
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol. , 22 March 2022
Sec. Neurorehabilitation
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.852848
This article is part of the Research Topic The use of repetitive peripheral magnetic stimulation (rPMS) in neurological disorders and neurorehabilitation View all 5 articles
 Cyril Schneider1,2*†
Cyril Schneider1,2*† Andrea Zangrandi1,2†
Andrea Zangrandi1,2† Nico Sollmann3,4,5,6
Nico Sollmann3,4,5,6 Michaela Veronika Bonfert7
Michaela Veronika Bonfert7 Louis-David Beaulieu8 and the rPMS Consensus Group‡
Louis-David Beaulieu8 and the rPMS Consensus Group‡An increasing number of clinical research studies have used repetitive peripheral magnetic stimulation (rPMS) in recent years to alleviate pain or improve motor function. rPMS is non-invasive, painless, and administrated over peripheral nerve, spinal cord roots, or a muscle using a coil affixed to the skin and connected to a rapid-rate magnetic stimulator. Despite the clinical impact and scientific interest, the methodological inconsistencies or incomplete details and findings between studies could make the rPMS demonstration difficult to replicate. Given the lack of guidelines in rPMS literature, the present study aimed at developing a checklist to improve the quality of rPMS methods in research. An international panel of experts identified among those who had previously published on the topic were enrolled in a two-round web-based Delphi study with the aim of reaching a consensus on the items that should be reported or controlled in any rPMS study. The consensual rPMS checklist obtained comprises 8 subject-related items (e.g., age, sex), 16 methodological items (e.g., coil type, pulse duration), and 11 stimulation protocol items (e.g., paradigm of stimulation, number of pulses). This checklist will contribute to new interventional or exploratory rPMS research to guide researchers or clinicians on the methods to use to test and publish rPMS after-effects. Overall, the checklist will guide the peer-review process on the quality of rPMS methods reported in a publication. Given the dynamic nature of a consensus between international experts, it is expected that future research will affine the checklist.
- A checklist was developed to assess the quality of rPMS methods in interventional or exploratory research
- By means of the Delphi method, an international panel of experts reached a consensus on the rPMS checklist items
- The checklist will help improve the quality of rPMS research, including reliability and reproducibility of stimulation protocols.
Repetitive peripheral magnetic stimulation (rPMS) is a technique used to deliver high-intensity magnetic pulses over spinal nerve roots, peripheral nerves, or muscles (1–4). It is non-invasive and painless with negligible side effects. Over the two last decades, this technique has gained popularity to reduce chronic pain or improve sensorimotor function in physiopathology and its clinical potential is currently being tested in several population (1, 5–24).
Despite a drastic increase of the number of publications listed in major research databases in the last years, the rPMS approach remains scarcely investigated as compared to noninvasive brain stimulation such as transcranial magnetic stimulation (TMS) or other peripheral stimulation techniques, such as transcutaneous electrical stimulation (TES) or transcranial direct current stimulation (tDCS). It is also noteworthy that most rPMS publications were preliminary or explorative in nature, reported single cases or case series (25–29), or small sample-sized (9, 16, 25, 30, 31), and explored different health conditions (22, 32) or different stimulation protocols (31, 33). Most importantly, rPMS studies lack of methodological information, which undermines the possibilities to replicate the findings and interpret data [for a comprehensive review, see (3)].
For example, several methods are used to identify the motor threshold of the peripheral target area and to set the stimulation intensity (i.e., subthreshold vs. suprathreshold stimulation). Most commonly, the muscle contraction threshold or the movement threshold has been used. The former is determined by visual inspection or by palpation of a slight muscle contraction (9, 25, 34, 35). The latter is obviously influenced by limb weight, inertia and position in relation to the gravity, resulting in a lower sensitivity and variability (14, 34–36). The surface electromyography (EMG) signal–which is a more objective outcome measure than the visual inspection or palpation–could be used to assess the motor threshold for rPMS (as it is done routinely for TMS of M1). However, when rPMS is applied close to the EMG electrodes, the artifacts produced contaminate the EMG signals and it remains challenging to determine an EMG response threshold. Nevertheless, despite the fact that stimulation intensity is pivotal for rPMS effects (3), terminology used and description of the methods to determine the motor threshold and the choice of rPMS intensity are sometimes confusing and difficult to understand, and not always reported.
Findings of some clinically important rPMS studies cannot be replicated due to unprecise description of the methods and the different stimulation protocol applied (i.e., continuous vs. intermittent stimulation, pulse shape, coil orientation, direction of the induced current in relation to the nerve axis or muscle fibers) [for a review see (3)]. Further, it is commonly acknowledged that some stimulation trials are needed prior to the intervention itself in order to determine the optimal location and the motor threshold (37). The potential impact of these prior stimuli on M1 excitability has already been suggested in TMS studies (38); however, this has never been addressed in relation to rPMS. The missing information on rPMS methods could plausibly lead to misinterpretation of clinical outcomes published and a misunderstanding of the mechanisms of action underlying the functional improvement. For instance, coil orientation could impact rPMS-driven neuromodulation, as studied by motor evoked potentials (MEP) for TMS over M1 (39–42) or by the amplitude and latency of somatosensory evoked potentials (SEP) (43–46).
The present study aimed at reaching a consensus among an international panel of rPMS experts regarding the items that should be reported or controlled in any interventional or exploratory publication on rPMS after-effects. The Delphi method was applied and the consensual checklist proposed a minimum set of items that may influence rPMS after-effects. Other methodological biases not related to rPMS methods were not considered and should be assessed by other critical appraisal tools. The use of our checklist will improve rPMS study designs, increase the quality of data collection when rPMS is administrated, and reduce reporting biases related to rPMS methods in any publication (47, 48). The checklist will also promote study reproducibility in rPMS and guide the peer-review process for the assessment of the quality of rPMS methods in future publications.
The Delphi method consists of a structured communication between experts within a specific field in order to reach a consensus of opinion on a designated topic by means of a series of items to assess in two rounds or more (48–50). The Delphi process thus reduces the variability of responses between experts who eventually meet a consensus. As compared to other techniques to reach a consensus among a group of experts, the Delphi method has at least two advantages (49, 51): respect of each expert's anonymity, which minimizes the possibility that an individual influences the outcome, and contribution of international experts (consensus worldwide) via virtual communication (e.g., via email or online questionnaires).
The present Delphi-based study was designed and conducted by a steering committee (the first five co-authors whose research has focused on rPMS in the last years). The initial list of items was developed by the members of the steering committee with respect to exhaustive literature and practical experience, as follows: they proposed an initial selection of items based on all rPMS studies published about either clinical administration (e.g., treatment of a health condition) or underlying mechanisms of action (e.g., with healthy people); this list was then enriched with personal experience with rPMS administration (either as researcher or clinician / end-user). Then they had to exchange on the resulting listing until agreement on which items were considered important to be reported or controlled to improve rPMS methods in any future publication. These items were grouped within four domains (subjects, methodology, stimulation protocol, analyses) that were transcribed on a Google form web-based questionnaire. The steering committee had then to identify a group of experts who will have to respond to the questionnaire and reach a consensus on the relevance of the items to be reported or monitored in any rPMS publication. The mandatory criterion to be eligible as an expert was to have already published in the field of rPMS (at least one publication in a peer-reviewed journal as first, senior, or corresponding author) or to be collaborators recommended by the steering committee and with an acknowledged expertise in rPMS. Publications used by the steering committee to develop the initial list of items but also to retrieve rPMS experts worldwide were selected in PubMed and Google Scholar databases. There was no restriction for time (year of publication) or methodological design and only studies on magnetic stimulation at the periphery in humans and published in English were selected by the title, then the abstract and the text. The following keywords were used: “peripheral magnetic stimulation”; “spinal magnetic stimulation”; “magnetic nerve stimulation”; “magnetic muscle stimulation”; “electromagnetic stimulation”. Additional studies were also hand-searched in the references of the selected publications. Figure 1 presents the number of all rPMS publications found per year until 2020.
The steering committee first compiled and tested the guidelines and items for univocal clarity plus well-functioning of the web-based questionnaire. The corresponding author then email-invited all eligible rPMS experts worldwide to participate in the Delphi study, the rationale, objective and procedures of the work (two-round, timelines) being detailed and the weblink of the questionnaire appended. Each recipient was blinded to the others to maintain anonymity within the panel, i.e., none of them was aware of the other experts invited to complete the questionnaire and who actually completed it. They were asked to declare potential conflict of interests (CoI) and none was declared. Furthermore, CoI could not interfere because the manuscript did no concern any specific medical devices or indications or applications. Precisely, the steering committee and the experts contributed in two rounds.
In the first round, they had to complete the web-based questionnaire, i.e., to judge each item (that has to be reported or controlled or both) by means of a 5-point Likert scale (different scale for reporting vs. monitoring, see below the section Questionnaire).
In the second round, all experts who had completed the first round were emailed again the weblink of the questionnaire, together with the feedback from the first-round responses in terms of an anonymous percent of responders per item (quantitative data) and the answers from the steering committee to specific comments made (qualitative data). It is noteworthy that the questionnaire used for the second round was identical as for the first round and included the items that were scored weaker after the first round or that did not reach the cut-off threshold chosen for analyzing the results (see details in Data reduction and analysis). This gave experts the possibility to adjust/revise/confirm their responses in the light of the first-round feedback and in order to reach a consensus per item.
Each survey round was open for 2 weeks (after the weblink was emailed) and no participant requested more time. Precisely, a reminder was emailed after the first week of the first round to those who agreed to participate but did not respond yet. A different reminder was sent after the first week of the second round if no response was received yet from those who took part in the first round. Another reminder was sent at the deadline of the second round (2 weeks) in the case of still missing responses from participants to the first round. This strategy was in accordance with a previous work using the same methodology but for a TMS checklist (38).
Figure 2 presents the first page of the questionnaire with guidelines and a custom picture for an example of rPMS administration (7-cm outer diameter per wing, biphasic waveform, 400-μs pulse width, device brand anonymized). The questionnaire was structured in three sections.
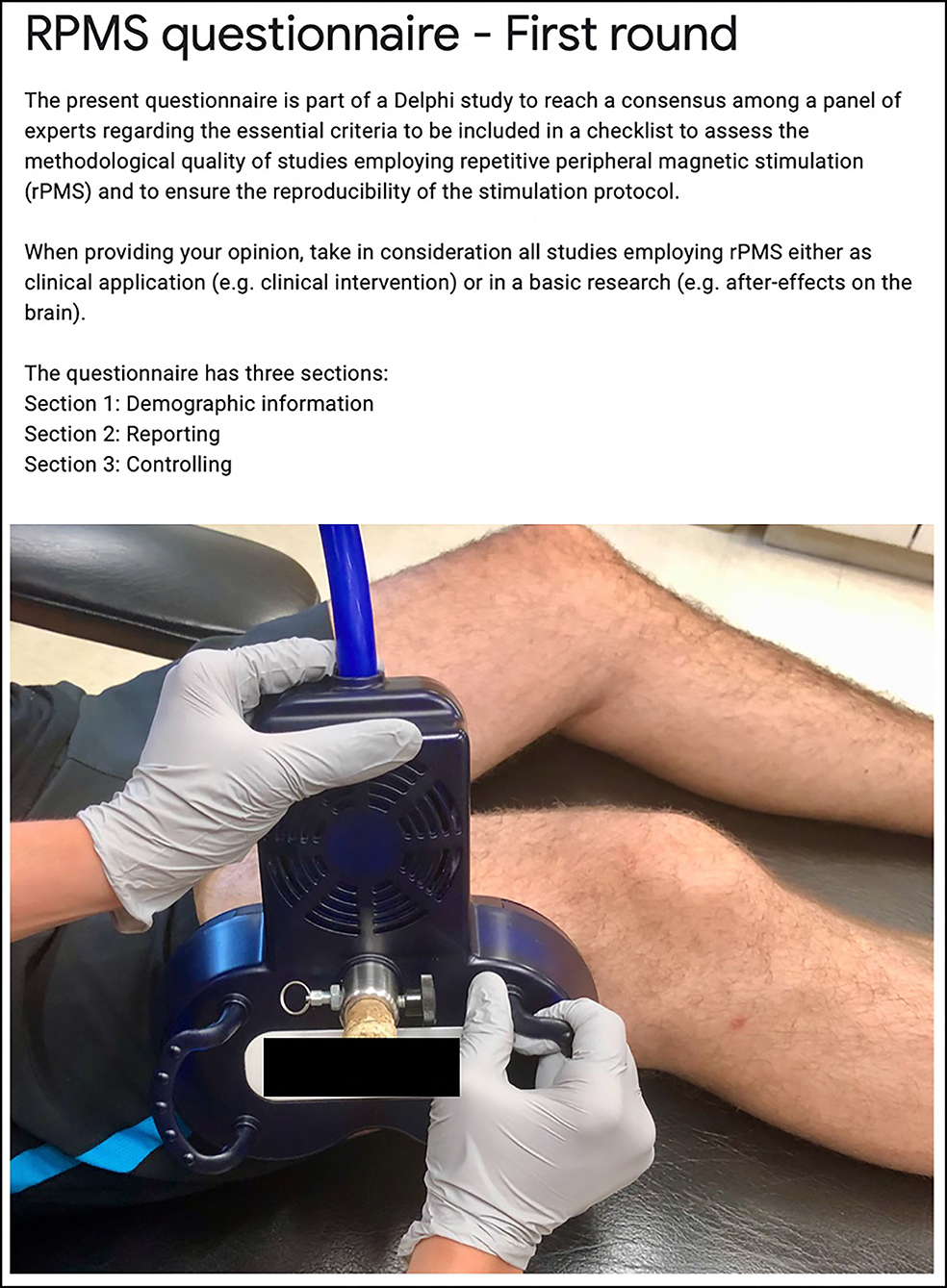
Figure 2. Extract of the first page of the questionnaire for the guidelines to the experts and a custom picture of an example of rPMS administration.
Section Introduction was used only for the first round to collect demographic information about the experts, including sex, main background, last diploma, place of research work, current affiliation and academic / professional position (see Table 1).
Section Methods was used to judge whether each item listed should be reported in any rPMS publication (to acknowledge their influence on rPMS outcomes), i.e., as instructed: “For each item of the list, precise how frequently it should be adequately and clearly reported in a journal publication. Please consider that this concerns any study with rPMS either as clinical application (e.g., clinical intervention) or in basic research (e.g., after-effects on the brain)”. A total of 46 items were categorized within four domains: subjects (14 items), methodology (19 items), stimulation protocol (11 items), and analyses (2 items). Figure 3 was inserted in the stimulation protocol section to illustrate the terminology and avoid any misunderstanding or doubtful signification of descriptive terms. The 5-point Likert scale used in this “reporting” section proposed the responses “unsure”, “never”, “some of the time”, “most of the time” and “always”, in ascending order with scores from 1 to 5, respectively.
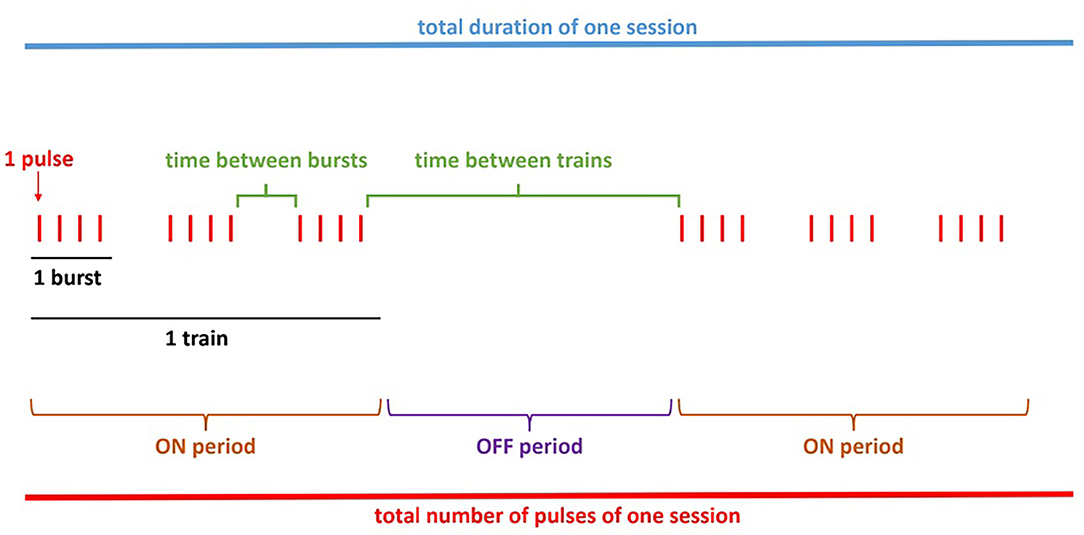
Figure 3. Scheme example of an rPMS stimulation protocol included in the questionnaire. These details aim at avoiding any misunderstanding related to the terminology used.
Section Results was used to judge whether the items should be controlled (to minimize their influence on rPMS outcomes), i.e., as instructed: “For each item of the list, precise how important it would be to control it, either by methodological consistency (e.g., by using the same equipment), research design (e.g., randomization or matching) or by statistical analysis (e.g., as a covariate factor).” This “monitoring” section proposed the same items as in the “reporting” section except two items that cannot be controlled (exclusion criteria related to rPMS; side effects of rPMS), thus a total of 44 items within the same four domains: subjects (13 items), methodology (18 items), stimulation protocol (11 items), and analyses (2 items). The 5-point Likert scale used in this “controlling” section proposed the responses “unsure”, “not important”, “somewhat important”, “important”, and “very important”, in ascending order with scores from 1 to 5, respectively.
The webform also enabled the participants to comment on the terminology used or to suggest other items not present in the questionnaire they completed (“if comments, please specify”) and, as already mentioned, feedback from the steering committee (qualitative data from the first-round responses) was provided for the second round.
Responses from all experts per round, i.e., data collected using the Google Form of the web-based questionnaire, were concatenated into a Microsoft Excel spreadsheet. Three indices were calculated per item: the percent of positive responses, the interquartile range of scores (IQR) and the coefficient of variation of scores (CoV). Statistical analysis was performed with Microsoft Excel Version 16.49 (2021).
The threshold of 75% of “positive” responses (scores 4 or 5 of the Likert scale) was used as a cut-off by the steering committee. This threshold was slightly higher (more severe) than the threshold used in a similar study for a TMS checklist (38). For section Methods, items were rejected (i.e. removed after the second round) if < 75% of the responders chose that they should be reported “most of the time” or “always” (scores 4 and 5 on the Likert scale). For section Results, items were rejected if < 75% of the responders considered them as “important” or “very important” to be controlled (scores 4 and 5 on the Likert scale).
After the second round, the IQR was calculated per item as the absolute value of the difference between the 75th and 25th percentiles of the scores. It is acknowledged that smaller IQR values for an item reflect a higher level of consensus for this item (52). According to previous studies (38, 53), an IQR ≤ 1.00 could be used to select an item for a checklist, in addition to the cut-off related to the percent of positive responses.
The CoV was calculated per item [CoV = standard deviation (SD) / mean score] and a Wilcoxon signed-rank test was used to compare mean CoV between the first and the second rounds. The mean CoV (all items CoV averaged) informed also on the overall variability of responses, i.e., on the general consensus per round.
Nineteen (n = 19) out of the 43 experts (including the members of the steering committee) who were invited to the survey completed the first round (44.1% responsiveness) and 18 participated to the second round (5.3% attrition). The demographic characteristics of the panel are reported in Table 1. The international panel represented seven countries (all affiliations considered), 66.7% held a PhD, 64% got a position in universities or other research institutes, all were trained in medicine, neuroscience, physiology or rehabilitation related fields (e.g., physical therapy).
In the first round, the cut-off of 75% positive responses was reached for 31 items in the Reporting section and 27 items in the Controlling section. Specifically, all responders scored the items from the “Stimulation protocol” with 4 or 5 points on the Likert scale, thus with an immediate maximal consensus to monitor and control them. A few minor changes of terminology were made before the second round according to comments provided. Also, the net effective depth of stimulation was questioned in relation to two potential additional items to report, namely skin thickness and contact pressure of the coil.
After the second round, the cut-off of 75% positive responses was reached for two more items in the Reporting section (total of 33) and four more items in the Controlling section (total of 31). The IQR of all these items was ≤ 1.00, thus informing on a sufficient level of consensus per item. These data are reported in Table 2.
The mean CoV (average of all items CoV) which informed on the overall level of consensus significantly decreased from the first round (mean CoV = 0.214) to the second (mean CoV = 0.160; Z = 514, p < 0.001). SD expressed against the mean score of each item score illustrates the decrease of variability between the first round (Figure 4A) and the second (Figure 4B).
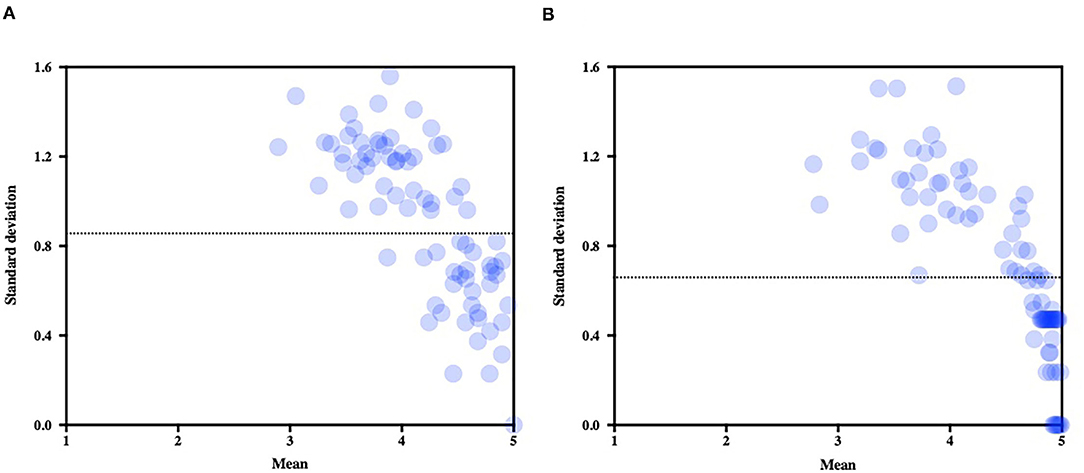
Figure 4. Mean scores (standard deviations expressed against the means) for each item of the questionnaire. Note the decrease of variability after the second round (B) as compared to the first round (A). The dotted line represents the average standard deviation in each round.
Some items did not reach the cut-off of 75% positive responses and were excluded (see Table 3): 2 items in the Reporting section, 2 other items in the Controlling section, and 11 other items in both sections. Thus, these 15 items were not included in the final checklist, which is composed of 8 subject factors, 16 methodological factors, and 11 stimulation protocol factors (see Table 4). A right column titled “Notes” was added in Table 4 to enable comments from the reviewers during the use of the checklist.
The present Delphi study was conducted to reach an international consensus on a checklist for rPMS studies. This checklist ought to represent the minimum dataset of items to be reported or controlled in any interventional or exploratory publication dealing with rPMS. The two rounds were sufficient to reach a consensus on the majority of items, as already suggested (49). The sample of experts obtained (n = 19 for the first round; n = 18 for the second, seven different countries) fitted the size of 10 to 50 experts usually recommended for conducting a Delphi study (54, 55).
The instructions given to the panel of experts (including members of the steering committee) were to provide an opinion for each item listed according to rPMS state-of-the-art and considering the methodological limitations or missing details related to rPMS methods in studies already published. Moreover, the experts were told that the checklist should be the minimum dataset of items to be reported or controlled. Consequently, items that were excluded (due to cut-off of 75% positive responses and IQR > 1.00) are not considered irrelevant and might be included as variables in future rPMS publications. It is indeed conceivable that future research will identify that some new factors can influence the rPMS after-effects or that others do not. This will eventually lead to update the list of factors listed in the present minimum dataset.
For example, the net effective depth of stimulation was questioned after the first round in relation to two potential additional items, one to report (skinfold thickness) and one to report and control (contact pressure of the coil on the skin). The reason was the empiric observation that the efficiency of rPMS of one muscle could be compromised not only by the amount of subcutaneous fat but also by the co-activation of synergistic and antagonistic muscles (e.g., around the knee joint). The latter situation can potentially occur if rPMS intensity is too high or pressure of the coil too strong. Nevertheless, the two potential additional items were not included in the minimum dataset checklist given the challenges to get a standardized measure of skinfold thickness or coil contact pressure. Future publications on rPMS are thus warranted to address this important issue of stimulation efficiency in depth. Similarly, reporting the stimulation intensity in % of maximum stimulator output of the device might not be optimal because this variable, which is not physical, remains difficult to compare between studies that use different stimulators. Rather, the use of the rPMS checklist will help to report more relevant information, such as the motor threshold relative to which the intensity could be expressed, or even the device brand and maximum magnetic field strength, in order to calculate the exact amount of energy delivered.
It is noteworthy that the present checklist focused on the items related to rPMS methods and not to other potential methodological biases (e.g., recruitment, attrition, type of publication, etc.) that could also contribute to variability of results between studies. These other factors could be appropriately assessed by other relevant critical appraisal tools (e.g., (https://casp-uk.net/casp-tools-checklists/) that could be used in combination with our rPMS checklist.
The checklist resulting from the second round's consensus included 35 items in three main domains, namely the subjects (n = 8), methodology (n = 16), and stimulation protocol (n = 11). This checklist is proposed as the minimum dataset to improve the methodological quality of rPMS research. It may be primarily used not only to design an rPMS study, i.e., to properly monitor all items known to potentially impact rPMS after-effects, but also to report the work done as accurately and rigorously as possible. That said, the checklist may guide the peer-review process in assessing the quality of any future study using rPMS. For instance, some factors should be reported while others should be only controlled. Specifically, current evidence on the topic, as it stands, does not provide yet any evidence of an effect of handedness/footedness or side of sensorimotor disorder or pain during an rPMS experiment; however, this should be reported in the manuscript to contribute to the demonstration of a possible impact on rPMS after-effects. Conversely, some items should be monitored for standardization of the methods (and not necessarily reported), as for example the expertise and training of any rPMS operator and the level of a subject's attention/arousal during stimulation. Of note, the number of stimuli required to determine the optimal location of rPMS and the intensity of rPMS (items listed initially in the analyses Section) were excluded from the checklist. As for the TMS checklist published previously (38), these factors were considered to have a potential impact on after-effects, but current evidence and experts of the present Delphi study did not suggest their relevance in the rPMS checklist. Overall, the final checklist warrants, with strong consensus between experts, to report and control items about subjects and related to rPMS application and methodology. This includes items that are rarely reported, such as the direction of the induced current (i.e., coil orientation) or the exact procedure to choose the rPMS intensity. Future research should refine the checklist given the dynamic nature of a consensus between international experts. It is expected also that sham settings will be adapted to the different disorders studied.
The present checklist should be tested, challenged and its usefulness and relevance confirmed by a few pilot studies before being included in upcoming rPMS publications. Also, given rPMS is a relatively newly emerging expensive technique in basic and clinical research, it is still mainly used in laboratories of most technologically advanced countries, thus the geographical distribution of experts was unbalanced, with a lack of representatives from low- and middle-income countries.
Our two-round Delphi study recommends a checklist of 35 items (minimum dataset of items for subjects, methodology, and stimulation protocol) that should be controlled in any rPMS research and reported in any scientific publication about rPMS. The checklist will thus assist researchers to improve methodological quality of rPMS research and will guide the peer-reviewing quality of future work. It is expected that this checklist will be refined in the future owing to new evidence on any parameter impacting rPMS after-effects. For instance, adequate sham settings
The original contributions presented in the study are included in the article. Further inquiries can directed to the corresponding author.
CS and AZ conceived and conducted the study. AZ analyzed the data and wrote the paper. CS revised and edited the manuscript. NS, MB, and L-DB contributed to study design and implementation of the questionnaire. All members of the steering committee and members of the panel of experts (rPMS Consensus Group) fulfilled the questionnaire for the two rounds. All authors approved the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The steering committee thank all experts of the rPMS Consensus Group that took part in the study (in alphabetical order): Akihiko Asao (Japan), Martin Behrens (Germany), Steffen Berweck (Germany), Corinna Börner (Germany), Véronique Flamand (Canada), Hitoshi Kagaya (Japan), Sandro Krieg (Germany), Hatice Kumru (Spain), Berthold Langguth (Germany), Hao Liu (China), Hugo Massé-Alarie (Canada), Martin Schecklmann (Germany), Nicola Smania (Italy), Volker Zschorlich (Germany). The authors acknowledge the support from the Chronic Pain Network (CS funds from the Canadian Institutes for Health Research – strategy for patient-oriented research, #358108) and the Neuroscience Thematic Research Center (part of AZ's PhD studentship).
1. Beaulieu LD, Masse-Alarie H, Camire-Bernier S, Ribot-Ciscar E, Schneider C. After-effects of peripheral neurostimulation on brain plasticity and ankle function in chronic stroke: the role of afferents recruited. Neurophysiol Clin. (2017) 47:275–91. doi: 10.1016/j.neucli.2017.02.003
2. Beaulieu LD, Schneider C. Effects of repetitive peripheral magnetic stimulation on normal or impaired motor control. A review. Neurophysiol Clin. (2013) 43:251–60. doi: 10.1016/j.neucli.2013.05.003
3. Beaulieu LD, Schneider C. Repetitive peripheral magnetic stimulation to reduce pain or improve sensorimotor impairments: a literature review on parameters of application and afferents recruitment. Neurophysiol Clin. (2015) 45:223–37. doi: 10.1016/j.neucli.2015.08.002
4. Massé-Alarie H, Schneider C. Cerebral reorganization in chronic low back pain and neurostimulation to improve motor control. Neurophysiol Clin. (2011) 41:51–60. doi: 10.1016/j.neucli.2011.03.004
5. Allen Demers F, Zangrandi A, Schneider C. Theta burst stimulation over forearm muscles in complex regional pain syndrome (CRPS): influence on brain and clinical outcomes. Front Pain Res. (2021) 2:736806. doi: 10.3389/fpain.2021.736806
6. Arii Y, Sawada Y, Kawamura K, Miyake S, Taichi Y, Izumi Y, et al. Immediate effect of spinal magnetic stimulation on camptocormia in Parkinson's disease. J Neurol Neurosurg Psychiatry. (2014) 85:1221–6. doi: 10.1136/jnnp-2014-307651
7. Beaulieu LD, Masse-Alarie H, Brouwer B, Schneider C. Noninvasive neurostimulation in chronic stroke: a double-blind randomized sham-controlled testing of clinical and corticomotor effects. Top Stroke Rehabil. (2015) 22:8–17. doi: 10.1179/1074935714Z.0000000032
8. Börner C, Urban G, Beaulieu L-D, Sollmann N, Krieg SM, Straube A, et al. The bottom-up approach: non-invasive peripheral neurostimulation methods to treat migraine: a scoping review from the child neurologist's perspective. Eur J Paediatr Neurol. (2021) 32:16–28. doi: 10.1016/j.ejpn.2021.02.008
9. Flamand VH, Beaulieu LD, Nadeau L, Schneider C. Peripheral magnetic stimulation to decrease spasticity in cerebral palsy. Pediatr Neurol. (2012) 47:345–8. doi: 10.1016/j.pediatrneurol.2012.07.005
10. Fujimura K, Kagaya H, Endou C, Ishihara A, Nishigaya K, Muroguchi K, et al. Effects of repetitive peripheral magnetic stimulation on shoulder subluxations caused by stroke: a preliminary study. Neuromodulation. (2020) 23:847–51. doi: 10.1111/ner.13064
11. Krause P, Straube A, Cherington M. Reduction of spastic tone increase induced by peripheral repetitive magnetic stimulation is frequency-independent. Neuro Rehabilitation. (2005) 20:63–5. doi: 10.3233/NRE-2005-20111
12. Kumru H, Albu S, Vidal J, Tormos JM. Effectiveness of repetitive trancranial or peripheral magnetic stimulation in neuropathic pain. Disabil Rehabil. (2017) 39:856–66. doi: 10.3109/09638288.2016.1170213
13. Masse-Alarie H, Beaulieu LD, Preuss R, Schneider C. Repetitive peripheral magnetic neurostimulation of multifidus muscles combined with motor training influences spine motor control and chronic low back pain. Clin Neurophysiol. (2017) 128:442–53. doi: 10.1016/j.clinph.2016.12.020
14. Musaro A, Dobrowolny G, Cambieri C, Onesti E, Ceccanti M, Frasca V, et al. Neuromuscular magnetic stimulation counteracts muscle decline in ALS patients: results of a randomized, double-blind, controlled study. Sci Rep. (2019) 9:2837. doi: 10.1038/s41598-019-39313-z
15. Nielsen JF, Sinkjaer T, Jakobsen J. Treatment of spasticity with repetitive magnetic stimulation; a double-blind placebo-controlled study. Mult Scler. (1996) 2:227–32. doi: 10.1177/135245859600200503
16. Reis Menezes J, Bernhart Carra R, Aline Nunes G, da Silva Simoes J, Jacobsen Teixeira M, Paiva Duarte K, et al. Transcutaneous magnetic spinal cord stimulation for freezing of gait in Parkinson's disease. J Clin Neurosci. (2020) 81:306–9. doi: 10.1016/j.jocn.2020.10.001
17. Renner T, Sollmann N, Heinen F, Albers L, Trepte-Freisleder F, Klose B, et al. Alleviation of migraine symptoms by application of repetitive peripheral magnetic stimulation to myofascial trigger points of neck and shoulder muscles-a randomized trial. Sci Rep. (2020) 10:5954. doi: 10.1038/s41598-020-62701-9
18. Renner T, Sollmann N, Trepte-Freisleder F, Albers L, Mathonia NM, Bonfert MV, et al. Repetitive peripheral magnetic stimulation (rPMS) in subjects with migraine-setup presentation and effects on skeletal musculature. Front Neurol. (2019) 10:738. doi: 10.3389/fneur.2019.00738
19. Sakai K, Yasufuku Y, Kamo T, Ota E, Momosaki R. Repetitive peripheral magnetic stimulation for patients after stroke. Stroke. (2020) 51:e105–6. doi: 10.1161/STROKEAHA.120.029373
20. Smania N, Corato E, Fiaschi A, Pietropoli P, Aglioti SM, Tinazzi M. Repetitive magnetic stimulation: a novel therapeutic approach for myofascial pain syndrome. J Neurol. (2005) 252:307–14. doi: 10.1007/s00415-005-0642-1
21. Sollmann N, Trepte-Freisleder F, Albers L, Jung NH, Mall V, Meyer B, et al. Magnetic stimulation of the upper trapezius muscles in patients with migraine-a pilot study. Eur J Paediatr Neurol. (2016) 20:888–97. doi: 10.1016/j.ejpn.2016.07.022
22. Werner C, Schrader M. Repetitive peripheral magnetic stimulation (rpMS) in combination with muscle stretch decreased the wrist and finger flexor muscle spasticity in chronic patients after CNS Lesion. Int J Phys Med Rehabil. (2016) 4:352. doi: 10.4172/2329-9096.1000352
23. Zangrandi A, Allen Demers F, Schneider C. Complex regional pain syndrome (CRPS). A comprehensive review on neuroplastic changes supporting the use of noninvasive neurostimulation in clinical settings. Front Pain Res. (2021) 2:732343. doi: 10.3389/fpain.2021.732343
24. Zschorlich VR, Hillebrecht M, Tanjour T, Qi F, Behrendt F, Kirschstein T, et al. Repetitive peripheral magnetic nerve stimulation (rPMS) as adjuvant therapy reduces skeletal muscle reflex activity. Front Neurol. (2019) 10:930. doi: 10.3389/fneur.2019.00930
25. Flamand VH, Schneider C. Noninvasive and painless magnetic stimulation of nerves improved brain motor function and mobility in a cerebral palsy case. Arch Phys Med Rehabil. (2014) 95:1984–90. doi: 10.1016/j.apmr.2014.05.014
26. Kinoshita S, Ikeda K, Hama M, Suzuki S, Abo M. Repetitive peripheral magnetic stimulation combined with intensive physical therapy for gait disturbance after hemorrhagic stroke: an open-label case series. Int J Rehabil Res. (2020) 43:235–9. doi: 10.1097/MRR.0000000000000416
27. Leung A, Fallah A, Shukla S. Transcutaneous magnetic stimulation (TMS) in alleviating post-traumatic peripheral neuropathic pain States: a case series. Pain Med. (2014) 15:1196–9. doi: 10.1111/pme.12426
28. Momosaki R, Abo M, Watanabe S, Kakuda W, Yamada N, Kinoshita S. Repetitive peripheral magnetic stimulation with intensive swallowing rehabilitation for poststroke dysphagia: an open-label case series. Neuromodulation. (2015) 18:630–5. doi: 10.1111/ner.12308
29. Savulescu SE, Grozoiu L, Popa F, Dumitru L, Berteanu M. Peripheral repetitive magnetic stimulation: a novel approach for hand rehabilitation in carpal tunnel syndrome - a pilot study. International Journal of Social Science and Humanity. (2016) 6:604–7. doi: 10.7763/IJSSH.2016.V6.718
30. Heldmann B, Kerkhoff G, Struppler A, Havel P, Jahn T. Repetitive peripheral magnetic stimulation alleviates tactile extinction. Neuroreport. (2000) 11:3193–8. doi: 10.1097/00001756-200009280-00029
31. Sato T, Nagai H. Sacral magnetic stimulation for pain relief from pudendal neuralgia and sciatica. Dis Colon Rectum. (2002) 45:280–2. doi: 10.1007/s10350-004-6162-8
32. Pujol J, Pascual-Leone A, Dolz C, Delgado E, Dolz JL, Aldoma J. The effect of repetitive magnetic stimulation on localized musculoskeletal pain. Neuroreport. (1998) 9:1745–8. doi: 10.1097/00001756-199806010-00014
33. Bustamante V, Lopez de Santa Maria E, Gorostiza A, Jimenez U, Galdiz JB. Muscle training with repetitive magnetic stimulation of the quadriceps in severe COPD patients. Respir Med. (2010) 104:237–45. doi: 10.1016/j.rmed.2009.10.001
34. Jia Y, Liu X, Wei J, Li D, Wang C, Wang X, et al. Modulation of the corticomotor excitability by repetitive peripheral magnetic stimulation on the median nerve in healthy subjects. Front Neural Circuits. (2021) 15:616084. doi: 10.3389/fncir.2021.616084
35. Nito M, Katagiri N, Yoshida K, Koseki T, Kudo D, Nanba S, et al. Repetitive peripheral magnetic stimulation of wrist extensors enhances cortical excitability and motor performance in healthy individuals. Front Neurosci. (2021) 15:632716. doi: 10.3389/fnins.2021.632716
36. Khedr EM, Ahmed MA, Alkady EA, Mostafa MG, Said HG. Therapeutic effects of peripheral magnetic stimulation on traumatic brachial plexopathy: clinical and neurophysiological study. Neurophysiol Clin. (2012) 42:111–8. doi: 10.1016/j.neucli.2011.11.003
37. Rossi S, Antal A, Bestmann S, Bikson M, Brewer C, Brockmoller J, et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: expert guidelines. Clin Neurophysiol. (2021) 132:269–306. doi: 10.1016/j.clinph.2020.10.003
38. Chipchase L, Schabrun S, Cohen L, Hodges P, Ridding M, Rothwell J, et al. A checklist for assessing the methodological quality of studies using transcranial magnetic stimulation to study the motor system: an international consensus study. Clinical Neurophysiology. (2012) 123:1698–704. doi: 10.1016/j.clinph.2012.05.003
39. Al-Mutawaly N, de Bruin H, Hasey G. The effects of pulse configuration on magnetic stimulation. J Clin Neurophysiol. (2003) 20:361–70. doi: 10.1097/00004691-200309000-00008
40. Goetz SM, Herzog HG, Gattinger N, Gleich B. Comparison of coil designs for peripheral magnetic muscle stimulation. J Neural Eng. (2011) 8:056007. doi: 10.1088/1741-2560/8/5/056007
41. Maccabee PJ, Amassian VE, Cracco RQ, Cracco JB, Eberle L, Rudell A. Stimulation of the human nervous system using the magnetic coil. J Clin Neurophysiol. (1991) 8:38–55. doi: 10.1097/00004691-199101000-00006
42. Matsumoto H, Hanajima R, Terao Y, Ugawa Y. Magnetic-motor-root stimulation: review. Clin Neurophysiol. (2013) 124:1055–67. doi: 10.1016/j.clinph.2012.12.049
43. Kunesch E, Knecht S, Classen J, Roick H, Tyercha C, Benecke R. Somatosensory evoked potentials (SEPs) elicited by magnetic nerve stimulation. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section. (1993) 88:459–67. doi: 10.1016/0168-5597(93)90035-N
44. Maccabee PJ, Eberle L, Amassian VE, Cracco RQ, Rudell A. Spatial distribution of the electric field induced in volume by round and figure ‘8' magnetic coils: relevance to activation of sensory nerve fibers. Electroencephalogr Clin Neurophysiol. (1990) 76:131–41. doi: 10.1016/0013-4694(90)90211-2
45. Olney RK, So YT, Goodin DS, Aminoff MJ. A comparison of magnetic and electrical stimulation of peripheral nerves. Muscle and Nerve. (1990) 13:957–63. doi: 10.1002/mus.880131012
46. Tsuji S, Murai Y, Yarita M. Cortical somatosensory potentials evoked by magnetic stimulation of thoracic and lumbar roots. Neurology. (1993) 43:391–6. doi: 10.1212/WNL.43.2.391
47. Minnerup J, Dirnagl U, Schäbitz W-R. Checklists for authors improve the reporting of basic science research. Stroke. (2020) 51:6–7. doi: 10.1161/STROKEAHA.119.027626
48. Okoli C, Pawlowski SD. The Delphi method as a research tool: an example, design considerations and applications. Inf Manag. (2004) 42:15–29. doi: 10.1016/j.im.2003.11.002
49. Hasson F, Keeney S, McKenna H. Research guidelines for the Delphi survey technique. J Adv Nurs. (2000) 32:1008–15. doi: 10.1046/j.1365-2648.2000.t01-1-01567.x
50. Vernon W. The Delphi technique: a review. Int J Ther Rehabil. (2009) 16:69–76. doi: 10.12968/ijtr.2009.16.2.38892
51. Avella JR. Delphi panels: research design, procedures, advantages, and challenges. Int J Dr Stud. (2016) 11:305–21. doi: 10.28945/3561
52. Rayens MK, Hahn EJ. Building consensus using the policy Delphi method. Policy Polit Nurs Pract. (2000) 1:308–15. doi: 10.1177/152715440000100409
53. Raskin MS. The Delphi study in field instruction revisited: expert consensus on issues and research priorities. J Soc Work Educ. (1994) 30:75–89. doi: 10.1080/10437797.1994.10672215
54. Ogbeifun E, Agwa-Ejon J, Mbohwa C, Pretorius J. The Delphi technique: A credible research methodology. In: International Conference on Industrial Engineering and Operations Management. Kuala Lumpur (2016).
Keywords: repetitive peripheral magnetic stimulation, Delphi study, rPMS checklist, non-invasive neurostimulation, peripheral neurostimulation
Citation: Schneider C, Zangrandi A, Sollmann N, Bonfert MV, Beaulieu L-D and the rPMS Consensus Group (2022) Checklist on the Quality of the Repetitive Peripheral Magnetic Stimulation (rPMS) Methods in Research: An International Delphi Study. Front. Neurol. 13:852848. doi: 10.3389/fneur.2022.852848
Received: 11 January 2022; Accepted: 28 February 2022;
Published: 22 March 2022.
Edited by:
Toshiyuki Fujiwara, Juntendo University, JapanReviewed by:
Michiyuki Kawakami, Keio University School of Medicine, JapanCopyright © 2022 Schneider, Zangrandi, Sollmann, Bonfert, Beaulieu and the rPMS Consensus Group. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cyril Schneider, Y3lyaWwuc2NobmVpZGVyQHJlYS51bGF2YWwuY2E=
†These authors have contributed equally to this work and share first authorship
‡See Acknowledgments
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.