
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Neurol. , 09 May 2022
Sec. Movement Disorders
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.855698
This article is part of the Research Topic Movement Disorders – Case Report Collection 2022 View all 16 articles
Migraine is a highly prevalent neurological disorder characterized by recurrent, unilateral, or bilateral throbbing severe headaches. Currently, there are extremely rare cases of migraine-induced dystonia. A 52-year-old woman was admitted for intractable migraine for about 5 days and walking difficulties for 1 day. The symptom of an inability to walk appeared on the fourth day of the headache attack lasting for 1 day and resolved on its own as the headache subsided. The same symptoms appeared once 6 years ago. Neurological examination, brain Magnetic resonance imaging (MRI), laboratory tests of blood and cerebrospinal fluid (CSF) were normal. The contrast transcranial Doppler echocardiography (cTCD) revealed a latent and massive right-to-left shunt (RLS) after the release of the Valsalva maneuver. The patient was diagnosed with migraine-induced dystonia of the lower limbs. Oral ibuprofen and flunarizine and avoidance of increased chest pressure maneuvers were used for treatment and prevention. During the 6-month follow-up, the patient was free of headaches and walking difficulties. Our study reported a rare case of migraine-induced dystonia of the lower extremities.
Migraine is the second most common neurological disease worldwide, with an annual incidence of up to 15% in the general population (1). Migraine is frequently characterized by recurrent, unilateral, or bilateral throbbing, severe headache. Dystonia is a movement disorder manifesting as abnormal movements or postures, or both, caused by continuous or intermittent muscle contractions, and the movements and postures are frequently repetitive with an annual incidence of 15–30 per 100,000 in the general population (2). There have been some reports of patients suffering from migraine and dystonia simultaneously (3, 4). However, reports of migraine-induced dystonia are particularly rare, and the mechanisms involved are unclear so far (5).
Here, we report a rare case of a patient with migraine-induced dystonia of the lower extremities manifested by the inability to walk. The dystonia symptoms resolved spontaneously with migraine resolution. Auxiliary examination revealed that she had a patent foramen ovale and a potentially large right-to-left shunt. The same symptoms appeared twice in 6 years. We also make reasonable guesses about possible etiologies.
A 52-year-old woman without a family history of migraine and dystonia was admitted to our department due to intractable migraine for about 5 days and walking difficulties for 1 day. She has had a history of headaches for over 20 years with one or two attacks per year described as a throbbing or swelling pain in the occipital region or even the whole brain with an intensity of 6 to 9/10. There was no significant aura before the attack. The headache attack was accompanied by numbness and coldness of the painful area and stiffness of the neck, and nausea and vomiting without photophobia or phonophobia. At worst, she could not take in any food or medicine because of vomiting. The headache usually lasted for 5–6 days and could last up to 15 days, and was slightly relieved by ibuprofen and flunarizine. On the fourth day of this attack, she suddenly suffered an inability to walk lasting for 1 day. She could stand on her own without any dizziness. However, every time she tried to start walking, she felt stiffness in both lower extremities and was unable to step and walk, but could only maintain an upright position. And, it did not change with prolonged standing time. In addition, no movement other than walking was affected. When she was lying down, the movement of both lower extremities was not restricted and she could raise and lower her legs and move them in any direction at will. The same symptom happened once 6 years ago, and it resolved on its own after lasting for 1 h.
The physical examination and vital signs were unremarkable, and the neurologic examination was normal. The patient showed a task-specific lower limbs dystonia, characterized by the appearance of sustained dystonic extension of both knees induced by stepping or walking attempts. Magnetic resonance imaging (MRI) scan of the brain showed no significant abnormalities (Figure 1). The patient underwent a lumbar puncture, the cerebrospinal fluid (CSF) pressure was 125 mm H2O (normal range: 80–180 mmH2O), and the biochemistry and cytology of the CSF were negative. Her blood tests were regular, including routine blood work, liver function, kidney function, ions, D-dimer, and markers of myocardial damage. The contrast transcranial doppler echocardiography (cTCD) showed a latent and massive right-to-left shunt (RLS), that was, more than 25 microbubbles were detected using insonation of the left middle cerebral artery (LMCA) after the release of the Valsalva maneuver (Figure 2). No microbubble was seen in the resting state. Right heart contrast echocardiography confirmed the result. Her echocardiography revealed no abnormalities in the structure and function of the heart at rest.
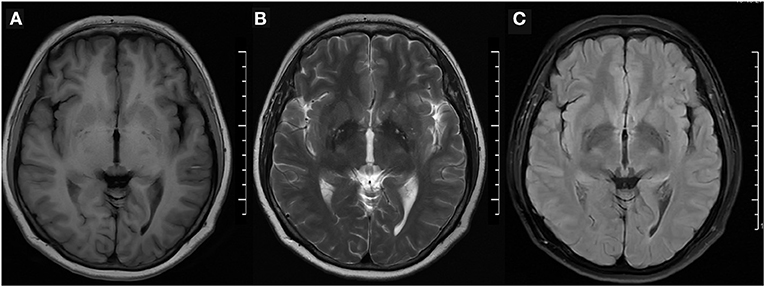
Figure 1. Brain MRI. (A) T1-weighted, (B) T2-weighted, and (C) fluid-attenuated inversion recovery (FLAIR) imaging were normal. MRI, magnetic resonance imaging.
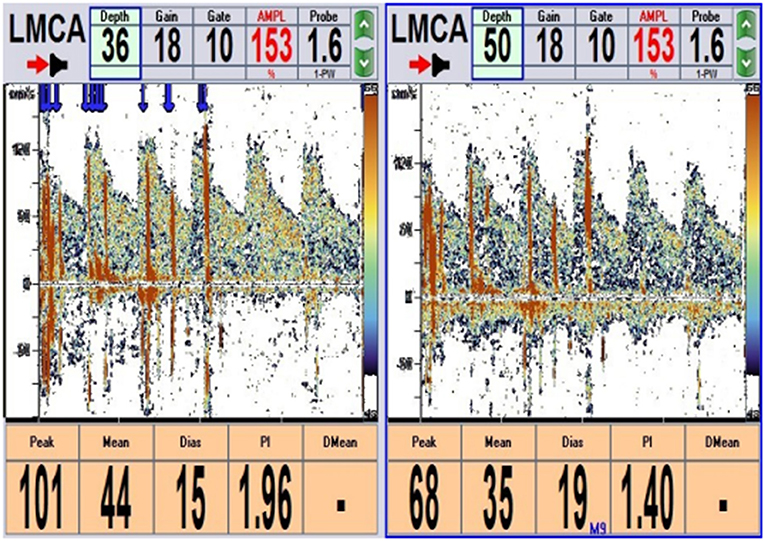
Figure 2. cTCD: More than 25 microbubbles were detected after 2 s of Valsalva maneuver detecting from the left middle cerebral artery (LMCA) (single-channel and double depth) (blue arrow). cTCD, contrast transcranial Doppler echocardiography.
Finally, the patient was diagnosed with migraine and dystonia of the lower extremities induced by it. The cTCD and right heart contrast echocardiography suggested that she had a patent foramen ovale (PFO). Transesophageal echocardiography (TEE) was required to clarify her heart condition further. The patient's headache lasted for 12 days and was relieved with oral ibuprofen (two capsules per day) and flunarizine (10 mg per day). The symptom of the inability to walk cleared spontaneously staying for 1 day, and she was capable of walking slowly and awkwardly during the consultation. Other than medicines for headaches, no additional treatment was given, similar to the situations 6 years ago. Considering the presence of PFO, we recommended she avoid actions that would increase chest pressure, such as diving, violent coughing, and strenuous exercise, which would be of considerable benefit for preventing her headaches. At a follow-up 6 months after the attack, the patient had no episodes of headache and walking difficulty. Unfortunately, owing to financial problems, the patient did not undergo a relevant genetic test, making it difficult to explore the etiology of her condition further.
In this case, we demonstrate a patient presenting with migraine combined with dystonia triggered by it, with the dystonia manifesting as a walking impediment. Migraine contains three main types: migraine with aura, migraine without aura, and chronic migraine. There is a strong association between migraine and PFO. The prevalence of PFO accounts for 46.3–88% of migraineurs with aura and 16.2–34.9% of migraineurs without aura (6). Recent studies suggest that PFO may trigger migraine and is positively correlated with attack frequency but are not significantly related to attack symptoms (7). Previous studies have confirmed that suffering from migraine can affect patients' gait and balance. Akdal et al. (8) discovered that migraineurs without manifesting vertigo had more incredible sway velocity when standing, more significant offset center of gravity (COG) alignment, wider step width, and slower speed. Machado Maciel et al. (9) found migraineurs experienced longer step width with increasing light and sound levels. However, in our case, the patient's twice walking disability within 6 years both occurred during the attack of migraine without dizziness and balance impairment and with normal motor and sensory examinations at rest, suggesting that the symptom was migraine-induced-dystonia of lower extremities.
The classification of dystonia is based on two main clinical features: the age of onset, distribution of symptoms, concomitant symptoms, and etiology (2). Combining the patient's medical history, we considered the patient's dystonia of the lower extremities as a task-specific walking dystonia. No significant geste antagoniste or sensory trick was found in the patient's description and examination. Kemp et al. (10) previously reported a case of delayed dystonia of the left leg secondary to traumatic brain injury, with some resemblance to our case. This patient presented with a persistent extension of the left knee and was task-specific, appearing only when he walked forward. Previous studies have revealed that focal task-specific lower extremity dystonia is associated with prolonged vigorous repetitive activity. Katz et al. (11) reported seven patients with task-specific lower extremity dystonia who had multiple exercise triggers. Their motor triggers included prolonged bicycling, hiking, long-distance running, and drumming. In addition, task-specific dystonia usually progresses continuously. This reminds us of the patient in this case, who has been persistently walking briskly 20,000 steps per day, about 14 km per week, for more than a decade. The duration of her two migraine-induced dystonic episodes that occurred within 6 years became longer, from an hour to a day, although the symptoms were the same on both occasions. Dystonia was elicited by walking attempt, in a task-specific manner. However, we found no significant geste antagoniste or sensory tricks, which may need to be detected in her subsequent episodes and long-term follow-up.
During the diagnosis of walking disability in the patient, we are not inclined to consider functional movement disorders or extrapyramidal symptoms caused by medication side effects as a diagnosis. Patients with functional movement disorders are often associated with anxiety and depression, and a higher proportion of patients have suffered psychological trauma (12, 13). The patient in this case had not experienced significant previous trauma, and her Hamilton Anxiety and Depression Scale test results did not indicate a tendency to suffer from anxiety or depression. Since the patient was capable of walking when she arrived at the hospital, we did not perform suggestive therapy on her. Studies have found that long-term oral administration of flunarizine significantly increases the risk of movement disorders in a dose-dependent manner, including dystonia, parkinsonism, akathisia, tremor, and tardive dyskinesia (14). The patient in our case did not take flunarizine to prevent migraine. She usually took ibuprofen (two capsules per day) and flunarizine (10 mg per day) orally to relieve her headache when it occurred. Furthermore, due to severe nausea and vomiting and even inability to take her medication during the first 2–3 days of the migraine attack, she usually took oral flunarizine for no more than 5 days during the course. Actually, ibuprofen was the more effective and more often chosen medication for her in most conditions. In addition, the patient's presentation differs from that of paroxysmal kinedigenic dystonia (PKD). PKD has a usual episode duration of <1 min, and the reported presentations of PKD do not cover walking disorders like this patient's (15). As a result, combining the patient's medical history and clinical manifestations, the final diagnosis was migraine-induced dystonia of the lower extremities.
Prior researches have reported several patients with migraine combined with dystonia and the relevant genetic mutation in these patients. Dale et al. (3) and Cuenca-León et al. (16) reported patients with benign paroxysmal torticollis (BPT) and hemiplegic migraine (HM) accompanied by PRRT2 and CACNA1A gene mutation respectively. Gardiner et al. (17) reported individuals with PKD and migraine accompanied by PRRT2 gene mutation. Weber et al. (4) and Gardiner et al. reported patients with paroxysmal exertion-induced dyskinesia (PED) and migraine accompanied by GLUT1 and SLC2A1 gene mutation respectively. In these cases, the dystonia usually manifests as abnormal movements, and there was no specific association between migraine and dystonia episodes. To our knowledge, only one study mentioned a patient with PED caused by migraine whose dystonia symptoms presented as face contraction with dysarthria, preceded the onset of migraine, and resolved with the relief of migraine (5). In our case, there was a definite association between the patient's two episodes of dystonia and migraine attacks, which occurred in the duration of migraine and disappeared with the reduction of the headache. Moreover, the dystonia presented as walking difficulties of the lower extremities, which was not previously documented.
The specific mechanisms of migraine and dystonia are not well identified so far. Cortical spreading depression (CSD) plays a vital role in the pathophysiology of both migraine and task-specific dystonia (18, 19). However, CDS is not specific and may also be an accompanying phenomenon in the disease process (20). Previously reported channel genes associated with migraine include CACNA1A, ATP1A2, ATP1A3, ATP1A4, SCN1A, PRRT2, PNKD, SLC2A1, SLC1A3 and SLC4A4 (21, 22). Different types of myotonia correspond to different pathogenic genes, such as the TOR1A gene mutation in DYT1, the TUBB4A gene mutation in DYT4, and the GNAL gene mutation in DYT25 (2). Among them, channel genes associated with paroxysmal dystonia include PRRT2, PNKD, ATP1A3, SLC2A1, and SCN8A (23). It is obvious that migraine and dystonia possess some genetic regulatory mechanisms in common. Given the mutation concerning genes, channel impairment may be an intrinsic mechanism commonly shared by both (24). Gene mutation results in disruption of neurotransmitter release, which in turn impairs synaptic release. The ATP1A2 and ATP1A3 genes, which are different isoforms encoding the Na+/K+-ATPase (NKA) alpha subunit, are associated with FHM2 (ATP1A2), childhood alternating hemiplegia (ATP1A2/A3), RDP (ATP1A3), cerebellar ataxia-reflex loss-progressive optic atrophy (ATP1A3), and recurrent encephalopathy with cerebellar ataxia (ATP1A3), respectively (25). The ATP1A4 mutation is a novel gene mutation that was detected associated with FHM (22). PRRT2 mutations lead to dysregulation of transmembrane calcium and sodium channels, resulting in diseases such as FHM2 and PKD (17). Mutations in SCN1A, which encodes the pore-forming a1 subunit of the neuronal voltage-gated sodium channel Nav1.1, are likely to cause neurological disorders such as FHM and HM in patients (21). Mutations in PNKD disrupt neurotransmitter regulation, which in turn is implicated in HM and PNKD (26). The possibility of phenotypic pleiotropy of genes associated with migraine and dystonia (Table 1), meanwhile, cannot be excluded. However, the significant temporal association between migraine and dystonia and the uniqueness of the dystonia manifestation in this case should still be acknowledged.
Management of migraine-induced dystonia is primarily focused on migraine prevention and treatment. Current randomized controlled studies revealed an unremarkable effect of PFO closure on migraineurs (27). A further genetic test is required, even though it has a limited impact on the patient's treatment, it may play a profound role in expanding our understanding of the underlying mechanisms of migraine and dystonia. Further follow-up is required as well.
In conclusion, our study suggests a rare case of migraine-induced dystonia of the lower extremities, which broadens our insight into migraine and dystonia. To date, only one case has been reported previously, and there are some discrepancies with our case. Further fundamental analyses are needed to explore the potential mechanisms.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
TJ and HM carried out the patient information acquisition and manuscript preparation and drafted the manuscript and prepared the figure and table. YX and BM checked the literature and developed the idea of the study. YC and ZL revised the manuscript and gave the final approval. HM contributed to the conception and design of the manuscript. All authors read the approved and final manuscript.
This study was supported by grants by the National Natural Science Foundation of China (No. 81871008).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank the patient and her family. We would also thank Editage for proofreading the manuscript.
1. Collaborators GBDN. Global, regional, and national burden of neurological disorders, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. (2019) 18:459–80.
2. Albanese A, Di Giovanni M, Lalli S. Dystonia: diagnosis and management. Eur J Neurol. (2019) 26:5–17. doi: 10.1111/ene.13762
3. Dale RC, Gardiner A, Antony J, Houlden H. Familial PRRT2 mutation with heterogeneous paroxysmal disorders including paroxysmal torticollis and hemiplegic migraine. Dev Med Child Neurol. (2012) 54:958–60. doi: 10.1111/j.1469-8749.2012.04394.x
4. Weber YG, Storch A, Wuttke TV, Brockmann K, Kempfle J, Maljevic S, et al. GLUT1 mutations are a cause of paroxysmal exertion-induced dyskinesias and induce hemolytic anemia by a cation leak. J Clin Invest. (2008) 118:2157–68. doi: 10.1172/JCI34438
5. Blakeley J, Jankovic J. Secondary paroxysmal dyskinesias. Mov Disord. (2002) 17:726–34. doi: 10.1002/mds.10178
6. Liu KM, Wang BZ, Hao YS, Song SJ, Pan MX. The correlation between migraine and patent foramen ovale. Front Neurol. (2020) 11. ARTN 543485 doi: 10.3389/fneur.2020.543485
7. He Q, Zhang YB, Wang FZ Li C, Guo R, Li XN, et al. Impact of right-to-left shunt and transcatheter closure on the clinical features of migraine. Int J Neurosci. (2020) 130:270–5. doi: 10.1080/00207454.2019.1672681
8. Akdal G, Donmez B, Ozturk V, Angin S. Is balance normal in migraineurs without history of vertigo? Headache. (2009) 49:419–25. doi: 10.1111/j.1526-4610.2008.01256.x
9. Machado Maciel N, Ferreira Carvalho G, Ferreira Pinheiro C, van Emmerik R, Moraes R, Bevilaqua Grossi D. Gait control of migraine patients with increasing light and sound levels. Gait Posture. (2021) 92:480–6. doi: 10.1016/j.gaitpost.2021.04.003
10. Kemp S, Kim SD, Cordato DJ, Fung VS. Delayed-onset focal dystonia of the leg secondary to traumatic brain injury. Journal Clin Neurosci. (2012) 19:916–7. doi: 10.1016/j.jocn.2011.08.025
11. Katz M, Byl NN, San Luciano M, Ostrem JL. Focal task-specific lower extremity dystonia associated with intense repetitive exercise: a case series. Parkinsonism Related Disord. (2013) 19:1033–38. doi: 10.1016/j.parkreldis.2013.07.013
12. Jacob AE, Smith CA, Jablonski ME, Roach AR, Paper KM, Kaelin DL, et al. Multidisciplinary clinic for functional movement disorders (FMD): 1-year experience from a single centre. J Neurol Neurosurg Psychiatry. (2018) 89:1011–2. doi: 10.1136/jnnp-2017-316523
13. Tinazzi M, Geroin C, Erro R, Marcuzzo E, Cuoco S, Ceravolo R, et al. Functional motor disorders associated with other neurological diseases: Beyond the boundaries of “organic” neurology. Eur J Neurol. (2021) 28:1752–8. doi: 10.1111/ene.14674
14. Jhang KM, Huang JY, Nfor ON, Tung YC, Ku WY, Lee CT, et al. Extrapyramidal symptoms after exposure to calcium channel blocker-flunarizine or cinnarizine. Eur J Clin Pharmacol. (2017) 73:911–6. doi: 10.1007/s00228-017-2247-x
15. Cao L, Huang X, Wang N, Wu Z, Zhang C, Gu W, et al. Recommendations for the diagnosis and treatment of paroxysmal kinesigenic dyskinesia: an expert consensus in China. Transl Neurodegener. (2021) 10:7. doi: 10.1186/s40035-021-00231-8
16. Cuenca-Leon E, Corominas R, Fernandez-Castillo N, Volpini V, Del Toro M, Roig M, et al. Genetic analysis of 27 Spanish patients with hemiplegic migraine, basilar-type migraine and childhood periodic syndromes. Cephalalgia. (2008) 28:1039–47. doi: 10.1111/j.1468-2982.2008.01645.x
17. Gardiner AR, Bhatia KP, Stamelou M, Dale RC, Kurian MA, Schneider SA, et al. PRRT2 gene mutations: from paroxysmal dyskinesia to episodic ataxia and hemiplegic migraine. Neurology. (2012) 79:2115–21. doi: 10.1212/WNL.0b013e3182752c5a
18. Beck S, Richardson SP, Shamim EA, Dang N, Schubert M, Hallett M. Short intracortical and surround inhibition are selectively reduced during movement initiation in focal hand dystonia. J Neurosci. (2008) 28:10363–9. doi: 10.1523/JNEUROSCI.3564-08.2008
19. Unekawa M, Ikeda K, Tomita Y, Kawakami K, Suzuki N. Enhanced susceptibility to cortical spreading depression in two types of Na+,K+-ATPase α2 subunit-deficient mice as a model of familial hemiplegic migraine 2. Cephalalgia. (2018) 38:1515–24. doi: 10.1177/0333102417738249
20. Sadnicka A, Kassavetis P, Pareés I, Meppelink AM, Butler K, Edwards M. Task-specific dystonia: pathophysiology and management. J Neurol Neurosurg Psychiatry. (2016) 87:968–74. doi: 10.1136/jnnp-2015-311298
21. Di Stefano V, Rispoli MG, Pellegrino N, Graziosi A, Rotondo E, Napoli C, et al. Diagnostic and therapeutic aspects of hemiplegic migraine. J Neurol Neurosurg Psychiatry. (2020) 91:764–71. doi: 10.1136/jnnp-2020-322850
22. Coppola G, Pastorino G, Vetri L, D'Onofrio F, Operto FF. Familial Hemiplegic Migraine with an ATP1A4 Mutation: Clinical Spectrum and Carbamazepine Efficacy. Brain Sci. (2020) 10:372. doi: 10.3390/brainsci10060372
23. Erro R, Bhatia KP. Unravelling of the paroxysmal dyskinesias. J Neurol Neurosurg Psychiatry. (2019) 90:227–34. doi: 10.1136/jnnp-2018-318932
24. Gardiner AR, Jaffer F, Dale RC, Labrum R, Erro R, Meyer E, et al. The clinical and genetic heterogeneity of paroxysmal dyskinesias. Brain 138(Pt 12). (2015) 3567–80. doi: 10.1093/brain/awv310
25. Vetro A, Nielsen HN, Holm R, Hevner RF, Parrini E, Powis Z, et al. ATP1A2- and ATP1A3-associated early profound epileptic encephalopathy and polymicrogyria. Brain. (2021) 144:1435–50. doi: 10.1093/brain/awab052
26. Sutherland HG, Albury CL, Griffiths LR. Advances in genetics of migraine. J Headache Pain. (2019) 20:72. doi: 10.1186/s10194-019-1017-9
Keywords: migraine, dystonia, movement disorder, patent foramen ovale, case report
Citation: Jiang T, Xie Y, Maimaiti B, Cheng Y, Li Z and Meng H (2022) Case Report: Migraine-Induced Dystonia of the Lower Extremities. Front. Neurol. 13:855698. doi: 10.3389/fneur.2022.855698
Received: 15 January 2022; Accepted: 05 April 2022;
Published: 09 May 2022.
Edited by:
Luigi M. Romito, IRCCS Carlo Besta Neurological Institute Foundation, ItalyReviewed by:
Antonio Emanuele Elia, IRCCS Carlo Besta Neurological Institute Foundation, ItalyCopyright © 2022 Jiang, Xie, Maimaiti, Cheng, Li and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongmei Meng, bWVuZ2htQGpsdS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.