understanding the significance of cerebellar lesions in the early stage of MS (47).
The role of the cerebellum in disability in patients with MS can be explained by the following. First, the cerebellum has important physiological functions. The deep involvement of the cerebellum in motor activities, including working memory, visuospatial function, language, procedural learning, and attention (49), as well as in cognitive (50) and affective processes can seriously affect the quality of life of patients. Second, the cerebellum does not exist in isolation and has extensive connections with other parts of the central nervous system. The cerebellum participates in functional loops with the frontal, superior temporal, limbic, and posterior parietal cortex (48), which is an important factor in the variety of manifestations and adverse outcomes of cerebellum lesions. However, very few studies have focused specifically on brainstem lesions in MS, and there is currently no systematic scientific knowledge.
This systematic review has some limitations. One of the main limitations is the heterogeneity among studies, with differences in patient characteristics, diagnostic criteria, methodology, and outcome reporting. Patient-level data were not available; thus, multivariable predictors of adverse outcomes could not be identified. The meta-analyses and subgroup analyses may be underpowered, and further validation is needed. Most of the studies were cross-sectional and thus provide little insight into the dynamics of the infratentorial involvement in MS. Furthermore, because some of the published studies are older, there are differences in the understanding of MS and infratentorial lesions, which may also influence the outcome.
XZ conceptualized the project. YY and MW performed the literature search and screening. LX and MZ evaluated the quality of the articles. YW and ML extracted and analyzed data from the included literature. YY wrote the manuscript with contribution from XZ. All authors read and approved the final manuscript.
This work was supported by Clinical +X of The Affiliated Hospital of Qingdao University (QDFY+X202101032).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CIS, clinically isolated syndromes; CDMS, clinically definite multiple sclerosis; EDSS, extended Disability Status Scale; FA, fractional anisotropy; MS, multiple sclerosis; RRMS, relapsing-remitting multiple sclerosis; SPMS, secondary progressive multiple sclerosis; WM, white matter.
1. Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sørensen PS, Thompson AJ, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. (2014) 83:278–86. doi: 10.1212/WNL.0000000000000560
2. Poser S, Wikström J, Bauer HJ. Clinical data and the identification of special forms of multiple sclerosis in 1271 cases studied with a standardized documentation system. J Neurol Sci. (1979) 40:159–68. doi: 10.1016/0022-510X(79)90201-6
3. Kalincik T, Buzzard K, Jokubaitis V, Trojano M, Duquette P, Izquierdo G, et al. Risk of relapse phenotype recurrence in multiple sclerosis. Mult Scler. (2014) 20:1511–22. doi: 10.1177/1352458514528762
4. Nakashima I, Fujihara K, Okita N, Takase S, Itoyama Y. Clinical and MRI study of brain stem and cerebellar involvement in Japanese patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. (1999) 67:153–7. doi: 10.1136/jnnp.67.2.153
5. Weier K, Banwell B, Cerasa A, Collins DL, Dogonowski AM, Lassmann H, et al. The role of the cerebellum in multiple sclerosis. Cerebellum. (2015) 14:364–74. doi: 10.1007/s12311-014-0634-8
6. Wing AC, Vasconcelos CC, Calvet J, Papais-Alvarenga RM, Thuler LC. Risk factors for convertion to clinically defined multiple sclerosis after clinically isolated syndrome in a racially mixed Brazilian cohort. Clin Neurol Neurosurg. (2016) 146:40–4. doi: 10.1016/j.clineuro.2016.04.022
7. de Groot V, Beckerman H, Uitdehaag BM, Hintzen RQ, Minneboo A, Heymans MW, et al. Physical and cognitive functioning after 3 years can be predicted using information from the diagnostic process in recently diagnosed multiple sclerosis. Arch Phys Med Rehabil. (2009) 90:1478–88. doi: 10.1016/j.apmr.2009.03.018
8. Sastre-Garriga J, Tintoré M, Nos C, Tur C, Río J, Téllez N, et al. Clinical features of CIS of the brainstem/cerebellum of the kind seen in MS. J Neurol. (2010) 257:742–6. doi: 10.1007/s00415-009-5403-0
9. Skorić MK, Adamec I, Madarić VN, Habek M. Evaluation of brainstem involvement in multiple sclerosis. Can J Neurol Sci. (2014) 41:346–9. doi: 10.1017/S0317167100017285
10. Banerjee TK, Saha M, Ghosh E, Hazra A, Das A, Choudhury D, et al. Conversion of clinically isolated syndrome to multiple sclerosis: a prospective multi-center study in Eastern India. Mult Scler J Exp Transl Clin. (2019) 5:2055217319849721. doi: 10.1177/2055217319849721
11. Çinar BP, Özakbaş S. Prediction of conversion from clinically isolated syndrome to multiple sclerosis according to baseline characteristics: a prospective study. Noro psikiyatri arsivi. (2018) 55:15–21. doi: 10.29399/npa.12667
12. Li R, Shiu KL, Tsoi TH. Clinically isolated syndrome in Hong Kong & long-term outcome. Multiple Sclerosis. (2010) 16:264. doi: 10.1177/1352458509356638
13. Fisniku LK, Brex PA, Altmann DR, Miszkiel KA, Benton CE, Lanyon R, et al. Disability and T2 MRI lesions: a 20-year follow-up of patients with relapse onset of multiple sclerosis. Brain. (2008) 131:808–17. doi: 10.1093/brain/awm329
14. Knobloch K, Yoon U, Vogt PM. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J Craniomaxillofac Surg. (2011) 39:91–2. doi: 10.1016/j.jcms.2010.11.001
15. Minneboo A, Barkhof F, Polman CH, Uitdehaag BM, Knol DL, Castelijns JA. Infratentorial lesions predict long-term disability in patients with initial findings suggestive of multiple sclerosis. Arch Neurol. (2004) 61:217–21. doi: 10.1001/archneur.61.2.217
16. Gaetani L, Fanelli F, Riccucci I, Eusebi P, Sarchielli P, Pozzilli C, et al. High risk of early conversion to multiple sclerosis in clinically isolated syndromes with dissemination in space at baseline. J Neurol Sci. (2017) 379:236–40. doi: 10.1016/j.jns.2017.06.008
17. Tintore M, Rovira A, Arrambide G, Mitjana R, Río J, Auger C, et al. Brainstem lesions in clinically isolated syndromes. Neurology. (2010) 75:1933–8. doi: 10.1212/WNL.0b013e3181feb26f
18. Jacome Sanchez C, Correa EP. Predictors of long term disability progression in patients with relapsing remitting multiple sclerosis in a cohort of patients from the Andean region of ecuador. Multiple Sclerosis Journal. (2019) 25:634.
19. Phadke JG. Clinical aspects of multiple sclerosis in north-east Scotland with particular reference to its course and prognosis. Brain. (1990) 113:1597–628. doi: 10.1093/brain/113.6.1597
20. Barzegar M, Najdaghi S, Afshari-Safavi A, Nehzat N, Mirmosayyeb O, Shaygannejad V. Early predictors of conversion to secondary progressive multiple sclerosis. Mult Scler Relat Disord. (2021) 54:103115. doi: 10.1016/j.msard.2021.103115
21. Nogales-Gaete J, Aracena R, Cepeda-Zumaeta S, Eloiza C, Agurto P, Díaz V, et al. [Clinical features of 314 patients with relapsing-remitting multiple sclerosis]. Rev Med Chil. (2014) 142:559–66. doi: 10.4067/S0034-98872014000500002
22. Riise T, Grønning M, Fernández O, Lauer K, Midgard R, Minderhoud JM, et al. Early prognostic factors for disability in multiple sclerosis, a European multicenter study. Acta Neurol Scand. (1992) 85:212–8. doi: 10.1111/j.1600-0404.1992.tb04031.x
23. Scalfari A, Neuhaus A, Daumer M, Muraro PA, Ebers GC. Onset of secondary progressive phase and long-term evolution of multiple sclerosis. J Neurol Neurosurg Psychiatry. (2014) 85:67–75. doi: 10.1136/jnnp-2012-304333
24. Akhtar S, Alroughani R, Ahmed SF, Al-Hashel JY. Prognostic indicators of secondary progression in a paediatric-onset multiple sclerosis cohort in Kuwait. Multiple Sclerosis. (2016) 22:1086–93. doi: 10.1177/1352458515608960
25. Miller DH, Chard DT, Ciccarelli O. Clinically isolated syndromes. The Lancet Neurology. (2012) 11:157–69. doi: 10.1016/S1474-4422(11)70274-5
26. Miller DH, Weinshenker BG, Filippi M, Banwell BL, Cohen JA, Freedman MS, et al. Differential diagnosis of suspected multiple sclerosis: a consensus approach. Mult Scler. (2008) 14:1157–74. doi: 10.1177/1352458508096878
27. Krupp LB, Banwell B, Tenembaum S. Consensus definitions proposed for pediatric multiple sclerosis and related disorders. Neurology. (2007) 68(16 Suppl. 2):S7–12. doi: 10.1212/01.wnl.0000259422.44235.a8
28. Miller D, Barkhof F, Montalban X, Thompson A, Filippi M. Clinically isolated syndromes suggestive of multiple sclerosis, part 2: non-conventional MRI, recovery processes, and management. Lancet Neurol. (2005) 4:341–8. doi: 10.1016/S1474-4422(05)70095-8
29. Mowry EM. Natural history of multiple sclerosis: early prognostic factors. Neurol Clin. (2011) 29:279–92. doi: 10.1016/j.ncl.2011.01.001
30. Alroughani R, Al Hashel J, Lamdhade S, Ahmed SF. Predictors of conversion to multiple sclerosis in patients with clinical isolated syndrome using the 2010 revised McDonald criteria. ISRN Neurol. (2012) 2012:792192. doi: 10.5402/2012/792192
31. Scalfari A, Neuhaus A, Degenhardt A, Rice GP, Muraro PA, Daumer M, et al. The natural history of multiple sclerosis: a geographically based study 10: relapses and long-term disability. Brain. (2010) 133:1914–29. doi: 10.1093/brain/awq118
32. Ingram G, Hirst CL, Robertson NP. What is the risk of permanent disability from a multiple sclerosis relapse? Neurology. (2010) 75:837. doi: 10.1212/WNL.0b013e3181eeea87
33. Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. national multiple sclerosis society (USA) advisory committee on clinical trials of new agents in multiple sclerosis. Neurology. (1996) 46:907–11. doi: 10.1212/WNL.46.4.907
34. Vukusic S, Confavreux C. Prognostic factors for progression of disability in the secondary progressive phase of multiple sclerosis. J Neurol Sci. (2003) 206:135–7. doi: 10.1016/S0022-510X(02)00426-4
35. Scalfari A, Neuhaus A, Daumer M, Deluca GC, Muraro PA, Ebers GC. Early relapses, onset of progression, and late outcome in multiple sclerosis. JAMA Neurol. (2013) 70:214–22. doi: 10.1001/jamaneurol.2013.599
36. Amato MP, Ponziani G, Bartolozzi ML, Siracusa G. A prospective study on the natural history of multiple sclerosis: clues to the conduct and interpretation of clinical trials. J Neurol Sci. (1999) 168:96–106. doi: 10.1016/S0022-510X(99)00143-4
37. Renoux C, Vukusic S, Mikaeloff Y, Edan G, Clanet M, Dubois B, et al. Natural history of multiple sclerosis with childhood onset. N Engl J Med. (2007) 356:2603–13. doi: 10.1056/NEJMoa067597
38. Alroughani R, Ahmed SF, Al-Hashel J. Pediatric-Onset multiple sclerosis disease progression in Kuwait: a retrospective analysis. Pediatr Neurol. (2015) 53:508–12. doi: 10.1016/j.pediatrneurol.2015.06.010
39. Ashtari F, Shaygannejad V, Farajzadegan Z, Amin A. Does early-onset multiple sclerosis differ from adult-onset form in Iranian people. J Res Med Sci. (2010) 15:94–9.
40. Derle E, Kurne AT, Konuşkan B, Karabudak R, Anlar B. Unfavorable outcome of pediatric onset multiple sclerosis: follow-up in the pediatric and adult neurology departments of one referral center, in Turkey. Mult Scler Relat Disord. (2016) 9:1–4. doi: 10.1016/j.msard.2016.06.002
41. Ghassemi R, Narayanan S, Banwell B, Sled JG, Shroff M, Arnold DL. Quantitative determination of regional lesion volume and distribution in children and adults with relapsing-remitting multiple sclerosis. PLoS ONE. (2014) 9:e85741. doi: 10.1371/journal.pone.0085741
42. De Meo E, Bonacchi R, Moiola L, Colombo B, Sangalli F, Zanetta C, Amato MP, Martinelli V, Rocca MA, Filippi M. Early predictors of 9-year disability in pediatric multiple sclerosis. Ann Neurol. (2021) 89:1011–1022. doi: 10.1002/ana.26052
43. Ormerod IE, Miller DH, McDonald WI, du Boulay EP, Rudge P, Kendall BE, et al. The role of NMR imaging in the assessment of multiple sclerosis and isolated neurological lesions. A quantitative study. Brain. (1987) 110:1579–616. doi: 10.1093/brain/110.6.1579
44. Prosperini L, Kouleridou A, Petsas N, Leonardi L, Tona F, Pantano P, et al. The relationship between infratentorial lesions, balance deficit and accidental falls in multiple sclerosis. J Neurol Sci. (2011) 304:55–60. doi: 10.1016/j.jns.2011.02.014
45. Hickman SJ, Brierley CM, Silver NC, Moseley IF, Scolding NJ, Compston DA, et al. Infratentorial hypointense lesion volume on T1-weighted magnetic resonance imaging correlates with disability in patients with chronic cerebellar ataxia due to multiple sclerosis. J Neurol Sci. (2001) 187:35–9. doi: 10.1016/S0022-510X(01)00519-6
46. Thömke F, Lensch E, Ringel K, Hopf HC. Isolated cranial nerve palsies in multiple sclerosis. J Neurol Neurosurg Psychiatry. (1997) 63:682–5. doi: 10.1136/jnnp.63.5.682
47. Kugler AV, Deppe M. Non-lesional cerebellar damage in patients with clinically isolated syndrome: DTI measures predict early conversion into clinically definite multiple sclerosis. NeuroImage Clinical. (2018) 19:633–9. doi: 10.1016/j.nicl.2018.04.028
48. Deppe M, Tabelow K, Krämer J, Tenberge JG, Schiffler P, Bittner S, et al. Evidence for early, non-lesional cerebellar damage in patients with multiple sclerosis: DTI measures correlate with disability, atrophy, and disease duration. Mult Scler. (2016) 22:73–84. doi: 10.1177/1352458515579439
49. Jansen A, Flöel A, Van Randenborgh J, Konrad C, Rotte M, Förster AF, et al. Crossed cerebro-cerebellar language dominance. Hum Brain Mapp. (2005) 24:165–72. doi: 10.1002/hbm.20077
Keywords: multiple sclerosis, cerebellum, brainstem, predictors, disability outcome
Citation: Yang Y, Wang M, Xu L, Zhong M, Wang Y, Luan M, Li X and Zheng X (2022) Cerebellar and/or Brainstem Lesions Indicate Poor Prognosis in Multiple Sclerosis: A Systematic Review. Front. Neurol. 13:874388. doi: 10.3389/fneur.2022.874388
Received: 12 February 2022; Accepted: 31 March 2022;
Published: 29 April 2022.
Edited by:
Susan A. Gauthier, Cornell University, United StatesReviewed by:
Neha Safi, Mount Sinai Hospital, United StatesCopyright © 2022 Yang, Wang, Xu, Zhong, Wang, Luan, Li and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xueping Zheng, c2ltcGxleHVlcGluZ0AxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.