- 1Department of Clinical Research, Biomedical Research Center, Lam Dong Medical College, Dalat, Vietnam
- 2Department of Intensive Care Unit, Covid-19 Unit of Phu Chanh, Binh Duong General Hospital, Binh Duong, Vietnam
- 3Division of Pulmonary, Allergy and Critical Care Medicine, Penn State College of Medicine, Hershey, PA, United States
- 4Department of Expert Consultation, Faculty of Medicine, Pham Ngoc Thach University of Medicine, Ho Chi Minh, Vietnam
- 5Department of Internal Medicine, Can Tho University of Medicine and Pharmacy, Can Tho, Vietnam
- 6Department of Family Medicine, Faculty of Medicine, University of Medicine and Pharmacy at Ho Chi Minh City, Ho Chi Minh, Vietnam
- 7Department of Respiratory Functional Exploration. University Medical Center, Ho Chi Minh, Vietnam
- 8Department of Pathology, Institute Gustave Roussy, Villejuif, France
Since the first case of Guillain-Barré syndrome (GBS)-associated SARS-CoV-2 (COVID-19) infection reported in 2020, a series of cases have been published in some countries. In this case report, we present a young patient with GBS, whose clinical and laboratory data were appropriate for the diagnosis of GBS due to COVID-19 infection. Neurological examination revealed the muscular weakness of lower limbs with Medical Research Council (MRC) scale of 2/5 associated with diminished reflexes. Laboratory studies showed the positive nasal swab RT-PCR test for COVID-19, leukopenia, increased ferritin and LDH levels, normal electrolyte and liver and kidney function, and normal chest X-ray. The result of cerebrospinal fluid showed the albuminocytologic dissociation. The patient was treated with remdesivir, dexamethasone, anticoagulation, and therapeutic plasma exchange (TPE). Patient's muscle weakness was significantly improved after 1 week of admission. He was discharged at 23rd days of hospitalization and followed-up in the out-patients department.
Introduction
Since the first COVID-19 patient reported in March 2020, until now, Vietnam has experienced four waves of COVID-19 with more than 2 million confirmed cases and nearly 36 thousand deaths due to COVID-19. During this outbreak of COVID-19 pandemic (September–October 2021), Ho Chi Minh City and Binh Duong Province, located in the South of Vietnam, were two main regions where the number of people who contracted COVID-19 and were hospitalized due to COVID-19 was highest. During this time, the national mortality rate from COVID-19 was 2.4% compared with 1.7% currently.
Although the main symptoms of patients with COVID-19 have been well described previously, COVID-19 patients can present with diverse neurological symptoms such as headache, dizziness, loss of smell and taste, inflammatory polyradiculoneuropathy or Guillain-Barré Syndrome (GBS) (1, 2). Since the first case of GBS-associated SARS-CoV-2 (Covid-19) infection reported by Zhao H. et al. (3), a series of cases have been published (4–9).
Here, we present a patient with GBS, whose clinical and laboratory data were appropriate for the diagnosis of GBS due to COVID-19 infection. The patient was treated successfully by therapeutic plasma exchange (TPE) in combination with standard treatment for Covid-19. This is the first case of GBS associated with the COVID-19 case in Vietnam treated by TPE. It appears that the intervention with TPE made a significant difference compared to most other previously reported patients with Covid-19 induced GBS worldwide during the pandemic outbreak.
Case Report
An 18-year-old man admitted to an intensive care unit (ICU of COVID-19 Unit of Phu Chanh, Binh Duong General Hospital, Binh Duong Province–Vietnam) during the middle of October 2021 because of fever, cough, sore throat, weakness, and pain of his legs for 4 days. He was otherwise in good health prior to this hospitalization. However, his roommate was diagnosed with COVID-19 and hospitalized two days before his admission. At the time of admission, his temperature was 38·6 °C, pulse was 80/min, respirations were 20/min, blood pressure was 120/80 mm Hg, and SpO2 was 95% with room air. He had no symptoms of chest pain or shortness of breath. Neurological examination revealed the muscular weakness of lower limbs with Medical Research Council (MRC) scale of 2/5 (vs 5/5 for upper limbs), diminished reflexes of lower limbs, normal sensation, no abnormal cranial nerve symptoms, and no meningeal signs. The rest of his clinical examination was normal.
Laboratory studies demonstrated a positive nasal swab RT-PCR test for COVID-19, leucopenia, increased ferritin and LDH levels, normal electrolyte and liver and kidney function, and normal chest X-ray. The result of cerebrospinal fluid showed the albuminocytologic dissociation (without an elevation in white blood cells; Table 1). The result of magnetic resonance imaging (MRI) of the spine was normal. The diagnosis of Guillain Barré Syndrome induced by COVID-19 was confirmed in this patient according to the hospital's medical experts. Some other differential diagnosis such as compressive myelopathy, transverse myelitis, and other infectious causes of acute myelitis were also eliminated due to lack of medical history and clinical features.
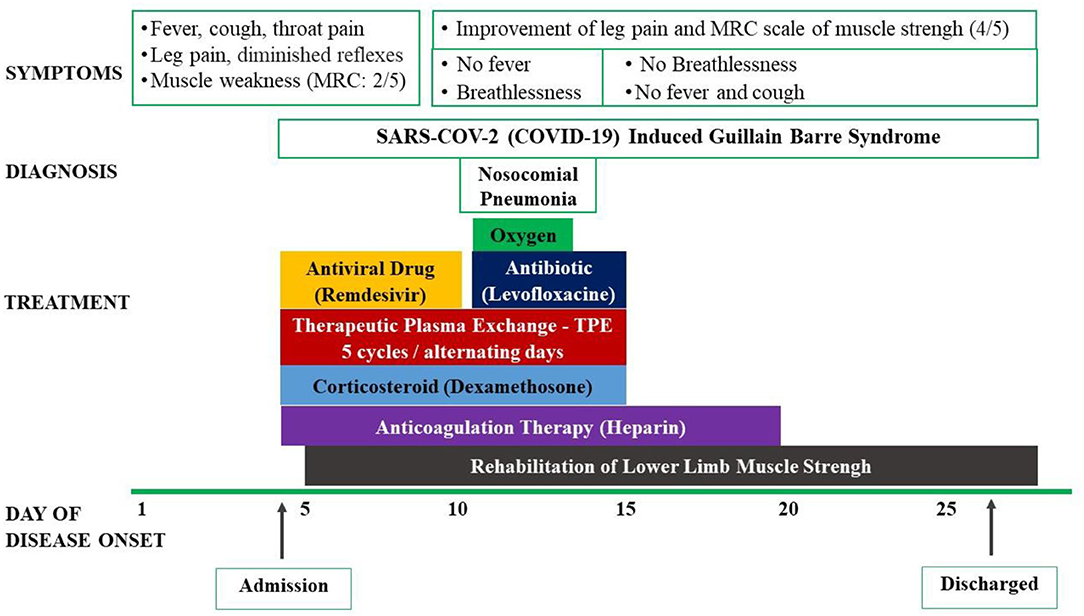
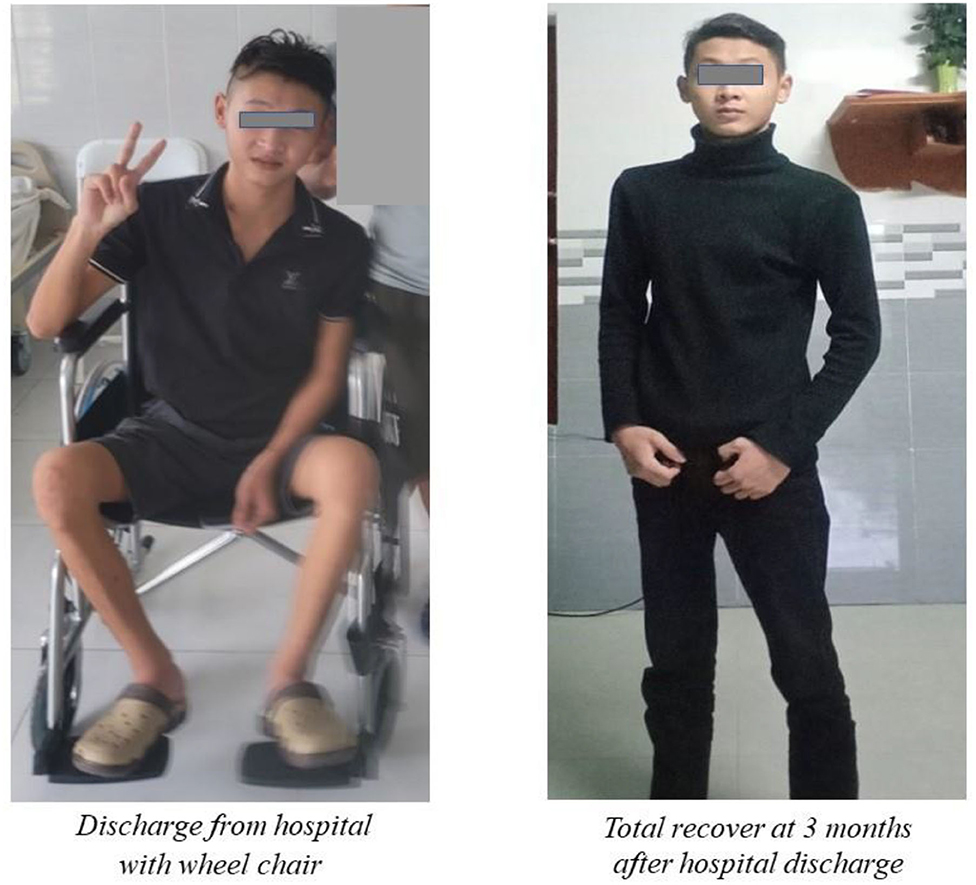
The patient was then treated with antiviral drug (remdesivir 200 mg loading dose on the first day and 100 mg in the next 4 days), intravenous corticosteroids (dexamethasone 9 mg/day in 3 days and 6 mg/day in the next 7 days), and anticoagulation. He was also treated with the therapeutic plasma exchange (TPE) for 5 alternating day cycles by using 5% human albumin replacement (3.200 ml/cycle). The patient's muscle weakness of the lower limbs improved at day 7 and 14 from 2/5 to 3/5 and then 4/5, respectively, according to the Medical Research Council (MRC) Scale. He also benefited from early rehabilitation to improve muscle strength during his hospitalization in the ICU. At day 7th, he developed a nosocomial lung infection, confirmed by chest X-ray and was treated successfully with short period of antibiotic (intravenous levofloxacin 750 mg/day for 5 days) and conventional oxygen therapy (nasal cannula 3 L/min). His RT-PCR for SARS-COV-2 (COVID-19) were negative at day 14th and 21th. Other laboratory tests were normalized at day 14th. The patient was discharged after 23 days from his hospitalized stay (Figure 1). He has been followed-up regularly at the outpatient Department for Long Covid-19 for his GBS and rehabilitation. At the 3rd month, the patient had a total recover from his GBS with MRC scale of 5/5 and he started to go to work (Figure 2).
Discussion
Guillain-Barré Syndrome (GBS) is an acute inflammatory polyradiculoneuropathy that results in muscle weakness and diminished reflexes. GBS may occur after immunization or viral infection, including the SARS-COV-2 (COVID-19) (9–14). After one of the first cases of GBS -associated SARS-CoV-2 infection presented by Zhao et al. in 2020 (3), other cases have been reported in the world literature during the COVID-19 pandemic (5–8, 10–14).
In 2020, Caress et al. analyzed 37 cases of GBS (mean age: 59 years and 65% of male gender), associated with COVID-19 and showed that the mean time from COVID-19 infection to GBS symptoms was 11 days. All 37 patients were free of severe acute respiratory distress syndrome. Albuminocytologic dissociation in cerebrospinal fluid was positive in 76% of cases. Most patients were treated with a single course of intravenous immunoglobulin and improved within 8 weeks. The authors also demonstrated the similar clinical manifestation and severity between non-COVID-19 and COVID-19 induced GBS in the study patients (15).
In 2021, Rumeileh et al. reviewed 73 patients reported in 52 publications, which demonstrated similar results to include the mean age of reported patients being 55 years old and the males accounting for 68.5% of cases (16). In this series of cases, cerebrospinal fluid albuminocytologic dissociation was found in 71 and 100% was negative for SARS-CoV-2. The treatment for GBS in this case series was intravenous immunoglobulin and more than 70% of cases had a good prognosis (16). The first case of GBS with severe acute respiratory distress syndrome induced by coronavirus-2 (SARS-CoV-2) was reported in a 50-year-old male in 2021. In this case report, the patient's cerebrospinal fluid was positive for SARS-CoV-2 (Covid-19), confirmed by RT-PCR (17).
In this case report, we presented a patient with acute progressive and symmetric muscle weakness of lower limbs associated with Covid-19 infection. The results of the clinical examination and laboratory data were appropriate for the diagnosis of Covid-19-induced GBS. It appears to be the first case of GBS associated with COVID-19 reported in Vietnam. Our patient was younger than most other patients in previous reports described above. Besides the treatment with antiviral therapy, anticoagulation and corticosteroids, our patient also received TPE (therapeutic plasma exchange). Although TPE is the treatment of choice for patients with GBS before COVID-19 pandemic, the use of TPE for COVID-19-induced GBS is still rare. The majority of the patients with COVID-19-associated GBS patients noted above were mainly treated with intravenous immunoglobulin. Fortunately, our patient had a total recover from his GBS with MRS scale of 5/5 at 3rd month after hospital discharge.
Both intravenous immunoglobulin and TPE are indicated to treat GBS within 2–4weeks after the onset of neuropathic symptoms to prevent further nerve damage, and both treatments have similar efficacy (16, 18–21). In GBS patients, TPE helps to remove antibodies and other potentially detrimental factors from the blood. TPE might be more effective when starting the treatment within seven days after symptoms onset, but data suggest it is still beneficial in patients treated up to 30 days after disease onset (19).
In addition, TPE is effective in decreasing inflammatory cytokines (including IL-6), acute phase proteins (ferritin, CRP), and improving tissue oxygenation and as such could be considered as an additional therapy to reduce the risk of cytokine storm-induced acute respiratory distress syndrome (ARDS) in COVID-19 patients (22). Moreover, in patients with COVID-19, TPE may help to repair coagulopathy and to restore endothelial membrane by decreasing blood viscosity (20, 23). The association between blood hyperviscosity and the tendency of thrombosis is also considered as a risk of COVID-19 infection.
However, it has been proven in various epidemiological studies, particularly from the United Kingdom, that the incidence of Guillain-Barre syndrome did not increase during the pandemic (24–26). In fact, GBS related COVID-19 is also a rare complication. Definitely, it has been currently more than 10 million people contracted COVID-19 in Vietnam but this case report is the first and only one which has been diagnosed by local experts and declared officially in the country.
One of the limitations of our case report is the lack of electromyography and nerve conduction studies to support the diagnosis of GBS during the acute phase. This was secondary to the lack of access to these tests in our COVID-19 ICU. However, similar to us, we suspect that during the COVID-19 pandemic many health care systems had limited procedures and transportation of patients for testing to decrease health care worker's exposure to COVID-19. In addition, another limitation was also related to the lack of negative results of other infectious test for supporting the diagnosis of GBS during COVID-19 pandemic.
Conclusion
Guillain-Barre Syndrome (GBS) is an acute inflammatory and demyelinating polyradiculoneuropathy disease. GBS may occur in young patients with COVID-19 as a main symptom of Covid-19 infection. The early management of Covid-19 induced GBS with therapeutic plasma exchange in combination with standard treatment improved the patient's prognosis.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
Written informed consent was obtained from the individual(s) and/or minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author Contributions
The literature search was done by SD-Q, DH-T-A, QT-X, and CN with significant contributions from TN-T-K, VN-N, and TC. Data collection was done by SD-Q, DH-T-A, TN-Q, TN-T-K, and TN-C. All authors contributed equally to analyzing and interpreting the data of case report. SD-Q, QT-X, and DH-T-A drafted the manuscript, with editing by TC and significant contributions by CN, VN-N, TN-C, and CN. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Losy J. Sars-Cov-2 infection: symptoms of the nervous system and implications for therapy in neurological disorders. Neurol Ther. (2020) 23:1–12. doi: 10.1007/s40120-020-00225-0
2. Romero-Sánchez CM, Díaz-Maroto I, Fernández-Díaz E, Sánchez-Larsen Á, Layos-Romero A, García-García J, et al. Neurologic manifestations in hospitalized patients with Covid-19: the albacovid registry. Neurology. (2020) 95:e1060–70. doi: 10.1212/WNL.0000000000009937
3. Zhao H, Shen D, Zhou H, Liu J. Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. (2020) 19:383–4. doi: 10.1016/S1474-4422(20)30109-5
4. Abrams RMC, Kim BD, Markantone DM, Reilly K, Paniz-Mondolfi AE, Gitman MR, et al. Severe rapidly progressive Guillain-Barré syndrome in the setting of acute COVID-19 disease. J Neurovirol. (2020) 26:797–9. doi: 10.1007/s13365-020-00884-7
5. Arnaud S, Budowski C, Tin SNW, Degos B. Post SARS-CoV-2 Guillain-Barré syndrome: case report. Clin Neurophysiol. (2020) 131:1652–54. doi: 10.7759/cureus.21246
6. Assini A, Benedetti L, Di Maio S, Schirinzi E, Del Sette M. New clinical manifestation of COVID-19 related Guillain-Barrè syndrome highly responsive to intravenous immunoglobulins: two Italian cases. Neurol Sci. (2020) 41:1657–8. doi: 10.1007/s10072-020-04484-5
7. Coen M, Jeanson G, Almeida LAC, et al. Guillain-Barré syndrome as a complication of SARS-CoV-2 infection. Brain Behav Immun. (2020) 87:111–2. doi: 10.1016/j.bbi.2020.04.074
8. Colonna S, Sciumé L, Giarda F, Innocenti A, Beretta G, Costa DD. Case report: postacute rehabilitation of Guillain-Barré syndrome and cerebral vasculitis-like pattern accompanied by SARS-CoV-2 infection. Front Neurol. (2021):11:602554. doi: 10.3389/fneur.2020.602554
9. Wilison HJ, Jacobs BC, van Doorn AP. Guillain-barré syndrome. Lancet. (2016) 388:717–27. doi: 10.1016/S0140-6736(16)00339-1
10. Ebrahimzadeh SA, Ghoreishi A, Rahimian N. Guillain-barré syndrome associated with the coronavirus disease 2019 (COVID-19). Neurol Clin Pract. (2021) 11:e196–198. doi: 10.1002/ccr3.5638
11. Farzia MA, Ayromloua H, Jahanbakhshb N, Bavil PH, Janzadeh A, Shayan FK. Guillain-Barré syndrome in a patient infected with SARS-CoV-2, a case report. J Neuroimmunol. (2020) 346:577294. doi: 10.1016/j.jneuroim.2020.577294
12. Hirayama T, Hongo Y, Kaida K, Kano O. Guillain-Barré syndrome after COVID-19 in Japan. BMJ Case Rep. (2020) 13:e239218. doi: 10.1136/bcr-2020-239218
13. Kilinc D, van de Pasch S, Doets AY, Jacobs BC, van Vliet J, Garssen MPJ. Guillain Barré syndrome after SARS-CoV-2 infection. Eur J Neurol. (2020) 27:1757–8. doi: 10.1111/ene.14398
14. Korem S, Gandhi H, Dayag DB. Guillain-Barré syndrome associated with COVID-19 disease. BMJ Case Rep. (2020) 13:e237215. doi: 10.1136/bcr-2020-237215
15. Caress JB, Castoro RJ, Simmons Z, Scelsa SN, Lewis RA, Ahlawat A, et al. COVID-19 – associated Guillain-Barré syndrome: the early pandemic experience. Muscle Nerve. (2020) 62:485–91. doi: 10.1002/mus.27024
16. Rumeileh SA, Abdelhak A, Foschi M, Tumani H, Otto M. Guillain-Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J Neurol. (2021) 268:1133–70. doi: 10.1007/s00415-020-10124-x
17. Khan F, Sharma P, Pandey S, Sharma D, Vijayavarman V, Kumar N, et al. COVID-19-associated Guillain-Barré syndrome: postinfectious alone or neuroinvasive too? J Med Virol. (2021) 93:6045–9. doi: 10.1002/jmv.27159
18. Plasma Exchange/ Sandoglobulin Guillain-Barré Syndrome Trial Group. Randomised trial of plasma exchange, intravenous immunoglobulin, and combined treatments in Guillain-Barré syndrome. Lancet. (1997) 349:225–60.
19. Raphael JC, Chevret S, Hughes RAC, Annane D. Plasma exchange for Guillain-Barré syndrome. Cochrane Database Syst Rev. (2012) 7:CD001798. doi: 10.1002/14651858.CD001798.pub3
20. Truong AD, Auld SC, Barker NA, Friend S, Wynn AT, Cobb J, et al. Therapeutic plasma exchange for COVID-19-asociated hyperviscosity. Transfusion. (2020) 61:1029–34. doi: 10.1111/trf.16218
21. Verboon C, van Doorn PA, Jacobs BC. Treatment dilemmas in Guillain-Barré syndrome. J Neurol Neurosurg Psychiatry. (2017) 88:346–52. doi: 10.1136/jnnp-2016-314862
22. Zaid I, Esaad O, El aidouni G, Aabdi M, Berrichi S, Taouihar S et al. Therapeutic plasma exchange in COVID-19 pnemonia in intensive care unit: cases series. Ann Med Surg (Lond). (2021) 71:102920. doi: 10.1016/j.amsu.2021.102920
23. Patidar GK, Land KJ, Vrielink H, Rahimi-Levene N, Dann EJ, Al-Humaidan, et al. Understanding the role of therapeutic plasma exchange in COVID-19: preliminary guidance and practices. Vox Sanguinis. (2021) 116:798–807. doi: 10.1111/vox.13067
24. González Del Castillo J, Porta-Etessam J, Miró Ò. Spanish Investigators on Emergency Situations TeAm (SIESTA) network. Currently available data regarding the potential association between COVID-19 and Guillain-Barré syndrome. Brain. (2021) 144:e46. doi: 10.1093/brain/awab066
25. Luijten LWG, Leonhard SE, van der Eijk AA, Doets AY, Appeltshauser L, Arends S, et al. IGOS consortium. Guillain-Barré syndrome after SARS-CoV-2 infection in an international prospective cohort study. Brain. (2021) 144:3392–404. doi: 10.1093/brain/awab279
Keywords: Guillain-Barré syndrome, SARS-CoV-2, COVID-19, MRC scale, therapeutic plasma exchange
Citation: Duong-Quy S, Huynh-Truong-Anh D, Nguyen-Thi-Kim T, Nguyen-Quang T, Nguyen-Chi T, Tran-Xuan Q, Nguyen-Nhu V, Ngo C and Craig T (2022) Guillain-Barré Syndrome in Patient With SARS-CoV-2 PCR Positivity Treated Successfully With Therapeutic Exchange Plasma: A First Case Report From Vietnam. Front. Neurol. 13:868667. doi: 10.3389/fneur.2022.868667
Received: 03 February 2022; Accepted: 21 April 2022;
Published: 25 May 2022.
Edited by:
Hans-Peter Hartung, Heinrich Heine University of Düsseldorf, GermanyReviewed by:
Anita Fletcher, National Institute of Neurological Disorders and Stroke (NIH), United StatesIvan Da Silva, Rush University Medical Center, United States
Copyright © 2022 Duong-Quy, Huynh-Truong-Anh, Nguyen-Thi-Kim, Nguyen-Quang, Nguyen-Chi, Tran-Xuan, Nguyen-Nhu, Ngo and Craig. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sy Duong-Quy, c2R1b25ncXV5LmpmdnBAZ21haWwuY29t
 Sy Duong-Quy
Sy Duong-Quy Duc Huynh-Truong-Anh
Duc Huynh-Truong-Anh Thanh Nguyen-Thi-Kim2
Thanh Nguyen-Thi-Kim2 Vinh Nguyen-Nhu
Vinh Nguyen-Nhu