
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Neurol. , 12 July 2022
Sec. Endovascular and Interventional Neurology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.862916
This article is part of the Research Topic Risk Assessment for Intracranial Aneurysm Rupture View all 8 articles
Background: Aneurysms of the cerebral vasculature are relatively common, which grow unpredictably, and even small aneurysms carry a risk of rupture. Rupture of intracranial aneurysms (IA) is a catastrophic event with a high mortality rate. Pieces of evidence have demonstrated that smoking is closely related to the formation and rupture of IA. However, the biological effect of smoking cigarettes on the formation and rupture of IA is still underrepresented.
Methods: The study protocol was prospectively registered in PROSPERO, registration number CRD42020203634. We performed a systematic search in PubMed and CNKI for studies exploring the biological effects of smoking on intracranial aneurysms published up to December 2021, and all studies were included in the analysis. The RevMan software was used for data analysis.
Results: A total of 6,196 patients were included in 14 original articles in this meta-analysis. The risk of ruptured IA in the current smoking group was significantly higher than that in the non-smoking group, with statistical significance (RRtotal = 1.23, 95% CI: 1.11–1.37). After heterogeneity among cohorts was removed by the sensitivity analysis, there was still a statistically significant difference in the risk of ruptured IA between the smoking and non-smoking groups (RR total = 1.26, 95% CI: 1.18–1.34). There was no statistically significant difference in the risk of ruptured IA between the former smoking (smoking cessation) group and the non-smoking group (RRtotal = 1.09, 95% CI: 0.50–2.38). After heterogeneity among cohorts was removed by sensitivity analysis, there was still no statistically significant difference in the risk of ruptured IA between the former smoking (smoking cessation) group and the non-smoking group (RRtotal = 0.75, 95% CI: 0.47–1.19). The risk of the ruptured IA in the current smoking group was significantly higher than that in the former smoking (smoking cessation) group, with a statistically significant difference (RRtotal=1.42, 95%CI: 1.27–1.59).
Conclusion: Although the biological effects of smoking on the formation and rupture of IA are unknown, this study suggests that current smoking is a risk factor for ruptured IA. Quitting smoking is very important for patients with IA.
An intracranial aneurysm (IA) is a partial expansion of cerebral blood vessels caused by the local abnormalities of blood vessels. The incidence of IA is 3–5% in the general population (1), and 85% of subarachnoid hemorrhage (SAH) is due to the rupture of IA, which could be seriously hazardous to health (2). Since most IAs are diagnosed after its rupture, it is of great importance to screen its risk factors.
At present, the diagnosis of IA in clinical settings mainly relies on neuroimaging technology (3). In addition, the use of MRI and CT as diagnostic tools has increased over the past 20 years as the quality of intracranial imaging techniques improved, and unruptured aneurysms have been detected with increasing frequency (4). Although the annual risk of ruptured incidental IA is relatively low (5), the prognosis of SAH caused by IA rupture is poor, with the 1 month case fatality rate still as high as 35% (6). About one-third of survivors require lifelong care, and another one-third have residual cognitive impairment that affects functional status and quality of life (7). Thus, preventing rupture of IA is vital. However, surgical clipping and endovascular intervention to prevent aneurysm rupture also have associated hemorrhagic and ischemic risks, which may exceed the natural risk of rupture (estimated total morbidity and mortality in the first month is 10–14%) (8, 9). Therefore, the prevention of IA by identifying and managing the modifiable risk factors, rather than surgical clipping and intravascular intervention, has a significant clinical and social value (10).
Many pieces of literature have reported that risk factors for the formation and rupture of IA include age, female sex, hypertension, and smoking. Among them, smoking is the most easily controlled risk factor (11). A previous studies has documented that cigarette smoking was significantly associated with the growth and rupture of IA (12). However, the mechanisms by which smoking causes the formation and rupture of IA are unclear. In the present meta-analysis, we aimed to determine the relationship between current smoking, former smoking (smoking cessation), and non-smoking and the risk of ruptured IA. Further, we summarized the potential biological effects of smoking on the formation, growth, and rupture of IA, which could be helpful in studying the pathophysiological mechanism of smoking and IA formation and rupture.
The inclusion criteria were as follows: (1) prospective and retrospective cohort studies; (2) research related to smoking and IA; and (3) availability of complete data on smoking and IA or data that can be extracted or calculated from the articles. The exclusion criteria were as follows: (1) studies with nonhuman experiments; (2) studies unrelated to smoking and IA; (3) repeated articles or data; and (4) articles of the type abstract, letter, editorial, expert opinion, review, case report, or laboratory study.
We searched PubMed and CNKI for studies on the relationship between smoking and IA published as of December 2021. The search formula is as follows: (((intracranial aneurysm[Title/Abstract]) OR (Aneurysms, Intracranial[Title/Abstract]) OR (Intracranial Aneurysms[Title/Abstract]) OR (Aneurysm, Intracranial[Title/Abstract]) OR (Aneurysm, Anterior Communicating Artery[Title/Abstract]) OR (Anterior Communicating Artery Aneurysm[Title/Abstract]) OR (Aneurysm, Basilar Artery[Title/Abstract]) OR (Aneurysms, Basilar Artery[Title/Abstract]) OR (Artery Aneurysm, Basilar[Title/Abstract]) OR (Artery Aneurysms, Basilar[Title/Abstract]) OR (Basilar Artery Aneurysms[Title/Abstract]) OR (Basilar Artery Aneurysm[Title/Abstract]) OR (Aneurysm, Middle Cerebral Artery[Title/Abstract]) OR (Middle Cerebral Artery Aneurysm[Title/Abstract]) OR (Aneurysm, Posterior Cerebral Artery[Title/Abstract]) OR (Posterior Cerebral Artery Aneurysm[Title/Abstract]) OR (Berry Aneurysm[Title/Abstract]) OR (Aneurysm, Berry[Title/Abstract]) OR (Aneurysms, Berry[Title/Abstract]) OR (Berry Aneurysms[Title/Abstract]) OR (Brain Aneurysm[Title/Abstract]) OR (Aneurysm, Brain[Title/Abstract]) OR (Aneurysms, Brain[Title/Abstract]) OR (Brain Aneurysms[Title/Abstract]) OR (Cerebral Aneurysm[Title/Abstract]) OR (Aneurysms, Cerebral[Title/Abstract]) OR (Cerebral Aneurysms[Title/Abstract]) OR (Aneurysm, Cerebral[Title/Abstract]) OR (Giant Intracranial Aneurysm[Title/Abstract]) OR (Aneurysm, Giant Intracranial[Title/Abstract]) OR (Aneurysms, Giant Intracranial[Title/Abstract]) OR (Giant Intracranial Aneurysms[Title/Abstract]) OR (Intracranial Aneurysm, Giant[Title/Abstract]) OR (Intracranial Aneurysms, Giant[Title/Abstract]) OR (Mycotic Aneurysm, Intracranial[Title/Abstract]) OR (Aneurysm, Intracranial Mycotic[Title/Abstract]) OR (Aneurysms, Intracranial Mycotic[Title/Abstract]) OR (Intracranial Mycotic Aneurysm[Title/Abstract]) OR (Intracranial Mycotic Aneurysms[Title/Abstract]) OR (Mycotic Aneurysms, Intracranial[Title/Abstract]) OR (Aneurysm, Anterior Cerebral Artery[Title/Abstract]) OR (Anterior Cerebral Artery Aneurysm[Title/Abstract]) OR (Aneurysm, Posterior Communicating Artery[Title/Abstract]) OR (Posterior Communicating Artery Aneurysm[Title/Abstract])) AND ((Smoking[Title/Abstract]) OR (Smoking Behaviors[Title/Abstract]) OR (Behavior, Smoking[Title/Abstract]) OR (Behaviors, Smoking[Title/Abstract]) OR (Smoking Behavior[Title/Abstract]) OR (Smoking Habit[Title/Abstract]) OR (Habit, Smoking[Title/Abstract]) OR (Habits, Smoking[Title/Abstract]) OR (Smoking Habits[Title/Abstract]))) AND (rupture[Title/Abstract]). The title and abstract of each study were independently screened by two researchers trained in standardization and uniformity. After our initial screening, we obtained the full text of all studies that were likely to meet our minimum inclusion criteria.
A systematic review and a meta-analysis were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Two evaluators independently assessed the quality of all included studies using the nine-star Newcastle-Ottawa Scale (NOS). The studies were scored according to the three aspects of the NOS assessment: selection, comparability, and outcome. Studies with NOS scores≥6 are considered to be of high quality. A reviewer extracts all study data. All disagreements were discussed, and a final decision by the two reviewers was obtained. The characteristics and scores of the studies included in this meta-analysis are shown in Tables 1, 2. We identified former smokers as quitters.
In this meta-analysis, the association between smoking status and IA was measured by estimating the relative risk (RR) with its 95% confidence interval (CI). Statistical heterogeneity was assessed using I2, and if I2 is more than 50% was analyzed using a random-effects model, and a sensitivity analysis was further performed. if I2 is <50% was analyzed using a fixed-effects model, and no sensitivity analysis was performed. Differences were considered significant if two-sided p < 0.05.
We found a total of 275 articles. After the elimination of duplicate literature, the literature that did not meet the inclusion criteria were excluded through preliminary screening of the title and abstract. There were 25 pieces of literature remaining, and the applicability of these 25 pieces of literature was evaluated by reading the full text. After the articles were further excluded according to the exclusion criteria, there were still 14 articles that met the inclusion criteria. These 14 articles were included in the meta-analysis. The flow chart of literature screening is shown in Figure 1. These 14 articles included 13 retrospective studies and 1 prospective study. A total of 6,196 patients were included in the 14 articles, including 2,558 current smokers, 1,216 former smokers, 2,422 non-smokers, and 3,014 ruptured IA. The characteristics of the studies included in this meta-analysis are shown in Table 1.
The relationship between smoking status (current smoking, former smoking, and non-smoking) and ruptured IA was evaluated based on relative risk. The risk of ruptured IA in the current smoking group was significantly higher than that in the non-smoking group, with statistical significance (RRtotal = 1.23, 95%CI: 1.11–1.37). The analysis was estimated using a random-effects model because significant heterogeneity was found between studies (p = 0.00001 < 0.05, I2 = 74% > 50%). These data are shown in Figure 2. There was no significant difference in the risk of ruptured IA between the former smoking (smoking cessation) group and the non-smoking group (RRtotal = 1.09, 95%CI: 0.50–2.38). The analysis was estimated using a random-effects model because significant heterogeneity was found between studies (p = 0.02 < 0.05, I2 = 69% > 50%). These data are shown in Figure 3. The risk of the ruptured IA in the current smoking group was significantly higher than that in the former smoking (smoking cessation) group, with a statistically significant difference (RRtotal = 1.42, 95%CI: 1.27–1.59). Since there was no significant heterogeneity between the studies, the fixed-effects models were used to estimate the analyses (p = 0.22 > 0.05, I2 = 30% < 50%). These data are shown in Figure 4.
Sensitivity analysis of the current smoking group and the non-smoking group found that Liu's study data were the main source of heterogeneity. These studies were removed, and there was no statistical heterogeneity among these cohorts (p = 0.21> 0.05, I2 = 24% < 50%). This meta-analysis, again calculated using the fixed-effect model, shows that current smoking remains a risk factor for ruptured IA (RRtotal = 1.26,95%CI: 1.18–1.34) (Figure 5). Sensitivity analysis of the former smoking group and the non-smoking group found that Feng's et al. study (2017) data were the main source of heterogeneity. These studies were removed, and there was no statistical heterogeneity among these cohorts (p = 0.15 > 0.05, I2 = 48% <50%). This meta-analysis, again calculated using the fixed-effects model, showed no statistical difference in the risk of ruptured IA between the former smokers and the non-smokers (RRtotal = 0.75, 95%CI: 0.47–1.19) (Figure 6). Potential publication bias was visually assessed using funnel plots generated in RevMan. However, the small amount of study data limits the interpretability of the findings, as shown in Figures 7–9.
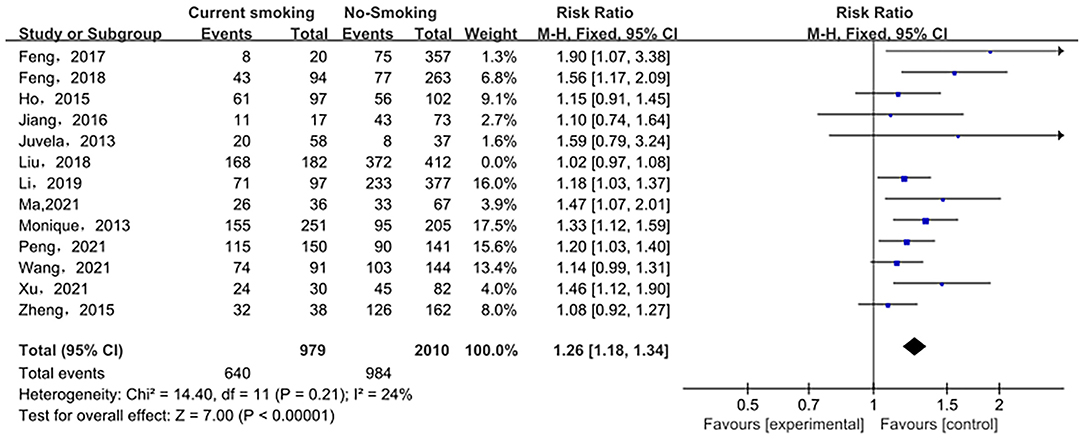
Figure 5. A Forest plot of the current smoking and non-smoking groups after heterogeneous cohorts were removed by sensitivity analysis.
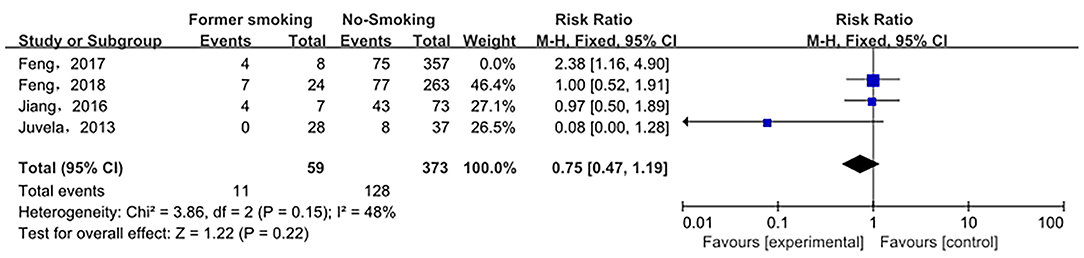
Figure 6. A Forest plot of the former smoking and non-smoking groups after heterogeneous cohorts were removed by sensitivity analysis.
Previous studies have documented that smoking is one of the most important risk factors for the formation of IA. However, there are still several studies with controversial results. We have presented an integrated overview and a systematic review of recent studies on the relationship between the current smoking, the former smoking (smoking cessation), and the non-smoking groups and the risk of ruptured IA. Further, we discussed the underlying mechanism of smoking on IA formation and rupture.
The global decline in the incidence of SAH parallels the decline in smoking prevalence. For every 1% point decrease in the smoking rate, the overall age- and gender-adjusted SAH incidence decreases by 2.4% (26), which clearly illustrates that the smoking is related to ruptured aneurysms. Moreover, genetic susceptibility to smoking leads to an increased risk of IA and drives most of the genetic association between IA and other cerebrovascular traits (27, 28). To better understand the relationship between smoking and ruptured IA, scholars analyzed the relationship between smoking time, smoking intensity, smoking index, and smoking status and ruptured IA. One case-control study showed that current smoking, smoking intensity, and smoking duration are significantly related to ruptured IA (25). There is also a dose-response relationship between the intensity, duration of smoking, and the incidence of ruptured IA (10). As the number of cigarettes smoked per day (CPD) and the number of years of smoking increased, the risk of IA also increased accordingly (25). Moreover, a positive correlation between the smoking index (CPD × years of tobacco use) and the risk of ruptured aneurysms were also reported (10). According to published data, current smokers are 2.5 times more likely to have a rupture than non-smokers, while former smokers are nearly 2 times more likely to have a rupture than non-smokers (25, 29). Compared with former smokers and non-smokers, current smokers are more likely to develop ruptured aneurysms, which is consistent with our results. Prospective studies showed that the risk of ruptured aneurysms will be reduced when patients quit smoking (12, 30). However, the study of Anil and his colleagues reported that the longer the smoking cessation period, the lower the risk of ruptured aneurysms in former smokers, but this association disappeared after adjusting for other confounding factors in the multivariate analysis (25). This indicates that the risk of ruptured IA caused by smoking could still exist even after smoking cessation. The reason for this phenomenon might be that smoking has caused irreversible damage. For example, the nicotine in cigarettes damages elastin, which has a low repair potential (31). Therefore, the elastin damage caused by long-term smoking may be permanent, even after smoking cessation. In addition, for a long time after quitting smoking, quitters showed persistent low-grade inflammation (32). These results obtained from Can et al. showed that smoking cessation only reduces the cumulative dose of tobacco in the body and that early quitting does not reduce the risk of SAH. This view has sparked debate about the relationship between smoking cessation and the risk of ruptured IA. Then, we further evaluated the association between smoking cessation (Former smoking) and the risk of ruptured IA. The results showed that there was no significant difference in the risk of ruptured IA between the former smoking (smoking cessation) group and the non-smoking group, suggesting that quitting smoking may be beneficial for patients with unruptured IA. Moreover, smoking cessation can also reduce the damage of delayed cerebral ischemia after SAH. Delayed cerebral ischemia is the leading cause of death and disability after ruptured IA (33). Therefore, smoking cessation is still indispensable in the treatment of IA. Moreover, those results emphasized the importance of quitting smoking in patients with unruptured IA.
The majority of previous research has focused on the effects of active smoking on IA. In contrast, people's understanding of the effects of passive smoking on IA is still incomplete. The two primary sources of exposure to passive smoking are the workplace and the home (such as a smoking spouse), and passive smoking is common among women. Previous studies have shown that, in non-smoking women with IA, passive smoking is not an independent risk factor for ruptured IA (10). However, passive smoking reduces eNOS activity and increases vascular endothelial inflammation similar to active smoking, which indicates that passive smoking has direct damage to the vascular system (34). There seems to be no harm in avoiding passive smoking in one's everyday life, although the association between passive smoking and the formation and rupture of IA remains indistinct. Further, we summarized the differences in aneurysm morphology between smokers and non-smokers (Table 3), and some aneurysm morphology parameters were significantly correlated with smoking status, such as larger daughter vessel diameters, larger size ratio, and the location at the basilar apex (19). Compared with non-smokers, current smokers may have larger daughter vessel diameters and size ratio, and smokers are more likely to develop basilar apex aneurysms (19). The size ratio is the ratio of the maximum aneurysm height to the mean diameter of the artery bearing the aneurysm (37). The larger size ratio means that large aneurysms formed by small blood vessels are more likely to rupture than small aneurysms formed by large blood vessels. Aneurysms at the Basilar apex have a higher risk of rupture, morbidity, and mortality (38, 39). Of course, the mechanism of smoking-induced changes in morphological parameters of these IA is indistinct, which requires further study. The morphological changes of the IA can reflect the process of its occurrence, growth, and rupture. The relationship between smoking and these specific morphological changes could provide a morphological basis for how smoking increases in aneurysm formation and rupture. However, not all morphological parameters of IA are related to smoking. The study by Juchler et al. found no association between the luminal shape of the IA and the patient's sex, age, smoking, or history of hypertension, even though these factors were strongly associated with aneurysm incidence (28, 40).
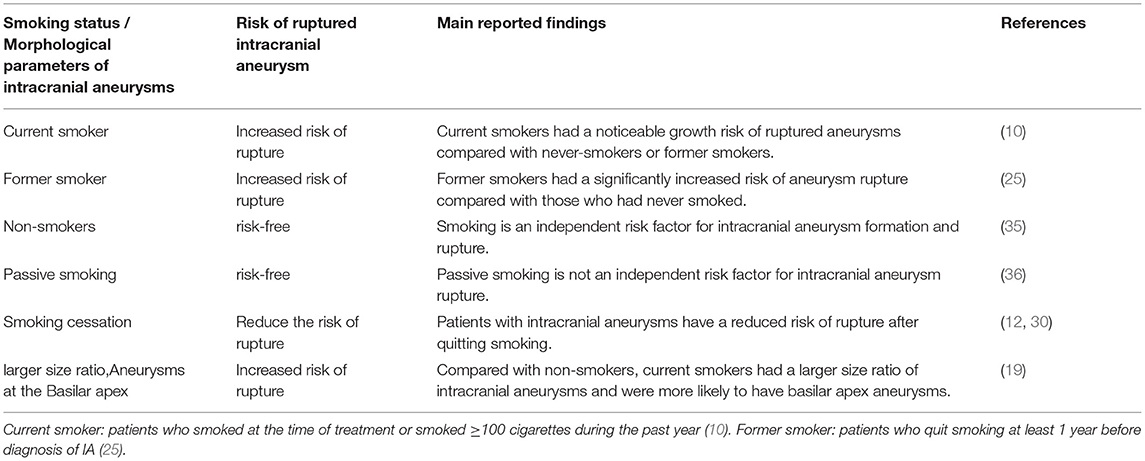
Table 3. Relationship between smoking and intracranial aneurysm rupture and intracranial aneurysm morphology.
It is well-known that IA formation is associated with hemodynamics, chronic inflammation, vascular endothelial dysfunction, and vascular remodeling (41–43) (Figure 10). Pieces of evidence have shown that smoking could induce an inflammatory response in blood vessels, hemodynamic stress, endothelial dysfunction, and eventually cause weakened blood vessel walls and rupture of blood vessel walls (25, 34, 44) (Figure 11). Smoke mainly contains chemicals such as tar, carbon monoxide, nicotine, nitrosamines, volatile organic compounds, and polycyclic aromatic hydrocarbons, which are characteristic of tobacco (46). Nicotine is a major biologically active constituent of tobacco products, which has deleterious effects on IA formation, and rupture (Table 4). Nicotine is also one of the most significant components that cause vascular inflammation (59). The early and key step of cigarette smoke-induced vascular inflammation is the activation of the NF-κB pathway (52), which might be related to nicotine (46). Nicotine also directly stimulates macrophages to secrete high levels of inflammatory factors such as TNF-α, IL-1β, and other chemokines (53). Moreover, the nicotine-induced endothelial dysfunction and inflammatory microenvironment also promote vascular smooth muscle cells (VSMCs) osteogenic transdifferentiation and vascular wall calcification. Experiments have shown that chronic nicotine exposure induces the expression of MMPs in the arterial wall and the destruction of the arterial wall structure (elastin damage) (54). Long-term smoking can impair vascular endothelial function (60), which may be also related to nicotine and oxidants in cigarette smoke. Nicotine promotes oxidative stress in VSMCs and endothelial cells by downregulating superoxide dismutase (SOD), catalase, and glutathione reductase (45). Together, smoking exposure could cause endothelial dysfunction, increase oxidative stress, and increase morbidity and mortality of cardiovascular diseases, which promotes the formation and rupture of IA. Therefore, to prevent the growth and rupture of IA, we underline that patients necessarily cease smoking, which is a valuable measure.
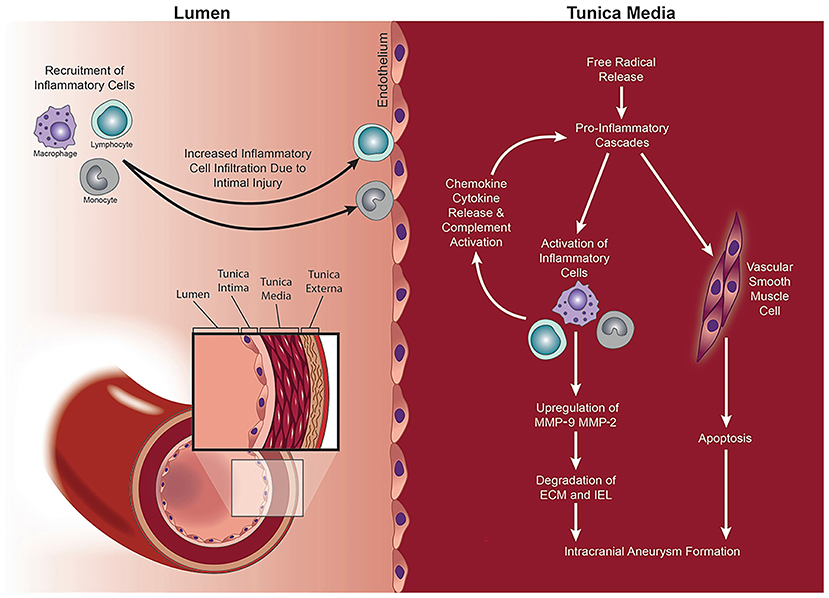
Figure 10. MMPs mediate IA formation via a process of vascular remodeling. Intimal injury and increased wall shear stress (WSS) lead to the recruitment and infiltration of inflammatory cells into the vessel wall. Release of free radicals, pro-inflammatory chemokines and cytokines, and activation of the complement cascade mediate the upregulation of MMP-2 and MMP-9. The consequent proteolytic process results in the degradation of the ECM within the tunica media and altered the structure of the vessel wall with IA formation. Copyright Department of Neurosurgery, The University of Utah. Published with permission (44).
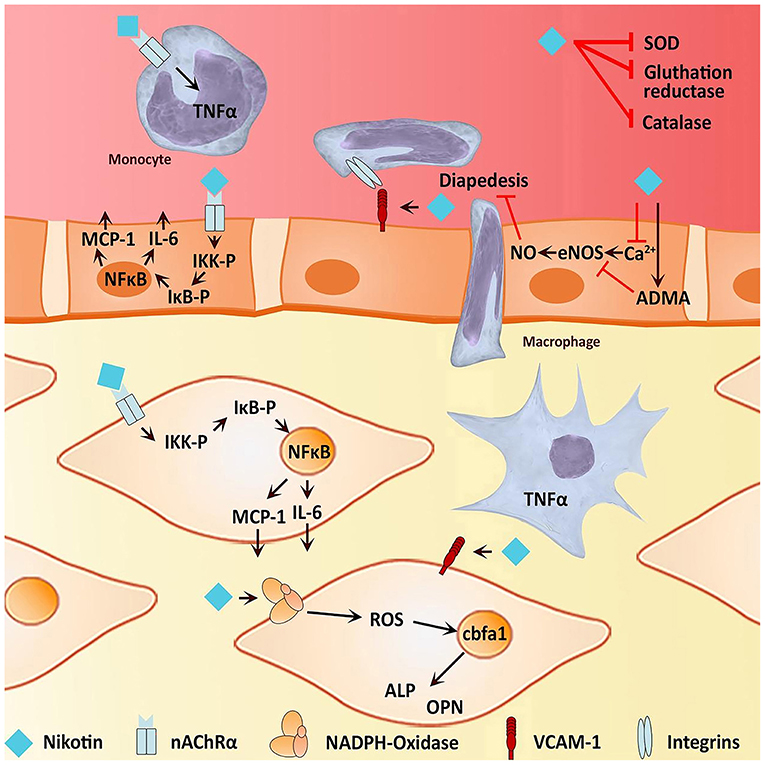
Figure 11. Effects of nicotine on osteogenic transdifferentiation. Nicotine could induce an environment that promotes or even initiates vascular calcification of the media vessel wall by osteogenic transdifferentiation of VSMC. On the one hand, nicotine is capable of inducing inflammation by stimulating the activity of NF-κB and therefore the expression of pro-inflammatory cytokines such as IL-6. Furthermore, there is evidence for nicotine to stimulate macrophages to express TNF-α, contributing to an inflammatory environment. On the other hand, nicotine increases oxidative stress, directly and indirectly, possibly leading to endothelial dysfunction Both endothelial dysfunction and an inflammatory environment facilitate an osteogenic phenotype of VSMC (45).
There are some limitations to this study. First, all the included studies are observational, and inevitably, there is an observational bias. Second, different studies use different criteria to define current smoking and former smoking. In addition, we did not consider gender, age, race, hypertension, and other factors in the study. Subgroup analyses were not performed because we could not extract relevant data for some factors from the included literature, which may increase heterogeneity among the included studies. These unspecified factors and other unmeasured or unknown confounding factors can also affect the reliability of these findings. We divided tobacco exposure into current smoking, former smoking, and non-smoking. To some extent, this may reduce heterogeneity between included studies. Finally, due to the relatively small number of studies included, the statistical power of these results is limited.
A larger and prospective cohort study should be conducted to validate our results in the future.
In conclusion, there is a dose-response relationship between the intensity and duration of smoking and the increased risk of ruptured IA. In terms of the morphology of IA, smoking causes IA to form morphologies that are more prone to rupture. The risk of ruptured IA was not statistically different between the former smoking (smoking cessation) group and the non-smoking group. To summarize, smoking is involved in the formation and rupture of IA, and it is indispensable for patients with IA to quit smoking. Despite numerous related studies on the formation and rupture of IA, the mechanism of IA formation and rupture is still inaccurate, which requires further investigation in our future.
CL and LZ: conception, design, and critically revising the article. HW, LW, and JW: acquisition of data. HW and LW: drafting the article. All authors reviewed submitted version of manuscript.
This work was supported by the Scientific Research Starting Foundation of Affiliated Hospital of Hebei University [Grant Number 31010413]; the Provincial Medical Talents Project funded of Hebei Province [Grant Number 361007]; the Key Scientific Research Projects of the Affiliated Hospital of Hebei University [Grant Number 2021Z001].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
IA, intracranial aneurysm; SAH, subarachnoid hemorrhage; WSS, wall shear stress; MMPs, matrix metalloproteinases; VSMCs, vascular smooth muscle cells; MCP1, macrophage chemotactic protein 1.
1. Chalouhi N, Hoh BL, Hasan D. Review of cerebral aneurysm formation, growth, and rupture. Stroke. (2013) 44:3613–22. doi: 10.1161/STROKEAHA.113.002390
2. Brown RD Jr, Broderick JP. Unruptured intracranial aneurysms: epidemiology, natural history, management options, and familial screening. The Lancet Neurol. (2014) 13:393–404. doi: 10.1016/S1474-4422(14)70015-8
3. Supriya M, Christopher R, Indira Devi B, Bhat DI, Shukla D. Circulating microRNAs as potential molecular biomarkers for intracranial aneurysmal rupture. Mol Diagn Ther. (2020) 24:351–64. doi: 10.1007/s40291-020-00465-8
4. Etminan N, Rinkel GJ. Unruptured intracranial aneurysms: development, rupture and preventive management. Nat Rev Neurol. (2016) 12:699–713. doi: 10.1038/nrneurol.2016.150
5. Macdonald RL, Schweizer TA. Spontaneous subarachnoid haemorrhage. Lancet. (2017) 389:655–66. doi: 10.1016/S0140-6736(16)30668-7
6. Roquer J, Cuadrado-Godia E, Guimaraens L, Conesa G, Rodríguez-Campello A, Capellades J, et al. Short- and long-term outcome of patients with aneurysmal subarachnoid hemorrhage. Neurology. (2020) 95:e1819–29. doi: 10.1212/WNL.0000000000010618
7. Smith M, Citerio G. What's new in subarachnoid hemorrhage. Intensive Care Med. (2015) 41:123–6. doi: 10.1007/s00134-014-3548-5
8. Broderick JP, Brown RD Jr, Sauerbeck L, Hornung R, Huston J 3rd, Woo D, et al. Greater rupture risk for familial as compared to sporadic unruptured intracranial aneurysms. Stroke. (2009) 40:1952–7. doi: 10.1161/STROKEAHA.108.542571
9. Robertson AM, Duan X, Aziz KM, Hill MR, Watkins SC, Cebral JR. Diversity in the strength and structure of unruptured cerebral. Ann Biomed Eng. (2015) 43:1502–15. doi: 10.1007/s10439-015-1252-4
10. Feng X, Qian Z, Zhang B, Guo E, Wang L, Liu P, et al. Number of cigarettes smoked per day, smoking index, and intracranial aneurysm rupture: a case-control study. Front Neurol. (2018) 9:380. doi: 10.3389/fneur.2018.00380
11. Flor LS, Reitsma MB, Gupta V, Ng M, Gakidou E. The effects of tobacco control policies on global smoking prevalence. Nat Med. (2021) 27:239–43. doi: 10.1038/s41591-020-01210-8
12. Juvela S. Treatment scoring of unruptured intracranial aneurysms. Stroke. (2019) 50:2344–50. doi: 10.1161/STROKEAHA.119.025599
13. Xu C, Li Y, Qin B, Cheng C, Guo Z, He Z, et al. Analysis of intracranial cystic aneurysms in the young: clinical characteristics and risk factors of rupture. Chin J Stroke Cerebrovasc Dis. (2021) 18:438–45.
14. Wang G, Guo T, Wang S, Chen L. Risk factors for rupture of small intracranial aneurysm. J Prac Clin Med. (2021) 25:1–3.
15. Peng H, Chen J, Liu Z, Cai R, Zhang M. Risk factors of ruptured intracranial aneurysm. J Hainan Med. (2020) 31:491–93.
16. Li B, Yuan D, Jiang W, Liu J, Xu L. The influence factors of intracranial aneurysm rupture analysis. J Neurol Neurosurg Psychiatry. (2019) 46–48:246–50. doi: 10.16636/j.cnki.jinn.2019.03.003
17. Zheng H, Gao J, Wu J. Correlation analysis of intracranial aneurysm and vascular risk factors. Guide Chin Med. (2015) 13:198.
18. Liu J, Chen Y, Lan L, Lin B, Chen W, Wang M, et al. Prediction of rupture risk in anterior communicating artery aneurysms with a feed-forward artificial neural network. Eur Radiol. (2018) 28:3268–75. doi: 10.1007/s00330-017-5300-3
19. Ho AL, Lin N, Frerichs KU, Du R. Smoking and intracranial aneurysm morphology. Neurosurgery. (2015) 77:59–66. doi: 10.1227/NEU.0000000000000735
20. Vlak MH, Rinkel GJ, Greebe P, Greving JP, Algra A. Lifetime risks for aneurysmal subarachnoid haemorrhage: multivariable risk stratification. J Neurol Neurosurg Psychiatry. (2013) 4:619–23. doi: 10.1136/jnnp-2012-303783
21. Jiang H, Weng YX, Zhu Y, Shen J, Pan JW, Zhan RY. Patient and aneurysm characteristics associated with rupture risk of multiple intracranial aneurysms in the anterior circulation system. Acta Neurochir (Wien). (2016) 158:1367–75. doi: 10.1007/s00701-016-2826-0
22. Feng X, Wang L, Guo E, Zhang B, Qian Z, Wen X, et al. Passive smoking is not associated with risk of intracranial aneurysm rupture in nonsmoking women. World Neur. (2017) 107:716–23.
23. Juvela S, Poussa K, Lehto H, Porras M. Natural history of unruptured intracranial aneurysms: a long-term follow-up study. Stroke. (2013) 44:2414–21.
24. Ma L. Clinical study on factors of intracranial aneurysm rupture. (dissertation/master's thesis). Ningxia Medical University, Yinchuan, China (2021).
25. Can A, Castro VM, Ozdemir YH, Dagen S, Yu S, Dligach D, et al. Association of intracranial aneurysm rupture with smoking duration, intensity, and cessation. Neurology. (2017) 89:1408–15. doi: 10.1212/WNL.0000000000004419
26. Etminan N, Chang HS, Hackenberg K, de Rooij NK, Vergouwen M, Rinkel G, et al. Worldwide incidence of aneurysmal subarachnoid hemorrhage according to region, time period, blood pressure, and smoking prevalence in the population: a systematic review and meta-analysis. JAMA Neurol. (2019) 76:588–97. doi: 10.1001/jamaneurol.2019.0006
27. Bakker MK, van der Spek R, van Rheenen W, Morel S, Bourcier R, Hostettler IC, et al. Genome-wide association study of intracranial aneurysms identifies 17 risk loci and genetic overlap with clinical risk factors. Nat Genet. (2020) 52:1303–13. doi: 10.1038/s41588-020-00725-7
28. Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. (2011) 10:626–36. doi: 10.1016/S1474-4422(11)70109-0
29. Feigin VL, Rinkel GJ, Lawes CM, Algra A, Bennett DA, van Gijn J, et al. Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies. Stroke. (2005) 36:2773–80. doi: 10.1161/01.STR.0000190838.02954.e8
30. Juvela S. PHASES score and treatment scoring with cigarette smoking in the long-term prediction of rupturing of unruptured intracranial aneurysms. J Neurosurg. (2021) 136:156–62. doi: 10.3171/2020.11.JNS203480
31. Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev. (2009) 89:957–89. doi: 10.1152/physrev.00041.2008
32. Csordas A, Bernhard D. The biology behind the atherothrombotic effects of cigarette smoke. Nat Rev Cardiol. (2013) 10:219–30. doi: 10.1038/nrcardio.2013.8
33. Ya X, Zhang C, Zhang S, Zhang Q, Cao Y, Wang S, et al. The relationship between smoking and delayed cerebral ischemia after intracranial aneurysm rupture: a systematic review and meta-analysis. Front Neurol. (2021) 12:625087. doi: 10.3389/fneur.2021.625087
34. Adams T, Wan E, Wei Y, Wahab R, Castagna F, Wang G, et al. Secondhand smoking is associated with vascular inflammation. Chest. (2015) 148:112–9. doi: 10.1378/chest.14-2045
35. Juvela S. Growth and rupture of unruptured intracranial aneurysms. J Neurosurg. (2018) 131:843–51. doi: 10.3171/2018.4.JNS18687
36. Feng X, Wang L, Guo E, Zhang B, Qian Z, Wen X, et al. Passive smoking is not associated with risk of intracranial aneurysm rupture in nonsmoking women. World Neurosurg. (2017) 107:716–23. doi: 10.1016/j.wneu.2017.07.120
37. Zhang J, Can A, Lai P, Mukundan S Jr, Castro VM, Dligach D, et al. Morphological variables associated with ruptured basilar tip aneurysms. Sci Rep. (2021) 11:2526. doi: 10.1038/s41598-021-81364-8
38. Wiebers DO, Whisnant JP, Huston J 3rd, Meissner I, Brown RD Jr, Piepgras DG, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. (2003) 362:103–10. doi: 10.1016/S0140-6736(03)13860-3
39. Molyneux A, Kerr R. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomized trial. J Stroke Cerebrovasc Dis. (2002) 11:304–14. doi: 10.1053/jscd.2002.130390
40. Juchler N, Schilling S, Bijlenga P, Morel S, Rüfenacht D, Kurtcuoglu V, et al. Shape irregularity of the intracranial aneurysm lumen exhibits diagnostic value. Acta Neurochir. (2020) 162:2261–70. doi: 10.1007/s00701-020-04428-0
41. Signorelli F, Sela S, Gesualdo L, Chevrel S, Tollet F, Pailler-Mattei C, et al. Hemodynamic stress, inflammation, and intracranial aneurysm development and rupture: a systematic review. World Neurosurg. (2018) 115:234–44. doi: 10.1016/j.wneu.2018.04.143
42. Czekajło A. Role of diet-related factors in cerebral aneurysm formation and rupture. Rocz Panstw Zakl Hig. (2019) 70:119–26. doi: 10.32394/rpzh.2019.0061
43. Frösen J, Cebral J, Robertson AM, Aoki T. Flow-induced, inflammation-mediated arterial wall remodeling in the formation and progression of intracranial aneurysms. Neurosurg Focus. (2019) 47:E21. doi: 10.3171/2019.5.FOCUS19234
44. Zhang X, Ares WJ, Taussky P, Ducruet AF, Grandhi R. Role of matrix metalloproteinases in the pathogenesis of intracranial aneurysms. Neurosurg Focus. (2019) 47:E4. doi: 10.3171/2019.4.FOCUS19214
45. Babic M, Schuchardt M, Tölle M, van der Giet M. In times of tobacco-free nicotine consumption: the influence of nicotine on vascular calcification. Eur J Clin Invest. (2019) 49:e13077. doi: 10.1111/eci.13077
46. Talhout R, Schulz T, Florek E, van Benthem J, Wester P, Opperhuizen A. Hazardous compounds in tobacco smoke. Int J Environ Res Public Health. (2011) 8:613–28. doi: 10.3390/ijerph8020613
47. Benowitz NL, Fraiman JB. Cardiovascular effects of electronic cigarettes. Nat Rev Cardiol. (2017) 14:447–56. doi: 10.1038/nrcardio.2017.36
49. Benowitz NL, Burbank AD. Cardiovascular toxicity of nicotine: Implications for electronic cigarette use. Trends Cardiovasc Med. (2016) 26:515–23. doi: 10.1016/j.tcm.2016.03.001
50. Wu Y, Song P, Zhang W, Liu J, Dai X, Liu Z, et al. Activation of AMPK?2 in adipocytes is essential for nicotine-induced insulin resistance in vivo. Nat Med. (2015) 21:373–82. doi: 10.1038/nm.3826
51. Luo HL, Zang WJ, Lu J, Yu XJ, Lin YX, et al. The protective effect of captopril on nicotine-induced endothelial dysfunction in rat. Basic Clin Pharmacol Toxicol. (2006) 99:237–45. doi: 10.1111/j.1742-7843.2006.pto_494.x
52. Golbidi S, Edvinsson L, Laher I. Smoking and endothelial dysfunction. Curr Vasc Pharmacol. 18 (2020) 1–11. doi: 10.2174/1573403X14666180913120015
53. Lau PP, Li L, Merched AJ, Zhang AL, Ko KW, Chan L. Nicotine induces proinflammatory responses in macrophages and the aorta leading to acceleration of atherosclerosis in low-density lipoprotein receptor (-/-) mice. Arterioscler Thromb Vasc Biol. (2006) 26:143–9. doi: 10.1161/01.ATV.0000193510.19000.10
54. Wagenhäuser MU, Schellinger IN, Yoshino T, Toyama K, Kayama Y, Deng A, et al. Chronic nicotine exposure induces murine aortic remodeling and stiffness segmentation—implications for abdominal aortic aneurysm susceptibility. Front Physiol. (2018) 9:1459. doi: 10.3389/fphys.2018.01459
55. Gu Z, Fonseca V, Hai CM. Nicotinic acetylcholine receptor mediates nicotine-induced actin cytoskeletal remodeling and extracellular matrix degradation by vascular smooth muscle cells. Vascul Pharmacol. (2013) 58:87–97. doi: 10.1016/j.vph.2012.08.003
56. Jacob-Ferreira AL, Palei AC, Cau SB, Moreno H Jr, Martinez ML, et al. Evidence for the involvement of matrix metalloproteinases in the cardiovascular effects produced by nicotine. Eur J Pharmacol. (2010) 627:216–22. doi: 10.1016/j.ejphar.2009.10.057
57. Hashimoto K, Zaima N, Sekiguchi H, Kugo H, Miyamoto C, Hoshino K, et al. Dietary DNA attenuates the degradation of elastin fibers in the aortic wall in nicotine-administrated mice. J Nutr Sci Vitaminol (Tokyo). (2018) 64:271–6. doi: 10.3177/jnsv.64.271
58. Yoshiyama S, Chen Z, Okagaki T, Kohama K, Nasu-Kawaharada R, Izumi T, et al. Nicotine exposure alters human vascular smooth muscle cell phenotype from a contractile to a synthetic type. Atherosclerosis. (2014) 237:464–70. doi: 10.1016/j.atherosclerosis.2014.10.019
59. Wang Z, Wang D, Wang Y. Cigarette smoking and adipose tissue: the emerging role in progression of atherosclerosis. Mediators Inflamm. (2017) 2017:3102737. doi: 10.1155/2017/3102737
Keywords: intracranial aneurysm, ruptured, smoking, nicotine, morphology
Citation: Wang H, Wang L, Wang J, Zhang L and Li C (2022) The Biological Effects of Smoking on the Formation and Rupture of Intracranial Aneurysms: A Systematic Review and Meta-Analysis. Front. Neurol. 13:862916. doi: 10.3389/fneur.2022.862916
Received: 26 January 2022; Accepted: 25 May 2022;
Published: 12 July 2022.
Edited by:
Xin Zhang, Southern Medical University, ChinaReviewed by:
Marialuisa Zedde, IRCCS Local Health Authority of Reggio Emilia, ItalyCopyright © 2022 Wang, Wang, Wang, Zhang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunhui Li, bGljaHVuaHVpMDg2MDMxMkBzaW5hLmNvbQ==; Lijian Zhang, bGlqaWFuLnpoYW5nQGFsaXl1bi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.