- 1Department of Rehabilitation Sciences, School of Public Health and Health Professions, State University of New York at Buffalo, Buffalo, NY, United States
- 2Department of Radiology, Nanjing First Hospital, Nanjing Medical University, Nanjing, China
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a high transmissible infectious disease that primarily impacts the respiratory system and leads to death as it worsens. Ever since the World Health Organization declared the disease as a global pandemic, the pathophysiology, clinical manifestations, and disease prognosis has been discussed in various literature. In addition to impaired respiratory health, the symptoms also indicated the involvement of the cardiovascular and neurological system after SARS-CoV-2 infection. Despite the pulmonary, cardiovascular, and neurological complications, many reports also revealed the prevalence of vestibulocochlear symptoms like dizziness, vertigo, vestibular neuritis, sudden sensorineural hearing loss, and tinnitus. Though many clinical reports and scientific reviews reported the vestibular and cochlear impairments associated with coronavirus disease 2019 (COVID-19) infection, the underlying pathological mechanisms are still unclear and unexplored. In this review, we discussed the published clinical reports, research articles, and literature reviews related to vestibulocochlear manifestations following SARS-CoV-2 infections. We also summarized the current knowledge about the prevalence, epidemiological and clinical features, and potential pathological mechanisms related to vestibular and cochlear manifestations resulting from COVID-19 infections.
Introduction
The novel coronavirus disease 2019 (COVID-19) infection caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), severely impairs the human respiratory system and its clinical manifestation includes cough, fever, and fatigues (1). In December 2019, the outbreak was identified as pneumonia cases with an unknown cause in Wuhan, China, the WHO declared the infection as a pandemic in March 2020 (2). As of March 2022, the pandemic has caused 440 million confirmed cases with 5.9 million deaths worldwide and is still persistent (https://covid19.who.int/).
Many countries are developing vaccines that mainly target the SARS-CoV-2 spike protein (S protein), a viral surface protein that enters the human cells (3). Even with significant protective measures, the disease is spreading relentlessly worldwide and causing health and socioeconomic burden. The COVID-19 pandemic also impacts the mental health of a larger population due to lockdowns and lifestyle modification-mediated stress, anxiety, depression, and insomnia (4).
The majority of COVID-19 infected cases are asymptomatic in the early stage of disease progression and affected predominantly with respiratory or extrapulmonary symptoms in subsequent stages (5). It is becoming apparent through clinical observations that patients with COVID-19 infection also exhibit neurological and otological symptoms (6, 7). With a high prevalent rate along with its asymptomatic and atypical nature, the COVID-19 is posing a huge burden and challenge to the healthcare communities. So there is a critical need for a detailed understanding of its demographics, transmission risk, symptoms, clinical outcomes, and manifestations in a broader manner.
In this review, we summarize the research reports and findings related to neurological, vestibular, and auditory complications in the patients affected with COVID-19 and discuss the potential pathological mechanisms.
Review Methods
Case reports, case series, and multicentral studies were sought from PubMed and Google using keywords, “coronavirus or COVID and neurological symptoms, audiological symptoms including hearing loss, tinnitus, vestibular symptoms including dizziness and vertigo” were included for this review. Self-reported case reports and observational studies without any clinical examination or tests were excluded from this review.
Neurological Manifestations in COVID-19 Patients
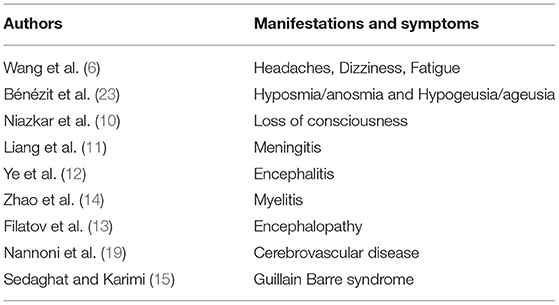
While COVID-19 infections primarily affect the respiratory system, many clinical observations and symptoms demonstrate its extrapulmonary involvement in cardiovascular, digestive, hematological, endocrine, excretory, and neurological systems (5, 8, 9). Recent evidence are indicating the increasing neurological manifestations in central and peripheral nervous systems with symptoms, including headaches, dizziness, fatigue, and loss of consciousness (10). In addition, other neurological manifestations like meningitis (11), encephalitis (12), encephalopathy (13), myelitis (14), and Guillain Barre syndrome (15), in patients with COVID-19, suggesting its neuroinfectious properties. Many clinical cases demonstrated the potential involvement of COVID-19 in acute ischemic stroke (AIS) (16–19). It is predicted that the stroke risk is increased to 3.2- to 7.8-fold after the first three days of COVID-19 infection (20, 21). Evidence also implied that COVID-19 infection may lead to arterial thrombosis through endothelial dysfunction, thrombin formation, and platelet activation (22). The neurological symptoms are identified as an initial COVID-19 presentation in many patients and the prevalence increases with the severity of the disease. The neurological manifestations and symptoms associated with COVID-19 are listed in Table 1.
Otological Manifestations in COVID-19 Patients
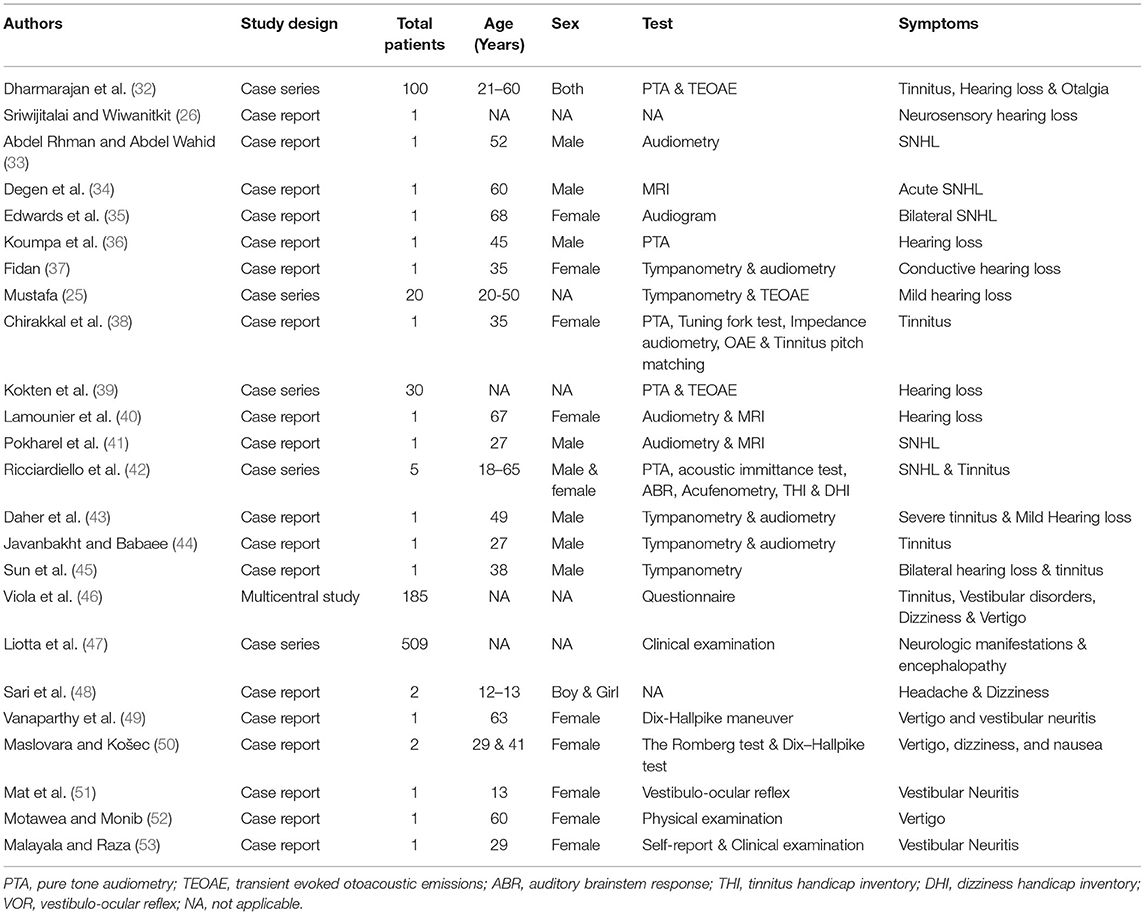
The COVID-19 infection also presented with many early otolaryngological symptoms, like throat infections, dyspnoea, cough, along the sudden onset of anosmia and ageusia (23, 24). In addition, few case reports have documented the adverse otologic (25–28) and vestibular manifestations (29–31) after COVID-19 infection. The detailed otological information in each report was shown in Table 2.
Auditory Manifestations
Hearing loss after viral infections are conductive or sensorineural types caused and being hypothesized to cause direct or indirect damage to inner ear structures. Studies have highlighted the neurotropic and neuroinvasive nature of the COVID-19 virus (54), and the infection has been considered a plausible cause for hearing loss. Auditory symptoms, including hearing loss and tinnitus, are being frequently reported along with other usual symptoms in patients with COVID-19 (32, 55, 56). Being a neglected symptom, screening for hearing is being encouraged in the patients with COVID-19. The first case of sensorineural hearing loss (SNHL), in a COVID-19-recovered elderly female patient, was reported in Thailand (26). Following this, a few other reports also stated the association between the SNHL and COVID-19 infection (33–36). Similarly, a case report demonstrated a unilateral conductive hearing loss and tinnitus in a 35-year-old female asymptomatic patient with COVID-19. The otoscopic examination revealed the acute otitis media mediated bulging tympanic membrane (37). In another study, asymptomatic patients with COVID-19 have significantly worsened high-frequency pure tone thresholds and transient evoked otoacoustic emission (TEOAE) amplitudes when compared to control subjects (25). In addition, a recent also study confirmed hearing impairment especially at 1,000 Hz through TEOAE tests (39).
Tinnitus is another significant clinical symptom additional to hearing loss, vertigo, and dizziness in patients with COVID-19 infection. Chirakkal et al. (38) have reported that unilateral tinnitus was observed at 4 kHz at 10 dB using frequency and intensity matching evaluation in a 35-year-old female patient with COVID-19. This study also demonstrated the detrimental effects of COVID-19 infection on cochlea outer hair cells, which was evident through TEOAE and distortion-product otoacoustic emissions amplitudes. Many case reports and reviews have reported sudden hearing loss and disabling tinnitus in a patient with severe COVID-19 infection (27, 40–42, 57). Furthermore, sudden unilateral hearing loss with worsening tinnitus was observed in a 52-year-old male physician affected with COVID-19 without any history of head trauma or ototoxic medications (33). Another study has reported bilateral tinnitus in a 60-year-old patient with COVID-19 having signs of inflammation in both cochleae through MRI findings (34). Multiple case reports and meta-analysis reviews have shown the prevalence of tinnitus in patients with COVID-19 (43–46, 56, 58, 59).
A case of sensorineural hearing loss and tinnitus was reported in the unilateral ear 2 days after administration of the Oxford-AstraZeneca (VAXZEVRIA) vaccine in a 57-year-old male patient (60). In another case study, the SSHL was demonstrated through pure tone audiometry 2 days after the second dose of Oxford-AstraZeneca vaccine in a 61-year-old female (61). But the hearing loss was recovered after 15 days with a treatment of glucocorticoids and acetylsalicylic acid. Furthermore, a 37-year-old male patient was diagnosed with tinnitus and cochleopathy after receiving his first AstraZeneca COVID-19 vaccine dose, which was reversed by dexamethasone and prednisolone treatment (62). Another case series have reported the transient sudden unilateral tinnitus after BNT162b2 mRNA-vaccine (e.g., pfizer), which resolved rapidly in 2 out of 3 cases (63). Recently, many case reports have reported a prevalence of sudden hearing loss and tinnitus after the COVID-19 vaccinations (64–68).
Vestibular Manifestations
Though the involvement of COVID-19 infection in otologic manifestations has not been confirmed yet, many case reports are providing preliminary evidence to emphasize the potential association between the COVID-19 infection and ear disorders. Along with auditory manifestations, a few vestibular symptoms like dizziness, vertigo, and tinnitus are described as the common clinical manifestations in patients with COVID-19 (47, 69). Many case studies have reported dizziness as a prevalent neurological symptom post-COVID-19 infection (47, 69, 70). In addition, many other reviews also reported dizziness as a common clinical manifestation along with other vertigo, hearing loss, and tinnitus (56, 71). A recent case report has stated the manifestation of dizziness in two COVID-19-affected children (12–13 years), which resolved in a week (48). Vestibular neuritis is a vestibular disorder that causes vertigo, dizziness, and balance problems, and is diagnosed in patients with COVID-19 (49). In addition, a few case reports also demonstrated vertigo as an important clinical manifestation of COVID-19 (50–53).
Discussion
Association between various types of viral infections and hearing loss have been implied for years. Hearing loss is a well-known complication of bacterial and viral meningitis (72) and some viral infections can cause SNHL (73, 74).
Many researchers have discussed the neuroinvasive and neurotropic properties of SARS-CoV-2 (54, 75, 76), which has been linked the post-COVID neurological manifestations. Almost every coronavirus variant has a similar structure and infection mechanisms. Earlier reports have confirmed the presence of SARS-CoV in cerebrospinal fluid of infected patients (77, 78).
SARS-CoV-2 primarily enters the body through the angiotensin-converting enzyme-2 receptor (ACE2) in the respiratory epithelium. The virus is replicated and enters the circulation by attaching to the β chain of hemoglobin in erythrocytes, and transported and binds to several organs (79).
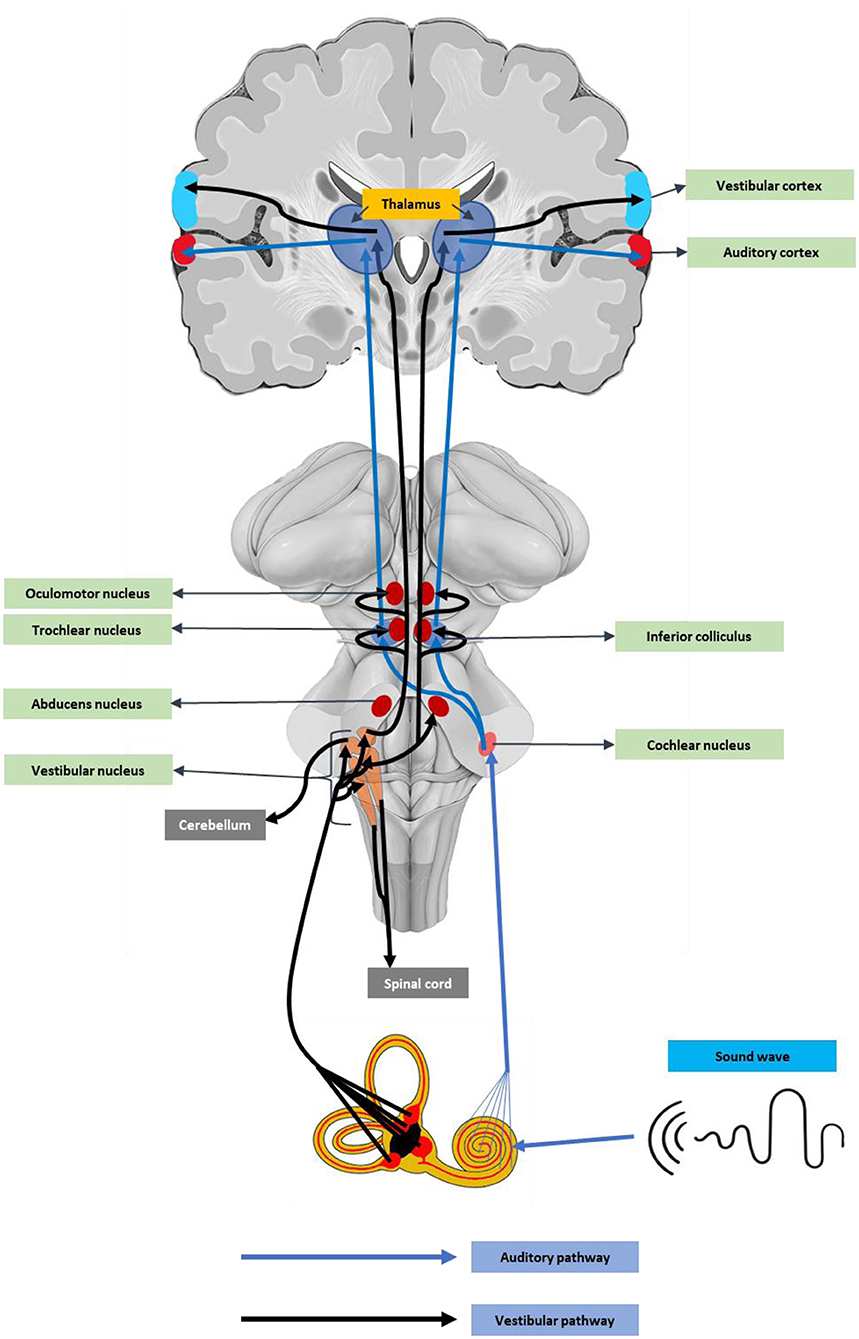
Angiotensin-converting enzyme-2 receptor (ACE2) receptors are abundant in neurons and glial cells of various brain regions like the cortex, striatum, substantia nigra, and the brainstem (80), suggesting the neuronal damage potential of SARS-CoV-2. In addition, the ACE2 receptors, including the medulla oblongata and temporal lobe (81), are key auditory regions. These auditory centers could be affected by cytokine release-mediated inflammatory responses (82). Recent reports also stated that SARS-CoV-2 can directly enter the brain through the olfactory epithelium or the cribriform bone (83). An earlier experimental study using a human ACE2 overexpressed mouse model, the intranasal SARS, SARS-CoV-1 infection caused neuronal death in the brainstem regions (84, 85). As the brainstem contains vital components of the auditory pathway (Figure 1), damage to these regions can cause hearing complications and deafness.
The blood-brain barrier is a physical barrier that prevents the entry of any harmful and infectious substances. Recent evidence also demonstrated that the spike protein of SARS-CoV-2 was able to cross the blood-brain barrier in male mice when it is administrated intravenously and intranasally (86). Adding to this, many research reports and review articles also cite the evidence of blood-brain barrier disruption caused by SARS-CoV-2 infection through upregulation of proinflammatory mediators (87–90). With a compromised blood-brain barrier, the SARs-CoV-2 can easily enter the brain parenchyma and lead to exacerbated brain pathology and neurological manifestations, including hearing and vestibular complications.
Another possible hypothesis of hearing loss and vestibular complications is hypoxia caused by the hyperfusion-mediated ischemia in the inner ear structures. Numerous clinical and experimental reports have confirmed that the SARS-CoV-2 infection enhances the chances of thrombus formation in the circulation and leads to increased risk for cerebrovascular diseases (91–96). SARS-CoV-2 can cause hypoxia by deoxygenating the binding erythrocytes. As vascular smooth muscles contain ACE2 receptors, the SARS-CoV-2 infection can form a blood clot in the blood vessels supplying the inner ear, thus leading to ischemic damage and subsequent hearing loss and vestibular impairments. According to the ischemia theory, the geriatric population is mainly prone to SARS-CoV-2-mediated otological complications (97).
A recent research finding has demonstrated the involvement of direct viral infections in the inner ear tissues as a potential cause for auditory and vestibular dysfunctions after COVID-19 infection (98). Varying degree of hearing impairment by direct or indirect damage to inner ear components following viral infections can be reversed with antiviral drugs. But, ototoxicity by specific drugs to treat SARS-CoV-2 infection, could be a potential cause for negative auditory and vestibular manifestations of COVID-19 treatments. Drugs like hydroxychloroquine used in early pandemics were proved to be ototoxic and cause SNHL, tinnitus, and balance issues (99). In addition, other drugs like azithromycin (100), Remdesivir, Favipiravir (101), and Lopinavir (102) used to combat COVID-19 have been proved to cause ototoxicity.
Conclusion
The novel coronavirus disease 2019 (COVID-19) pandemic became the major healthcare challenge in recent human history. Although respiratory and cardiovascular are identified as characteristic features, neurological and otological manifestations are being frequently reported in patients with COVID-19. These atypical symptoms can severely affect the long-term outcomes and impair the post-COVID-19 life quality. However, current data available on inner ear disorders associated with COVID-19 and studies describing the possible pathophysiology remain unclear and limited. Thus, there is a critical need for early screening and clinical laboratory diagnosis to identify the auditory and vestibular disorders to manage the disease effectively. Furthermore, it is crucial to ascertain the potential ototoxic properties of the drug used to manage COVID-19 to avoid permanent hearing and vestibular disorders.
Author Contributions
KK checked the references and wrote the manuscript. Y-CC and VK contributed to the discussion and manuscript revision. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Natural Science Foundation of Jiangsu Province (No. BK20211008) and Medical Science and Technology Development Foundation of Nanjing Department of Health (No. ZKX20037).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
2. Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Bio Medica: Atenei Parmensis. (2020) 91:157. doi: 10.23750/abm.v91i1.9397
3. Huang Y, Yang C, Xu Xf, Xu W, Liu S. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacologica Sinica. (2020) 41:1141–1149. doi: 10.1038/s41401-020-0485-4
4. Torales J, O'Higgins M, Castaldelli-Maia JM, Ventriglio A. The outbreak of COVID-19 coronavirus and its impact on global mental health. Int J Soc Psychiatry. (2020) 66:317–20. doi: 10.1177/0020764020915212
5. Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med. (2020) 26:1017–32. doi: 10.1038/s41591-020-0968-3
6. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. Jama. (2020) 323:1061–9. doi: 10.1001/jama.2020.1585
7. Korkmaz MÖ, Egilmez OK, Özçelik MA. Otolaryngological manifestations of hospitalised patients with confirmed COVID-19 infection. Euro Archiv Oto-Rhino-Laryngol. (2021) 278:1675–85. doi: 10.1007/s00405-020-06396-8
8. Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. New Engl J Med. (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
9. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
10. Niazkar HR, Zibaee B, Nasimi A, Bahri N. The neurological manifestations of COVID-19: a review article. Neurologic Sci. (2020) 41:1667–71. doi: 10.1007/s10072-020-04486-3
11. Liang W, Jia CAI, Huating LUO, Hongzhi G, Hongzhi W, Huang C, et al. A case of coronavirus disease 2019 with tuberculous meningitis. Chinese J Neurol. (2020) 20:E004. doi: 10.3760/cma.j.cn113694-20200302-00134
12. Ye M, Ren Y, Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav Immun. (2020) 88:945–6. doi: 10.1016/j.bbi.2020.04.017
13. Filatov A, Sharma P, Hindi F, Espinosa PS. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. (2020) 12. doi: 10.7759/cureus.7352
14. Zhao K, Huang J, Dai D, Feng Y, Liu L, Nie S. Acute myelitis after SARS-CoV-2 infection: a case report. MedRxiv. (2020). doi: 10.1101/2020.03.16.20035105
15. Sedaghat Z, Karimi N. Guillain Barre syndrome associated with COVID-19 infection: a case report. J Clinic Neurosci. (2020) 76:233–5. doi: 10.1016/j.jocn.2020.04.062
16. Beyrouti R, Adams ME, Benjamin L, Cohen H, Farmer SF, Goh YY, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatr. (2020) 91:889–91. doi: 10.1136/jnnp-2020-323586
17. Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. New Engl J Med. (2020) 382:e60. doi: 10.1056/NEJMc2009787
18. Yaghi S, Ishida K, Torres J, Mac Grory B, Raz E, Humbert K, et al. SARS-CoV-2 and stroke in a New York healthcare system. Stroke. (2020) 51:2002–11. doi: 10.1161/STROKEAHA.120.030335
19. Nannoni S, de Groot R, Bell S, Markus HS. Stroke in COVID-19: a systematic review and meta-analysis. Int J Stroke. (2021) 16:137–49. doi: 10.1177/1747493020972922
20. Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. New Engl J Med. (2004) 351:2611–8. doi: 10.1056/NEJMoa041747
21. Warren-Gash C, Blackburn R, Whitaker H, McMenamin J, Hayward AC. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Euro Respirat J. (2018) 18:51. doi: 10.1183/13993003.01794-2017
22. Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. (2020) 75:2950–73. doi: 10.1016/j.jacc.2020.04.031
23. Bénézit F, Le Turnier P, Declerck C, Paillé C, Revest M, Dubée V, et al. Utility of hyposmia and hypogeusia for the diagnosis of COVID-19. Lancet Infect Dis. (2020) 20:1014–5. doi: 10.1016/S1473-3099(20)30297-8
24. Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Euro Archiv Oto-Rhino-Laryngol. (2020) 277:2251–61. doi: 10.1007/s00405-020-05965-1
25. Mustafa MWM. Audiological profile of asymptomatic Covid-19 PCR-positive cases. Am J Otolaryngol. (2020) 41:102483. doi: 10.1016/j.amjoto.2020.102483
26. Sriwijitalai W, Wiwanitkit V. Hearing loss and COVID-19: A note. Am J Otolaryngol. (2020) 41:102473. doi: 10.1016/j.amjoto.2020.102473
27. Chern A, Famuyide AO, Moonis G, Lalwani AK. Bilateral Sudden Sensorineural Hearing Loss and Intralabyrinthine Hemorrhage in a Patient With COVID-19. Otol Neurotol. (2021) 42:e10–4. doi: 10.1097/MAO.0000000000002860
28. Zahran M, Ghazy R, Ahmed O, Youssef A. Atypical otolaryngologic manifestations of COVID-19: a review. Egypt J Otolaryngol. (2021) 37:1–4. doi: 10.1186/s43163-021-00075-z
29. Giannantonio S, Scorpecci A, Montemurri B, Marsella P. Case of COVID-19-induced vestibular neuritis in a child. BMJ Case Reports CP. (2021) 14:e242978. doi: 10.1136/bcr-2021-242978
30. Malayala SV, Mohan G, Vasireddy D, Atluri P. A case series of vestibular symptoms in positive or suspected COVID-19 patients. Infez Med. (2021) 29:117–22.
31. Tan M, Cengiz DU, Demir I, Demirel S, Çolak SC, Karakaş O, et al. Effects of Covid-19 on the audio-vestibular system. Am J Otolaryngol. (2021) 43:103173. doi: 10.1016/j.amjoto.2021.103173
32. Dharmarajan S, Bharathi M, Sivapuram K, Prakash B, Madhan S, Madhu A, et al. Hearing Loss-a Camouflaged Manifestation of COVID 19 Infection. Indian J Otolaryngol Head Neck Surg. (2021) 11:1–5. doi: 10.1007/s12070-021-02581-1
33. Abdel Rhman S, Abdel Wahid A. COVID−19 and sudden sensorineural hearing loss, a case report. Otolaryngology Case Reports. (2020) 16:100198–100198. doi: 10.1016/j.xocr.2020.100198
34. Degen C, Lenarz T, Willenborg K. Acute Profound Sensorineural Hearing Loss After COVID-19 Pneumonia. Mayo Clin Proc. (2020) 95:1801–3. doi: 10.1016/j.mayocp.2020.05.034
35. Edwards M, Muzaffar J, Naik P, Coulson C. Catastrophic bilateral sudden sensorineural hearing loss following COVID-19. BMJ Case Rep. (2021) 14:2315. doi: 10.1136/bcr-2021-243157
36. Koumpa FS, Forde CT, Manjaly JG. Sudden irreversible hearing loss post COVID-19. BMJ Case Rep. (2020) 13. doi: 10.1136/bcr-2020-238419
37. Fidan V. New type of corona virus induced acute otitis media in adult. Am J Otolaryngol. (2020) 41:102487. doi: 10.1016/j.amjoto.2020.102487
38. Chirakkal P, Al Hail AN, Zada N, Vijayakumar DS. COVID-19 and Tinnitus. Ear, Nose and Throat J. (2021) 100:160S-162S. doi: 10.1177/0145561320974849
39. Kokten N, Celik S, Mutlu A, Pektas E, Icten S, Kalcioglu MT. Does COVID-19 have an impact on hearing? Acta Otolaryngol. (2022) 142:48–51. doi: 10.1080/00016489.2021.2020897
40. Lamounier P, Franco Gonçalves V, Ramos HVL, Gobbo DA, Teixeira RP, Dos Reis PC, et al. A 67-Year-Old Woman with Sudden Hearing Loss Associated with SARS-CoV-2 Infection. Am J Case Rep. (2020) 21:e927519. doi: 10.12659/AJCR.927519
41. Pokharel S, Tamang S, Pokharel S, Mahaseth RK. Sudden sensorineural hearing loss in a post-COVID-19 patient. Clinical Case Rep. (2021) 9:e04956. doi: 10.1002/ccr3.4956
42. Ricciardiello F, Pisani D, Viola P, Cristiano E, Scarpa A, Giannone A, et al. Sudden sensorineural hearing loss in mild covid-19: case series and analysis of the literature. Audiol Res. (2021) 11:313–26. doi: 10.3390/audiolres11030029
43. Daher GS, Nassiri AM, Vanichkachorn G, Carlson ML, Neff BA, Driscoll CLW. New onset tinnitus in the absence of hearing changes following COVID-19 infection. Am J Otolaryngol. (2022) 43:103208. doi: 10.1016/j.amjoto.2021.103208
44. Javanbakht M, Babaee S. Tinnitus in COVID-19 Patients; a Case Study. Iran J War Public Health. (2021) 13:79−83. doi: 10.29252/acadpub.ijwph.13.1.79
45. Sun R, Liu H, Wang X. Mediastinal emphysema, giant bulla, and pneumothorax developed during the course of COVID-19 pneumonia. Korean J Radiol. (2020) 21:541. doi: 10.3348/kjr.2020.0180
46. Viola P, Ralli M, Pisani D, Malanga D, Sculco D, Messina L, et al. Tinnitus and equilibrium disorders in COVID-19 patients: preliminary results. Euro Archiv Oto-Rhino-Laryngol. (2021) 278:3725–30. doi: 10.1007/s00405-020-06440-7
47. Liotta EM, Batra A, Clark JR, Shlobin NA, Hoffman SC, Orban ZS, et al. Frequent neurologic manifestations and encephalopathy-associated morbidity in Covid-19 patients. Ann Clinic Translat Neurol. (2020) 7:2221–30. doi: 10.1002/acn3.51210
48. Sari E, Külcü NU, Erdede O, Yalçin EU, Yamanel RGS. New-Onset Dizziness Associated With COVID-19. Pediatr Neurol. (2021) 115:72. doi: 10.1016/j.pediatrneurol.2020.11.007
49. Vanaparthy R, Malayala SV, Balla M. COVID-19-induced vestibular neuritis, hemi-facial spasms and Raynaud's phenomenon: a case report. Cureus. (2020) 12. doi: 10.7759/cureus.11752
50. Maslovara S, Košec A. Post-COVID-19 benign paroxysmal positional vertigo case reports. Medicine. (2021) 221:555. doi: 10.1155/2021/9967555
51. Mat Q, Noël A, Loiselet L, Tainmont S, Chiesa-Estomba CM, Lechien JR, et al. Vestibular neuritis as clinical presentation of COVID-19. Ear, Nose and Throat Journal. (2021) 0145561321995021. doi: 10.1177/0145561321995021
52. Motawea KR, Monib FA. New Onset Vertigo After COVID-19 infection. a case report. Indian J Otolaryngol Head Neck Surg. (2021) 21:1–3. doi: 10.1007/s12070-021-02715-5
53. Malayala SV, Raza A. A case of COVID-19-induced vestibular neuritis. Cureus. (2020) 12. doi: 10.7759/cureus.8918
54. Sahin A-R, Erdogan A, Agaoglu PM, Dineri Y, Cakirci A-Y, Senel M-E, et al. 2019 novel coronavirus (COVID-19) outbreak: a review of the current literature. EJMO. (2020) 4:1–7. doi: 10.14744/ejmo.2020.12220
55. De Luca P, Scarpa A, Ralli M, Tassone D, Simone M, Cassandro C, et al. Auditory Disturbances and SARS-CoV-2 infection: brain inflammation or cochlear affection? systematic review and discussion of potential pathogenesis. Front Neurol. (2021) 12:1234. doi: 10.3389/fneur.2021.707207
56. Jafari Z, Kolb BE, Mohajerani MH. Hearing loss, tinnitus, and dizziness in COVID-19: a systematic review and meta-analysis. Canadian J Neurologic Sci. (2021) 21:1–33. doi: 10.1017/cjn.2021.63
57. McIntyre KM, Favre NM, Kuo CC, Carr MM. Systematic Review of Sensorineural Hearing Loss Associated With COVID-19 Infection. Cureus. (2021) 13. doi: 10.7759/cureus.19757
58. Cui C, Yao Q, Zhang D, Zhao Y, Zhang K, Nisenbaum E, et al. Approaching Otolaryngology Patients During the COVID-19 Pandemic. Otolaryngol Head Neck Surg. (2020) 163:121–31. doi: 10.1177/0194599820926144
59. Lechien JR, Chiesa-Estomba CM, Place S, Van Laethem Y, Cabaraux P, Mat Q, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. (2020) 288:335−44. doi: 10.1111/joim.13089
60. Pisani D, Leopardi G, Viola P, Scarpa A, Ricciardiello F, Cerchiai N, et al. Sudden sensorineural hearing loss after covid-19 vaccine; A possible adverse reaction? Otolaryngol Case Rep. (2021) 21:100384. doi: 10.1016/j.xocr.2021.100384
61. Tsetsos N, Poutoglidis A, Vlachtsis K, Kilmpasanis A, Gougousis S. Sudden Sensorineural Hearing Loss Following the Second Dose of COVID-19 Vaccine. Cureus. (2021) 13. doi: 10.7759/cureus.17435
62. Tseng PT, Chen TY, Sun YS, Chen YW, Chen JJ. The reversible tinnitus and cochleopathy followed first-dose AstraZeneca COVID-19 vaccination. QJM: Int J Med. (2021) 21:210. doi: 10.1093/qjmed/hcab210
63. Parrino D, Frosolini A, Gallo C, De Siati RD, Spinato G, de Filippis C. Tinnitus following COVID-19 vaccination: report of three cases. Int J Audiol. (2021) 21:1–4. doi: 10.1080/14992027.2021.1931969
64. Briggs SE, Brenner MJ, Chandrasekhar SS. Sudden Sensorineural Hearing Loss and COVID-19 Vaccination. JAMA Otolaryngol Head Neck Surg. (2021) 21:3384. doi: 10.1001/jamaoto.2021.3384
65. Ciorba A, Bianchini C, Caranti A, Skarzyński PH, Pelucchi S, Hatzopoulos S. Incidence of audiological adverse effects induced by COVID-19 Vaccines: a preliminary study. Ear Nose Throat J. (2021) 21:01455613211048975. doi: 10.1177/01455613211048975
66. Formeister EJ, Chien W, Agrawal Y, Carey JP, Stewart CM, Sun DQ. Preliminary Analysis of Association Between COVID-19 Vaccination and Sudden Hearing Loss Using US Centers for Disease Control and Prevention Vaccine Adverse Events Reporting System Data. JAMA Otolaryngol Head Neck Surg. (2021) 21:869. doi: 10.1001/jamaoto.2021.0869
67. Jeong J, Choi HS. Sudden sensorineural hearing loss after COVID-19 vaccination. Int J Infectious Dis. (2021) 113:341–3. doi: 10.1016/j.ijid.2021.10.025
68. Wichova H, Miller ME, Derebery MJ. Otologic manifestations after COVID-19 vaccination: the house ear clinic experience. Otol Neurotol. (2021) 42:e1213. doi: 10.1097/MAO.0000000000003275
69. Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. (2020) 77:683–90. doi: 10.1001/jamaneurol.2020.1127
70. Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. Bmj. (2020) 368:m1091. doi: 10.1136/bmj.m1091
71. Saniasiaya J, Kulasegarah J. Dizziness and COVID-19. Ear, Nose Throat J. (2021) 100:29–30. doi: 10.1177/0145561320959573
72. Rosenhall U, Kankkunen A. Hearing alterations following meningitis. 1. Hearing improvement. Ear Hear. (1980) 1:185–90. doi: 10.1097/00003446-198007000-00002
73. Cohen BE, Durstenfeld A, Roehm PC. Viral causes of hearing loss: a review for hearing health professionals. Trends Hear. (2014) 18:2331216514541361. doi: 10.1177/2331216514541361
74. Young Y-H. Contemporary review of the causes and differential diagnosis of sudden sensorineural hearing loss. Int J Audiol. (2020) 59:243–53. doi: 10.1080/14992027.2019.1689432
75. Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dubé M, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses. (2020) 12:14. doi: 10.3390/v12010014
76. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. (2020) 92:552–5. doi: 10.1002/jmv.25728
77. Hung EC, Chim SS, Chan PK, Tong YK, Ng EK, Chiu RW, et al. Detection of SARS coronavirus RNA in the cerebrospinal fluid of a patient with severe acute respiratory syndrome. Clin Chem. (2003) 49:2108. doi: 10.1373/clinchem.2003.025437
78. Lau K-K, Yu W-C, Chu C-M, Lau S-T, Sheng B, Yuen K-Y. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis. (2004) 10:342. doi: 10.3201/eid1002.030638
79. Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. (2020) 11:995–8. doi: 10.1021/acschemneuro.0c00122
80. Chen R, Wang K, Yu J, Howard D, French L, Chen Z, et al. The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in the human and mouse brains. Front Neurol. (2021) 11:1860. doi: 10.3389/fneur.2020.573095
81. Krasniqi S, Daci A. Role of the angiotensin pathway and its target therapy in epilepsy management. Int J Mol Sci. (2019) 20:726. doi: 10.3390/ijms20030726
82. Saniasiaya J. Hearing loss in SARS-CoV-2: what do we know? Ear, Nose Throat J. (2021) 100:152S-154S. doi: 10.1177/0145561320946902
83. Butowt R, von Bartheld CS. < ? covid19?> Anosmia in COVID-19: Underlying Mechanisms and Assessment of an Olfactory Route to Brain Infection. The Neuroscientist. (2020) 20:1073858420956905. doi: 10.1177/1073858420956905
84. McCray PBJr, Pewe L, Wohlford-Lenane C, Hickey M, Manzel L, Shi L, et al. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J Virol. (2007) 81:813–21. doi: 10.1128/JVI.02012-06
85. Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. (2008) 82:7264–75. doi: 10.1128/JVI.00737-08
86. Rhea EM, Logsdon AF, Hansen KM, Williams LM, Reed MJ, Baumann KK, et al. The S1 protein of SARS-CoV-2 crosses the blood–brain barrier in mice. Nat Neurosci. (2021) 24:368–78. doi: 10.1038/s41593-020-00771-8
87. Achar A, Ghosh C. COVID-19-Associated neurological disorders: the potential route of CNS invasion and blood-brain barrier relevance. Cells. (2020) 9:2360. doi: 10.3390/cells9112360
88. Buzhdygan TP, DeOre BJ, Baldwin-Leclair A, Bullock TA, McGary HM, Khan JA, et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood–brain barrier. Neurobiol Dis. (2020) 146:105131. doi: 10.1016/j.nbd.2020.105131
89. Burks SM, Rosas-Hernandez H, Ramirez-Lee MA, Cuevas E, Talpos JC. Can SARS-CoV-2 infect the central nervous system via the olfactory bulb or the blood-brain barrier? Brain, Behavior, and Immun. (2021) 21:31. doi: 10.1016/j.bbi.2020.12.031
90. Welcome MO, Mastorakis NE. Neuropathophysiology of coronavirus disease 2019: neuroinflammation and blood brain barrier disruption are critical pathophysiological processes that contribute to the clinical symptoms of SARS-CoV-2 infection. Inflammopharmacology. (2021) 2:1–25. doi: 10.1007/s10787-021-00806-x
91. Aid M, Busman-Sahay K, Vidal SJ, Maliga Z, Bondoc S, Starke C, et al. Vascular disease and thrombosis in SARS-CoV-2-infected rhesus macaques. Cell. (2020) 183:1354–66. e1313. doi: 10.1016/j.cell.2020.10.005
92. Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. (2020) 46:1089–98. doi: 10.1007/s00134-020-06062-x
93. Kashi M, Jacquin A, Dakhil B, Zaimi R, Mahé E, Tella E, et al. Severe arterial thrombosis associated with Covid-19 infection. Thromb Res. (2020) 192:75–7. doi: 10.1016/j.thromres.2020.05.025
94. Mackman N, Antoniak S, Wolberg AS, Kasthuri R, Key NS. Coagulation abnormalities and thrombosis in patients infected with SARS-CoV-2 and other pandemic viruses. Arterioscler Thromb Vasc Biol. (2020) 40:2033–44. doi: 10.1161/ATVBAHA.120.314514
95. Mowla A, Shakibajahromi B, Shahjouei S, Borhani-Haghighi A, Rahimian N, Baharvahdat H, et al. Cerebral venous sinus thrombosis associated with SARS-CoV-2; a multinational case series. J Neurol Sci. (2020) 419:117183. doi: 10.1016/j.jns.2020.117183
96. Nougier C, Benoit R, Simon M, Desmurs-Clavel H, Marcotte G, Argaud L, et al. Hypofibrinolytic state and high thrombin generation may play a major role in SARS-COV2 associated thrombosis. J Thrombosis Haemostasis. (2020) 18:2215–9. doi: 10.1111/jth.15016
97. Cure E, Cure MC. Comment on “Hearing loss and COVID-19: a note”. Am J Otolaryngol. (2020) 41:102513. doi: 10.1016/j.amjoto.2020.102513
98. Jeong M, Ocwieja KE, Han D, Wackym PA, Zhang Y, Brown A, et al. Direct SARS-CoV-2 infection of the human inner ear may underlie COVID-19-associated audiovestibular dysfunction. Commun Med. (2021) 1:1–14. doi: 10.1038/s43856-021-00044-w
99. Bortoli R, Santiago M. Chloroquine ototoxicity. Clin Rheumatol. (2007) 26:1809–10. doi: 10.1007/s10067-007-0662-6
100. Cianfrone G, Pentangelo D, Cianfrone F, Mazzei F, Turchetta R, Orlando MP, et al. Pharmacological drugs inducing ototoxicity, vestibular symptoms and tinnitus: a reasoned and updated guide. Eur Rev Med Pharmacol Sci. (2011) 15:601–36.
101. Kakuda TN. Pharmacology of nucleoside and nucleotide reverse transcriptase inhibitor-induced mitochondrial toxicity. Clin Ther. (2000) 22:685–708. doi: 10.1016/S0149-2918(00)90004-3
Keywords: auditory symptoms, vestibular symptoms, COVID-19, hearing loss, tinnitus
Citation: Kaliyappan K, Chen Y-C and Krishnan Muthaiah VP (2022) Vestibular Cochlear Manifestations in COVID-19 Cases. Front. Neurol. 13:850337. doi: 10.3389/fneur.2022.850337
Received: 07 January 2022; Accepted: 15 February 2022;
Published: 18 March 2022.
Edited by:
Arianna Di Stadio, University of Catania, ItalyReviewed by:
Zhenyu Xiong, University of Texas Southwestern Medical Center, United StatesMarije de Jong, Hadassah Medical Center, Israel
Copyright © 2022 Kaliyappan, Chen and Krishnan Muthaiah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu-Chen Chen, Y2hlbnl1Y2hlbjE5ODlAMTI2LmNvbQ==; Vijaya Prakash Krishnan Muthaiah, dmlqYXlhcHJAYnVmZmFsby5lZHU=
 Kathiravan Kaliyappan
Kathiravan Kaliyappan Yu-Chen Chen
Yu-Chen Chen Vijaya Prakash Krishnan Muthaiah
Vijaya Prakash Krishnan Muthaiah