- 1Department of Psychiatry and Behavioral Sciences, Johns Hopkins University, Baltimore, MD, United States
- 2Department of Neurology, Johns Hopkins University, Baltimore, MD, United States
- 3Department of Neurology, University of Washington, Seattle, WA, United States
- 4Department of Physical Medicine and Rehabilitation, Johns Hopkins University, Baltimore, MD, United States
An emerging body of evidence suggests that changes in cognitive and emotional function are common aspects of stiff person spectrum disorders (SPSD). We sought to examine the pattern of cognitive impairment and psychiatric symptoms in SPSD.
Methods: A retrospective review of medical records was conducted for patients seen at the Johns Hopkins Stiff Person Syndrome (SPS) center from 1997 to January 1st, 2020. Individuals who had received formal cognitive testing as part of routine clinical care for patient-reported cognitive changes were included. Demographics, prevalence of cognitive impairment, psychoactive medication use, and clinically significant psychiatric symptoms were described.
Results: Out of 205 patients screened, 20 completed cognitive testing (75% female, mean age 47.4 years). The most common domains of impairment were verbal learning and recall memory (n = 14, 70%), verbal fluency (n = 10, 50%), processing speed (n = 8, 40%), and attention (n = 8, 40%). 9/11 patients assessed for depression reported clinically significant symptoms, and 4/9 patients assessed for anxiety reported clinically significant symptoms.
Conclusions: Screening for cognitive impairment in SPSD should utilize testing that assesses verbal learning and recall, phonemic verbal fluency, attention, and processing speed. Moreover, it is important to evaluate for co-existing depression and anxiety symptoms, as these are common in SPSD.
Introduction
Stiff person spectrum disorders (SPSD) are immune-mediated disorders most often characterized by rigidity, unpredictable and painful spasms, and heightened sensitivity to external stimuli (1). Anti-glutamic acid decarboxylase 65 (anti-GAD65) antibodies are thought to play a role in the GABAergic dysfunction in SPSD. While it is classified as a neurologic disorder, research is limited regarding the effects of stiff person syndrome (SPS) on cognitive and emotional function (1, 2).
SPSD has been associated with lower than expected performance on cognitive testing relative to estimated premorbid intelligence (3). Furthermore, the presence of anti-GAD65 antibody has been associated with cognitive impairment in patients with neurological conditions (4), type 2 diabetes (5), and in animal models (6). In addition to cognitive dysfunction, patients with SPSD are also more likely than the general population to report anxiety and depressive symptoms, and to regularly use prescription benzodiazepines and muscle relaxants (7), all of which may contribute to poor performance on cognitive testing (8–10). To our knowledge, only two prior studies have assessed cognitive symptoms in patients with SPSD (3, 11). While one also included measures of psychiatric symptoms (3), neither study reported on psychiatric symptoms or patterns of medication use in the context of cognitive performance.
The aims of this case series were to: (1) describe the pattern of cognitive impairment in patients with SPSD who reported concerns of cognitive impairment and participated in cognitive testing as part of routine clinical care; and (2) examine the frequency of mood symptoms and use of benzodiazepines and muscle relaxants in the most commonly impaired cognitive domains.
Methods
A retrospective review of medical records was conducted for patients seen at the Johns Hopkins SPS center from 1997 to January 1st, 2020. All patients had provided informed consent to participate in a longitudinal observational study of clinical characteristics in SPS, approved by the Johns Hopkins Institutional Review Board.
Medical records were reviewed for formal cognitive testing, performed by either a licensed psychologist or a speech and language pathologist, as part of routine clinical care for patient-reported cognitive changes. Information on demographics, clinical characteristics, medical comorbidities, and medications at the time of cognitive testing were extracted. Patients with limbic encephalitis, co-existing intractable epilepsy, and/or other neurological conditions known to affect cognitive performance (e.g., Alzheimer's disease, multiple sclerosis, etc.) were excluded.
As a retrospective review of cognitive testing performed as part of routine clinical care, cognitive testing batteries used were determined at the discretion of the provider and therefore not standardized. Details of cognitive testing reports were extracted; results were interpreted as “impaired” if records included descriptive labels of “abnormal”, “extremely low”, or “weak”. If no descriptive interpretation was offered, an adjusted percentile score of <2 or z-score of < −2 (e.g., more than 2 standard deviations below mean) was interpreted as “impaired” (12). If standardized instruments of psychological symptoms (e.g., depression and/or anxiety) were administered, the scores and descriptive labels (e.g., “clinically significant”) were extracted.
Demographic and clinical characteristics were evaluated using descriptive statistics, t-test for continuous variables and chi-squared test for dichotomous variables using R Studio Version 1.2.5033 (13). Significance was set at p < 0.05. Frequency of domain-specific cognitive impairment across individuals with cognitive testing was examined. For the 4 most commonly impaired cognitive domains, frequency of prescription antidepressants (e.g., selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors), benzodiazepines (e.g., lorazepam, diazepam, clonazepam) and non-benzodiazepine muscle relaxants (e.g., cyclobenzaprine, baclofen, dantrolene), and clinically significant depression and anxiety were assessed.
Results
Out of 205 patients, 66 reported cognitive concerns, of which 20 completed cognitive testing (Table 1). There was no statistically significant difference in gender, age, or duration of illness in individuals included in this case series vs. the remainder of the cohort, or between those included in the case series vs. those who reported cognitive concerns but did not have cognitive testing (all p > 0.05). Three participants completed testing with a speech and language pathologist using the Repeatable Battery for the Assessment of Neuropsychological Status [RBANS; (20)], and 17 completed testing with a psychologist using a wide array of instruments (Supplementary Table 1). Our cohort was mostly female (n = 15, 75%), had a mean age at time of cognitive testing of 47.4 years (SD = 12.4), and mean duration of illness of 10.1 years (SD = 7.6). Most had anti-GAD65 antibodies (17/20, 75%), and classic SPS phenotype (15/20, 75%). Three (15%) had a history of seizures, none of which were intractable or poorly controlled. Common classes of medications prescribed included benzodiazepines (n = 14, 70%), antidepressants (n = 13, 65%), non-benzodiazepine muscle relaxants (n = 10, 50%), and opioids (n = 4, 20%). Nine out of eleven (82%) patients assessed for depression reported clinically significant symptoms, and 4 out of 9 (44%) patients assessed for anxiety reported clinically significant symptoms.
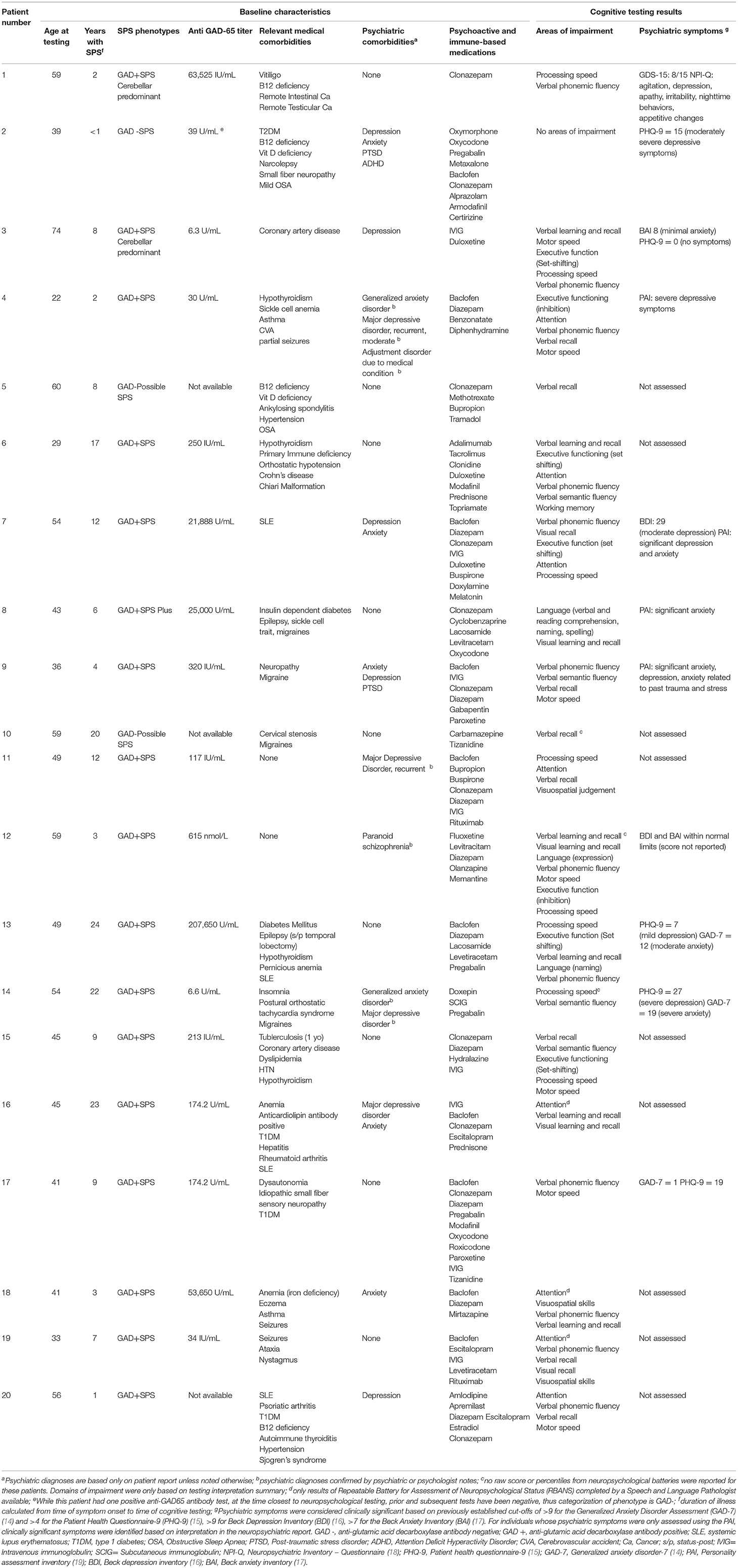
Table 1. Clinical and laboratory features of patients with stiff person syndrome spectrum disorders who received formal cognitive testing as part of routine clinical care for patient-reported cognitive changes.
Of the 20 patients who completed cognitive testing, 19 performed in the “impaired” range in at least one cognitive domain. The most common domains of impairment were verbal learning and recall memory (n = 14, 70%), verbal fluency (n = 11, 55%), processing speed (n = 8, 40%), attention (n = 8, 40%), motor speed (n = 7, 35%), semantic verbal fluency (n = 6, 30%), visual learning and recall memory (n = 5, 25%), set-shifting (n = 5, 25%), inhibition control (n = 3, 15%), and visuospatial processing (n = 3, 15%).
Patterns of medication use and clinically significant depressive and anxiety symptoms are described in Figure 1.
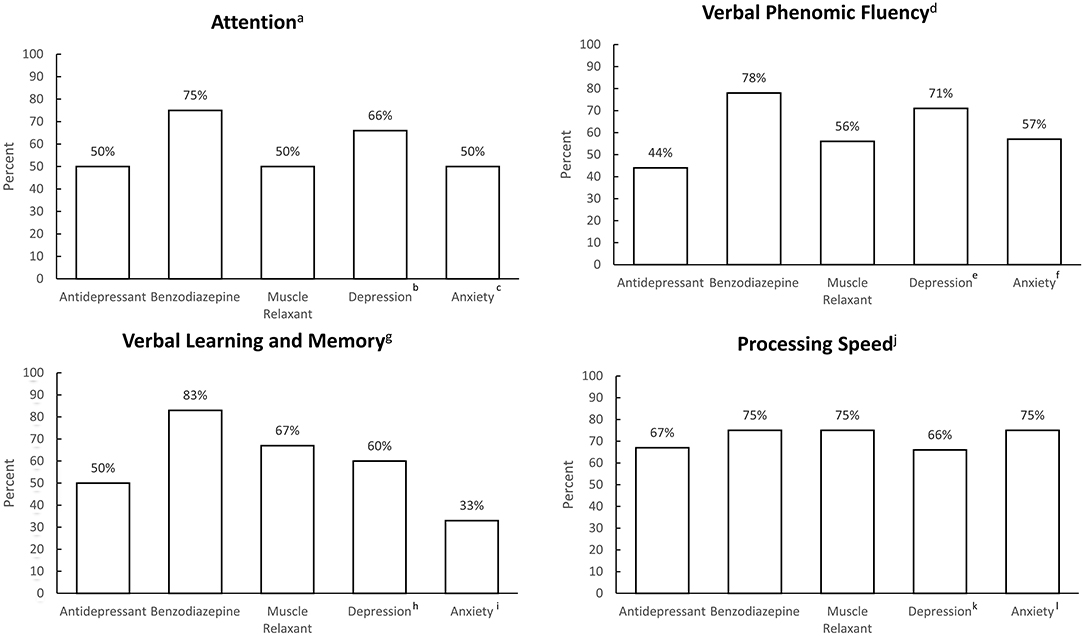
Figure 1. Frequency of antidepressant use, benzodiazepine use, non-benzodiazepine muscle relaxant use, and clinically significant depression and anxiety symptoms, grouped by most commonly impaired cognitive domains. an = 8, bn = 3, cn = 2; dn = 11, en = 7, fn = 7; gn = 14, hn = 11, in = 6; jn = 8, kn = 6; ln = 4.
Discussion
To our knowledge, this is the first detailed examination of cognitive and mood profiles in patients with SPSD who present with cognitive concerns. The most common cognitive domains exhibiting impairment were verbal recall, processing speed, attention, and phonemic verbal fluency. Additionally, results suggest an overlap of cognitive impairment with use of SPSD medications and presence of mood and anxiety symptoms. Reduced GABA levels have been associated with anxiety and depression (21), as well as cognitive impairment in schizophrenia (22), multiple sclerosis (23), and Alzheimer's disease (24). Metabolic abnormalities in the frontal cortex, temporal cortex, thalamus, and cingulate cortex (25) have been reported in classic SPS, regions that have previously been associated with psychiatric symptoms in cognitive disorders (26). Thus, there is a biological plausibility that cognitive impairment and mood and anxiety disorders are intrinsic to the disease process.
Our results expand on previously published work by Budhram et al. (11). Though cognitive findings specific to SPS phenotype were not reported separately, they found that 18% (n = 38) of their cohort with various anti-GAD65 associated neurological disorders had cognitive impairment as diagnosed by the Kokmen short test of mental status (11, 27). Consistent with our findings, the predominant cognitive domains impacted were verbal learning and recall memory (29/38, 76%), followed by working memory/attention (6/38, 16%), and verbal fluency/language processing (3/38, 8%). Similarly, another study of cognitive profiles in 21 patients with anti-GAD65-positive diabetes (without a co-existing neurological condition, severe psychiatric disorders or use of psychotropic medications) reported that performance on recall memory and phonemic verbal fluency tasks were significantly lower in anti-GAD65-positive individuals than in the control group (5). Psychiatric symptoms, however, were not evaluated in either study in relation to cognition.
Among the 20 patients included in our case series, 65% were prescribed antidepressants, and approximately half of those assessed for depression and anxiety reported clinically significant symptoms. This is consistent with prior studies (3, 28, 29), and a recent systematic review which found that the relative risk of psychiatric comorbidity in SPS was higher than that of the general population (7). Mood and anxiety disorders are associated with deficits in learning and memory, executive function, and attention—areas also impaired in SPSD and anti-GAD65 associated diseases (8, 9). Although the present findings are observational and cannot confirm causation, bidirectional pathways of mood and cognition have been established in longitudinal studies of other patient populations (30, 31).
Both benzodiazepines and muscle relaxants have been associated with increased risk of cognitive impairment (32–34). In particular, long-term benzodiazepine use has been associated with deficits in visuospatial processing, processing speed, and verbal learning (10). While we observed a high prevalence of these medications in individuals with cognitive impairment, future studies on the potential effects of these medications on cognition in SPSD are needed to establish causality. At a minimum, there should be increased consideration for their long-term use given the potentially harmful effects.
These findings should be interpreted within the context of their limitations. This was a convenience, retrospective sample of individuals who had completed cognitive testing following referral based on reported cognitive concerns. Testing was conducted at different sites and by different providers, without standardization of test selection or interpretation. Moreover, as previously noted, certain medications that are used in SPSD can influence cognitive function. Despite the aforementioned limitations, our present findings contribute to the limited literature on cognitive and mood profiles in patients with SPSD by identifying common domains of cognitive impairment and potential overlap of cognitive impairment with mood symptoms and medication use.
In summary, assessment of cognitive impairment in SPSD should include testing of verbal learning and recall, phonemic verbal fluency, attention, and processing speed. Cognitive screening tools that examine these domains, such as the Montreal Cognitive Test (MoCA), could be used in the clinical setting to help identify patients who may need additional cognitive evaluation. Psychiatric symptoms and use of medications that may affect cognition are common, and should be considered when evaluating cognitive impairment in this population. Further studies are needed to replicate these findings using longitudinal prospective study designs with consistent cognitive assessment tools and interpretive standards to further clarify the scope of neuropsychiatric disturbance in SPSD and their underlying mechanisms.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Johns Hopkins Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
CC: conceptualization of the study, data analysis, data interpretation, drafting, and revision of manuscript. DP, YW, and DO: data acquisition, data interpretation, and revision of manuscript. AH: data interpretation and revision of manuscript. SN: conceptualization of the study, data acquisition, data interpretation, study supervision, and revision of manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.865462/full#supplementary-material
References
1. Hadavi S, Noyce AJ, Leslie RD, Giovannoni G. Stiff person syndrome. Pract Neurol. (2011) 11:272–82. doi: 10.1136/practneurol-2011-000071
2. Dalakas MC. Stiff person syndrome: advances in pathogenesis and therapeutic interventions. Curr Treat Options Neurol. (2009) 11:102–10. doi: 10.1007/s11940-009-0013-9
3. Ameli R, Snow J, Rakocevic G, Dalakas MC. A neuropsychological assessment of phobias in patients with stiff person syndrome. Neurology. (2005) 64:1961–3. doi: 10.1212/01.WNL.0000163984.71993.FE
4. Lohmann T, Hawa M, Leslie RD, Lane R, Picard J, Londei M. Immune reactivity to glutamic acid decarboxylase 65 in Stiffman syndrome and type 1 diabetes mellitus. Lancet. (2000) 356:31–5. doi: 10.1016/S0140-6736(00)02431-4
5. Takagi M, Ishigaki Y, Uno K, Sawada S, Imai J, Kaneko K, et al. Cognitive dysfunction associated with anti-glutamic acid decarboxylase autoimmunity: a case-control study. BMC Neurol. (2013) 13:76. doi: 10.1186/1471-2377-13-76
6. Hampe CS, Petrosini L, De Bartolo P, Caporali P, Cutuli D, Laricchiuta D, et al. Monoclonal antibodies to 65kDa glutamate decarboxylase induce epitope specific effects on motor and cognitive functions in rats. Orphanet J Rare Dis. (2013) 8:82. doi: 10.1186/1750-1172-8-82
7. Caffrey D, Finn CT, Song SM, Burton F, Arsan C. Stiff-person syndrome and psychiatric comorbidities: a systematic review. J Acad Consult Liaison Psychiatry. (2021) 62:3–13. doi: 10.1016/j.psym.2020.08.005
8. Airaksinen E, Larsson M, Forsell Y. Neuropsychological functions in anxiety disorders in population-based samples: evidence of episodic memory dysfunction. J Psychiatr Res. (2005) 39:207–14. doi: 10.1016/j.jpsychires.2004.06.001
9. Rock P, Roiser J, Riedel W, Blackwell A. Rock PL, Roiser JP, Riedel WJ, Blackwell AD. Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med. (2013) 44: 2029–40. doi: 10.1017/S0033291713002535
10. Stewart SA. The effects of benzodiazepines on cognition. J Clin Psychiatry. (2005) 66(Suppl 2):9−13.
11. Budhram A, Sechi E, Flanagan EP, Dubey D, Zekeridou A, Shah SS, et al. Clinical spectrum of high-titre GAD65 antibodies. J Neurol Neurosurg Psychiatry. (2021) 92:645–54. doi: 10.1136/jnnp-2020-325275
12. Ingraham LJ, Aiken CB. An empirical approach to determining criteria for abnormality in test batteries with multiple measures. Neuropsychology. (1996) 10:120–4. doi: 10.1037/0894-4105.10.1.120
13. RStudio Team,. RStudio: Integrated Development Environment for R. Boston, MA: RStudio, PBC (2020). Available online at: http://www.rstudio.com/
14. Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Internal Med. (2006) 166:1092–7. doi: 10.1001/archinte.166.10.1092
15. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Internal Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x
16. Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation (1996).
17. Steer RA, Beck AT. Beck anxiety inventory. In: Zalaquett CP, Wood RJ, editors. Evaluating Stress: A Book of Resources. Scarecrow Education (1997). p. 23–40.
18. Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, et al. Validation of the NPI-Q, a brief clinical form of the neuropsychiatric inventory. J Neuropsychiatry Clin Neurosci. (2000) 12:233–9. doi: 10.1176/jnp.12.2.233
19. Morey LC, Lowmaster SE. Personality assessment inventory. In: Irving B, Weiner W, editors. The Corsini Encyclopedia of Psychology. Hoboken, NJ: Edward Craighead John Wiley & Sons Inc; American Cancer Society (2010). p. 1–4. doi: 10.1002/9780470479216.corpsy0663
20. Randolph C, Tierney MC, Mohr E, Chase TN. The repeatable battery for the assessment of neuropsychological status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. (1998) 20:310–9. doi: 10.1076/jcen.20.3.310.823
21. Kalueff AV, Nutt DJ. Role of GABA in anxiety and depression. Depress Anxiety. (2007) 24:495–517. doi: 10.1002/da.20262
22. Xu M, Wong AHC. GABAergic inhibitory neurons as therapeutic targets for cognitive impairment in schizophrenia. Acta Pharmacol Sin. (2018) 39:733–53. doi: 10.1038/aps.2017.172
23. Cao G, Edden RAE, Gao F, Li H, Gong T, Chen W, et al. Reduced GABA levels correlate with cognitive impairment in patients with relapsing-remitting multiple sclerosis. Eur Radiol. (2018) 28:1140–8. doi: 10.1007/s00330-017-5064-9
24. Solas M, Puerta E, Ramirez JM. Treatment options in Alzheimer's disease: the GABA story. Curr Pharmaceut Design. (2015) 21:4960–71. doi: 10.2174/1381612821666150914121149
25. Wang Y, Sadaghiani MS, Tian F, Fitzgerald KC, Solnes L, Newsome SD. Brain and muscle metabolic changes by FDG-PET in stiff person syndrome spectrum disorders. Front Neurol. (2021) 12:1479. doi: 10.3389/fneur.2021.692240
26. Chen Y, Dang M, Zhang Z. Brain mechanisms underlying neuropsychiatric symptoms in Alzheimer's disease: a systematic review of symptom-general and -specific lesion patterns. Mol Neurodegen. (2021) 16:38. doi: 10.1186/s13024-021-00456-1
27. Kokmen E, Naessens JM, Offord KP. A short test of mental status: description and preliminary results. Mayo Clin Proc. (1987) 62:281–8. doi: 10.1016/S0025-6196(12)61905-3
28. Gerschlager W, Schrag A, Brown P. Quality of life in stiff person syndrome. Movement Disord. (2002) 17:1064–7. doi: 10.1002/mds.10235
29. Newsome SD. Other Proven and Putative Autoimmune Disorders of the CNS: Anti-GAD Associated Neurological Disorders-Stiff-Person Syndrome, Cerebellar Ataxia, Progressive Encephalopathy with Rigidity and Myoclonus, and Encephalitis. New York, NY: Oxford University Press (2016).
30. Chruzander C, Johansson S, Gottberg K, Einarsson U, Fredrikson S, Holmqvist LW, et al. A 10-year follow-up of a population-based study of people with multiple sclerosis in Stockholm, Sweden: changes in disability and the value of different factors in predicting disability and mortality. J Neurol Sci. (2013) 332:121–7. doi: 10.1016/j.jns.2013.07.003
31. Petkus AJ, Gomez ME, Filoteo JV, Schiehser DM, Petzinger G. Worse cognitive performance predicts increased anxiety and depressive symptoms in patients with Parkinson's disease: a bidirectional analysis. Neuropsychology. (2018) 33:35–46. doi: 10.1037/neu0000498
32. Mancuso CE, Tanzi MG, Gabay M. Paradoxical reactions to benzodiazepines: literature review and treatment options. Pharmacotherapy. (2004) 24:1177–85. doi: 10.1592/phco.24.13.1177.38089
33. Picton JD, Marino AB, Nealy KL. Benzodiazepine use and cognitive decline in the elderly. Am J Health Syst Pharm. (2018) 75:e6–12. doi: 10.2146/ajhp160381
Keywords: stiff person syndrome, cognition, attention, verbal fluency, depression, anxiety
Citation: Chan CK, Pimentel Maldonado DA, Wang Y, Obando D, Hughes AJ and Newsome SD (2022) Cognitive and Mood Profiles Among Patients With Stiff Person Syndrome Spectrum Disorders. Front. Neurol. 13:865462. doi: 10.3389/fneur.2022.865462
Received: 29 January 2022; Accepted: 09 May 2022;
Published: 27 May 2022.
Edited by:
Pamela Ann McCombe, The University of Queensland, AustraliaReviewed by:
Marta Altieri, Sapienza University of Rome, ItalyEoin Flanagan, Mayo Clinic, United States
Copyright © 2022 Chan, Pimentel Maldonado, Wang, Obando, Hughes and Newsome. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Scott D. Newsome, c25ld3NvbTJAamhtaS5lZHU=
 Carol K. Chan
Carol K. Chan Daniela A. Pimentel Maldonado2
Daniela A. Pimentel Maldonado2 Abbey J. Hughes
Abbey J. Hughes Scott D. Newsome
Scott D. Newsome