
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Neurol., 21 March 2022
Sec. Neuroepidemiology
Volume 13 - 2022 | https://doi.org/10.3389/fneur.2022.846690
This article is part of the Research TopicSex Differences in Cerebrovascular DiseasesView all 13 articles
Background: Identification of sex- and age-related differences in the presentation of atypical symptoms at stroke onset may reduce prehospital delay and improve stroke treatment if acknowledged at first contact.
Aim: To explore sex- and age-related differences in patient-reported typical and atypical symptoms of a stroke.
Methods: We used data from a cross-sectional survey at two non-comprehensive stroke units in the Capital Region of Denmark. Patient-reported symptoms, stroke knowledge, and behavioral response were analyzed by the Chi-square test or a Fisher's exact test separated by sex. Multivariable logistic regression adjusted for covariates were used to explore sex- and age-related differences according to each patient-reported typical or atypical symptoms.
Results: In total, 479 patients with acute stroke were included (median age 74 years [25th to 75th percentile: 64–80], and 40.1% were women). Female sex was associated with higher odds of presenting with atypical symptoms, such as loss of consciousness (OR 2.12 [95% CI 1.08–4.18]) and nausea/vomiting (OR 2.33 [95% CI 1.24–4.37]), and lower odds of presenting with lower extremity paresis (OR 0.59 [95% CI 0.39–0.89). With each year of age, the odds decreased of presenting with sensory changes (OR 0.95 [95% CI 0.94–0.97]) and upper extremity paresis (OR 0.98 [95% CI 0.96–0.99]), whereas odds of presenting with dysphagia (OR 1.06 [95% CI 1.02–1.11]) increased.
Conclusions: Patients of female sex and younger age reported on admission more frequently atypical stroke symptoms. Attention should be drawn to this possible atypical first presentation to facilitate correct identification and early stroke revascularization treatment to improve the outcome for both sexes.
Identification of sex- and age-related differences in the presentation of atypical symptoms at stroke onset may improve stroke treatment if acknowledged at first contact and reduce prehospital delay. Failed recognition of stroke upon presentation may cause delayed treatment with reduced clinical outcome (1–4). Currently, a small but increasing proportion of all ischemic stroke patients are treated with revascularization therapy, a well-established treatment worldwide (5–7). A frequent cause of failure to receive revascularization therapy within the required time window is a prehospital delay, either patient or system related. Patient delay is often associated with failure in symptom recognition or reluctance to respond acutely to symptoms. In system delay, much has improved in door-to-needle time at comprehensive stroke units but is still largely affected by missed symptom recognition by the health professionals at first contact (8–11). Stroke symptoms can be categorized as typical (12) (e.g., hemiparesis, facial palsy, visual, or language disturbances) or atypical (e.g., headache, dizziness, confusion, or sensory symptoms) (13), where the latter induce a significant risk of missing the stroke diagnosis.
Minimizing response time and implementing fast-track treatment of stroke is the key to reducing the impact of stroke on death and disability worldwide (14). Previous studies suggest that patients of female sex present a different profile of stroke symptoms on admission compared to the male sex. However, these studies focused on symptoms identified by health professionals and not those reported by the patients or bystanders. Symptoms recognized by health professionals may be different from those experienced and reported by the patient and bystanders potentially unaware of stroke-related symptoms (15–19). Knowledge is scarce on which acute stroke symptoms are reported by the patient or the bystander on admission. In this study, we have focused on the sex- and age-related differences in patient-reported symptoms which cause them to react and present to the prehospital health system. Older patients (>80 years of age) seem to present more frequently with typical symptoms, such as aphasia and hemiplegia (18). Headache and nausea are reported more commonly in younger patients, even after controlling for concomitant migraine (19). It remains to be seen if increased age amplifies this difference in the patient-reported symptoms (9). We hypothesized that patient-reported atypical symptoms at the onset of stroke were more frequent in the female sex and that the distribution of typical and atypical stroke symptoms varied with increasing age. Accordingly, we aimed to explore the sex- and age-related differences in patient-reported symptoms of a stroke.
This study is a post-hoc analysis of data from a cross-sectional survey performed at two non-comprehensive stroke units in the Capital Region of Denmark, Herlev Gentofte Hospital, and Nordsjællands Hospital. Study design and methods have previously been published (11).
Patients with symptoms of acute stroke or transient ischemic attack (TIA) were enrolled immediately after admission to the stroke unit, albeit before completion of a full diagnostic workup for stroke. All diagnoses [International Classification of Diseases codes: I61: non-traumatic intracerebral hemorrhage (ICH); I63: ischemic stroke (IS); or G45. TIA] were confirmed by a neurologist supported by neuroimaging (CT and MRI scans). Enrolled patients fulfilled the following inclusion criteria: (1) admitted directly to a non-comprehensive stroke unit or transferred from a comprehensive or primary stroke center after revascularization therapy, (2) age ≥ 18 years, and (3) obtained written consent from the patient. Exclusion criteria were patients with (1) a subarachnoid hemorrhage, (2) an in-hospital stroke, (3) a non-stroke diagnosis, or (4) symptom onsets abroad. Only the first event was included in case of recurrent stroke during the inclusion period.
Data were collected from February 2018 to June 2018 and September 2018 to January 2019 at Herlev Gentofte Hospital and Nordsjællands Hospital, both located in The Capital Region of Denmark. Medical records and emergency medical service (EMS) data supported patients' responses. Data were managed in a Research Electronic Data Capture, REDCAP, system (REDCap consortium, Vanderbilt University, United States of America, v9.1.0 hosted by The Capital Region of Denmark) (20).
Typical patient-reported symptoms were defined according to the American Stroke Association's stroke warning signs and symptoms “BEFAST save a life,” covering Balance, Eyes, Face, Arm, Speech (and Time). BEFAST describes factors associated with the need to call EMS immediately for treatment evaluation (12). Atypical stroke symptoms were defined as symptoms not included in BEFAST (e.g., pain, loss of consciousness, unclassifiable neurological symptoms, and non-neurological symptoms) (13, 21). Patient-reported symptoms were categorized by the interviewer into predefined medical terms.
Baseline characteristics were summarized as frequencies with percentages or medians with interquartile ranges (IQR), and differences were tested using the Chi-square test or Fisher's exact test for categorical variables and the Wilcoxon test for continuous variables. To explore sex- and age-related differences in patient-reported typical and atypical stroke symptoms, multivariable logistic regression models were used to estimate odds ratios (OR) with 95% confidence intervals (CI) for each symptom. Models were adjusted for stoke severity assessed by the Scandinavian Stroke Scale (severe 0–25 points, moderate 26–42 points, and mild 43–58 points) (22), stroke localization (right hemisphere, left hemisphere, or bilateral, brainstem, cerebellum), a history of hypertension, diabetes (yes/no), atrial fibrillation (yes/no), and hypercholesterolemia (yes/no). Age was included as a continuous variable. Male sex was the reference group in all statistic models. All statistical analyses were performed using SAS statistical software version 9.4 (SAS Institute, Cary, NC, United States). A two-sided significance level was set at alpha < 0.05. There were no missing data on stroke symptoms.
To test the robustness of our findings, we conducted several sensitivity analyses. In the first analysis, all symptoms were grouped into two categories: typical and atypical symptoms and examined for differences in age and sex. In the second analysis, age was included as a categorical variable with the following age groups 18–59 years, 60–74 years, and 75+. In the third analysis, age was included in 5-year intervals.
The study was approved by the Capital Region's Ethics Committee (no. 2012-58-004) and the Danish Data Protection Agency (no. 2012-58-0004; internal reference: HGH-2017-110, I-Suite no. 06014). Patients provided written informed consent before interviews.
The process of enrollment is described in detail elsewhere (11). In total, 479 patients with stroke or TIA were included (40.1% female), and the median age was 74 years (64–80) with no significant age-related difference between sexes. Baseline characteristics are summarized in Table 1, and Table 2 summarizes patient-reported symptoms, stroke knowledge, behavioral response, arrival time, and treatment. The proportions of acute stroke symptoms are displayed in Figure 1. There were no differences between women and men with respect to stroke knowledge, behavioral response, hospital arrival within 180 min from symptom onset, or stroke treatment.
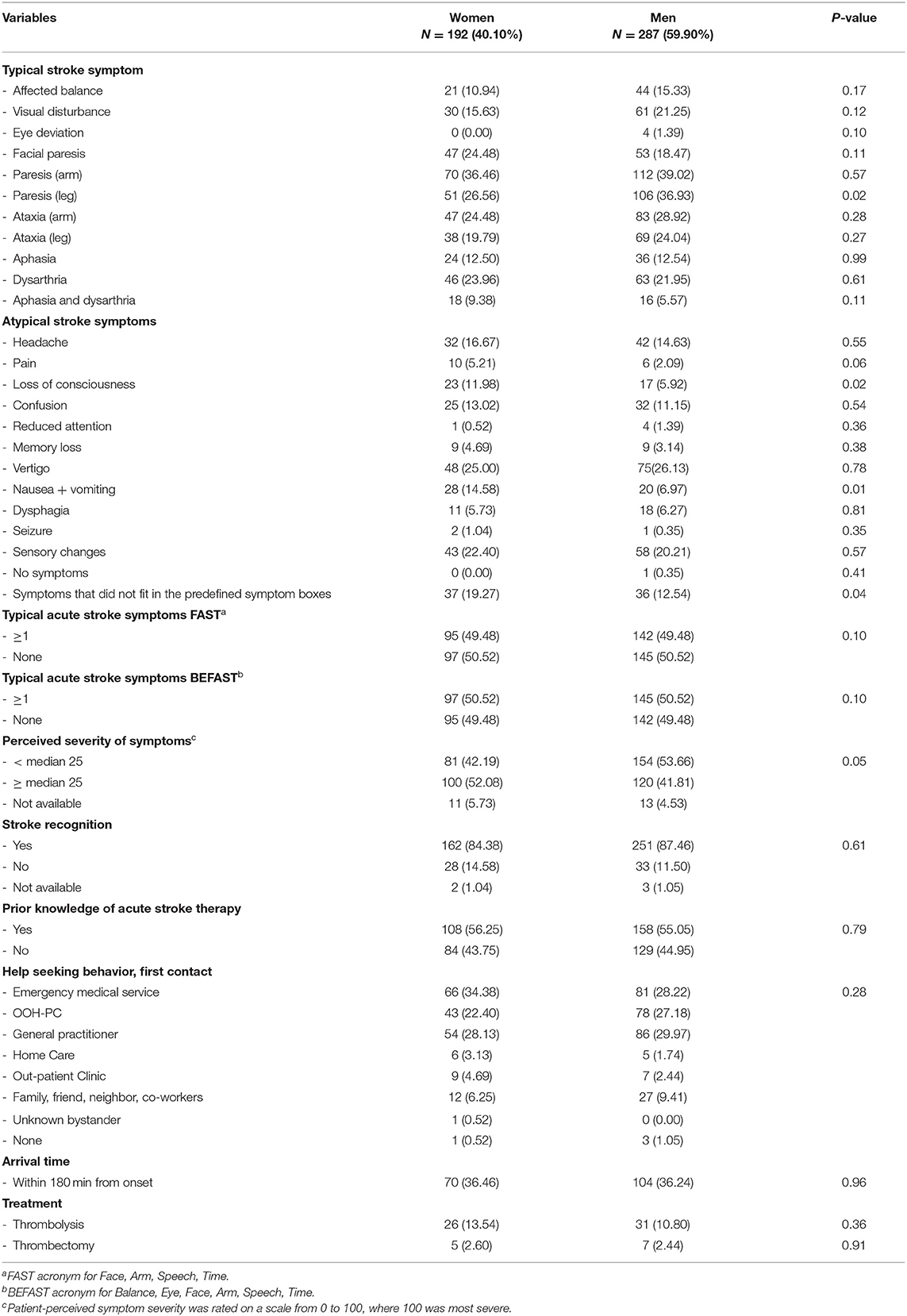
Table 2. Patient-reported symptoms, stroke knowledge, behavioral response, arrival time, and treatment.
In multivariable logistic regression analyses, female sex, compared with male sex, was associated with higher odds of presenting with loss of consciousness [adjusted OR 2.12 (95% CI 1.08–4.18)] and nausea/vomiting [adjusted OR 2.33 (95% CI 1.24–4.37)], but lower odds of presenting with lower extremity paresis [adjusted OR 0.59 (95% CI 0.39–0.89)] (Table 3). With increase in age each year, the odds were lower of presenting with sensory changes [adjusted, OR 0.95 (95% CI 0.94–0.97)] and upper extremity paresis [adjusted OR 0.98 (95% CI 0.96–0.99)]. The odds of presenting with dysphagia [adjusted OR 1.06 (95% CI 1.02–1.11)] increased with each year of age (Table 4).
When comparing typical vs. atypical symptoms, the male sex was not significantly associated with higher odds of presenting with either typical or atypical symptoms [adjusted OR 1.55, (95% CI 0.84–2.87)]. When age was included as a categorical variable or included in 5-year intervals (Supplementary Tables I, IIII online-only data supplement) our primary findings were confirmed. Including age as a categorical variable, the odds for presenting with sensory changes (atypical symptom) were lower when comparing the age group 18–59 years with 60–74 years [adjusted OR 0.52 (95% CI 0.30–0.92)], (Supplementary Table II online-only data supplement). When we compared the age group 18–59 years with 75+ years, the results from the primary analysis were confirmed (Supplementary Table III online-only data supplement). Including age in 5-year intervals, the odds of presenting with upper extremity paresis (typical symptom) and sensory changes (atypical symptom) were significantly lower with each 5-years increase in age [adjusted, OR 0.89 (95% CI 0.82–0.97)] and [adjusted, OR 0.79 (95% CI 0.72–0.87)] (Supplementary Table V online-only data supplement). We performed the sub-analysis according to the type of stroke by sex. In population I61, female sex was significantly associated with loss of consciousness (atypical; Supplementary Table VI online-only data supplement). In population I63, female sex was significantly associated with nausea + vomiting (atypical; Supplementary Table VII online-only data supplement). In population G45, male sex was significantly associated with affected balance (typical; Supplementary Table VIII online-only data supplement).
In this cross-sectional two-center survey, we explored sex- and age-related differences in patient-reported typical and atypical stroke symptoms in a stroke and TIA population. Our study yielded the following finding: Female sex was associated with higher odds of reporting atypical stroke symptoms, such as loss of consciousness and nausea/vomiting, and lower odds of reporting typical stroke symptoms, such as lower extremity paresis compared to the male sex, which confirmed our hypothesis. With increasing age, we found that sensory changes (atypical symptom) and upper extremity paresis (typical symptom) were less frequently reported, whereas dysphagia (atypical symptom) was more frequent with increasing age, which largely confirmed our hypothesis.
The chain of survival and the acute treatment of stroke and TIA have important similarities with that of acute coronary syndrome. To reduce morbidity and mortality, correct interpretation and action on acute symptoms are essential to ensure timely and correct revascularization therapy. In symptomatic manifestations of acute coronary syndrome, female sex is associated with frequent atypical presentations of symptoms, for example, unusual fatigue, dyspnea, neck and throat pain, and pain between the shoulder blades. This diversity of acute symptoms led to delay in identification and interpretation (23, 24). To which extent this diversity in symptom presentation may apply to stroke need to be addressed. In our study, female sex was associated with the initial presentation of atypical symptoms, such as loss of consciousness and nausea/vomiting at stroke onset. The findings on patient-reported symptoms were in line with studies where acute symptoms were identified by health professionals, hence female sex is associated with symptoms, such as loss of consciousness (17, 25, 26) and nausea/vomiting (27). Studies further reported that female sex, in comparison to the male sex, tended to present with other atypical symptoms identified by health professionals, such as dysphagia (25, 26, 28), headache (15, 16), mental status change (21, 29), and seizure (27). The findings that the female sex entailed a variety of atypical symptoms upon onset of stroke highlight the risk of misinterpretation symptoms as non–stroke related. In one study, female sex was associated with a longer hospital arrival time, including a decreased likelihood of reperfusion therapy, primarily due to patient-dependent delay (30). However, when adjusted for age, stroke severity, and co-habitant status, the sex difference in prehospital delay disappeared (30). In our univariate analysis 36% of both women and men arrived within 180 min from the onset. We could not confirm a significant sex difference in timely hospital arrival within 180 min. Some atypical presentations may be interpreted as severe, such as loss of consciousness or seizure, which caused rapid contact to emergency services. This may contribute to why sex differences in symptoms presentation did not significantly affect arrival times in the group. Having a bystander or co-habiting at the onset of stroke was previously associated with an increased chance of stroke recognition and immediate contact to emergency medical services (31, 32). In our study, the proportion of female sex living alone at stroke onset was significantly higher compared to the male sex, but this did not affect arrival times. Nonetheless, perception of symptom severity was associated with timely hospital arrival in other studies (32, 33).
The American Heart Association warning signs for myocardial infarction included a cautionary statement in their campaign, stressing the fact that the female sex is more likely to experience atypical symptoms (34). Previous stroke awareness campaigns focused on improving knowledge of typical symptoms and increasing behavioral response, but the effect on timely arrival has so far been inconclusive (35, 36). Future stroke awareness campaigns should be tailored to address that female sex may associate also with atypical presentations of stroke, to embrace sex differences in stroke care (37). We could not confirm any sex differences in stroke recognition, prior knowledge of stroke therapy, or help-seeking behavior. This could be due to no launch of a focused stroke awareness campaign before our study, and the number of included patients was relatively small.
Stroke patients above the age of 75 years presented more frequently with dysphagia (atypical symptom) when identified by health professionals (28). This was aligned when dysphagia was patient-reported in our study. Other atypical symptoms, such as coma, aphasia, and cerebellar dysfunction, have also been associated with stroke patients above 80 years (18). These findings were not confirmed in this study. Interestingly, headache, nausea/vomiting, and sensory deficits (atypical symptoms) appeared to be more common in the female sex between 18 and 44 years, even after controlling for a diagnosis of migraine and for age (19). Results on stroke patients younger than 55 years showed that almost 25% of strokes were not identified when clustering acute stroke symptoms according to typical symptoms (19). Furthermore, it should be stressed that the outcomes of atypically presenting stroke patients are not necessarily benign, if the symptoms are misdiagnosed (38).
We aimed to reduce recall bias by including patients as early as possible after stroke onset. A selection bias cannot be dismissed as there was an overrepresentation of patients with mild stroke, either due to early patient discharge, transfer to other departments if there was a change in clinical condition before inclusion was possible which could omit mild strokes or severely affected patients. The included population represented a broad stroke population arriving at non-comprehensive stroke units. The hypothesis of this study was devised after the overall study was designed but before the end of this study. Analyses were therefore exploratory and mainly applicable as hypothesis-generating.
The current study confirmed our hypothesis that sex differences in patient reporting of acute stroke symptoms exist. These findings were in accordance with previous studies investigating physician-reported symptoms, where the female sex presented with atypical symptoms, such as loss of consciousness and nausea/vomiting. Recognition of stroke and correct response to symptoms pose a particular challenge in early treatment when atypical stroke symptoms are reported. It needs to be further assessed to which extent sex- and age-related differences in symptoms of acute stroke influence interpretation of symptoms, behavioral motivators, and barriers for first contact to emergency medical service.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Capital Region's Ethics Committee, Danish Data Protection Agency. The patients/participants provided their written informed consent to participate in this study.
CK and AD were responsible for protocol development, gathering ethical approval, and data permission. Sub study on sex and age was conceived by HE and CK. HE and TC (only Nordsjællands Hospital) were involved in patient recruitment and data collection. HE, JB, and CK in data analysis. HE wrote the first draft of the manuscript. All authors made critical revisions to the manuscript and approved its final version.
This work was supported by TrygFonden, application ID 128669 and by the Novo Nordisk Foundation Borregaard stipend, grant number NNF18OC0031840.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2022.846690/full#supplementary-material
1. Fassbender K, Balucani C, Walter S, Levine SR, Haass A, Grotta J. Streamlining of prehospital stroke management: the golden hour. Lancet Neurol. (2013) 12:585–96. doi: 10.1016/S1474-4422(13)70100-5
2. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. (2018). 49:e46–110. doi: 10.1161/STR.0000000000000158
3. Lees KR, Emberson J, Blackwell L, Bluhmki E, Davis SM, Donnan GA, et al. Effects of alteplase for acute stroke on the distribution of functional outcomes: a pooled analysis of 9 trials. Stroke. (2016) 47:2373–9. doi: 10.1161/STROKEAHA.116.013644
4. Goyal M, Almekhlafi M, Dippel DW, Campbell BCV, Muir K, Demchuk AM, et al. Rapid alteplase administration improves functional outcomes in patients with stroke due to large vessel occlusions. Stroke. (2019) 50:645–51. doi: 10.1161/STROKEAHA.118.021840
5. Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. (2014) 384:1929–35. doi: 10.1016/S0140-6736(14)60584-5
6. Saver JL, Fonarow GC, Smith EE, Reeves MJ, Grau-Sepulveda MV, Pan W, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. (2013) 309:2480–8. doi: 10.1001/jama.2013.6959
7. Whiteley WN, Emberson J, Lees KR, Blackwell L, Albers G, Bluhmki E, et al. Risk of intracerebral haemorrhage with alteplase after acute ischaemic stroke: a secondary analysis of an individual patient data meta-analysis. Lancet Neurol. (2016) 15:925–33. doi: 10.1016/S1474-4422(16)30076-X
8. Kleindorfer D, Lindsell CJ, Moomaw CJ, Alwell K, Woo D, Flaherty ML, et al. Which stroke symptoms prompt a 911 call? A population-based study. Am J Emerg Med. (2010) 28:607–12. doi: 10.1016/j.ajem.2009.02.016
9. Bushnell C, Howard VJ, Lisabeth L, Caso V, Gall S, Kleindorfer D, et al. Sex differences in the evaluation and treatment of acute ischaemic stroke. Lancet Neurol. (2018) 17:641–50. doi: 10.1016/S1474-4422(18)30201-1
10. Fladt J, Meier N, Thilemann S, Polymeris A, Traenka C, Seiffge DJ, et al. Reasons for prehospital delay in acute ischemic stroke. J Am Heart Assoc. (2019) 8:e013101. doi: 10.1161/JAHA.119.013101
11. Eddelien HS, Butt JH, Amtoft AC, Nielsen NSK, Jensen ES, Danielsen IMK, et al. Patient-reported factors associated with early arrival for stroke treatment. Brain Behav. (2021) 11:e2225. doi: 10.1002/brb3.2225
12. American Stroke Associations. The American Stroke Associations Stroke Campaign BEFAST. (2021). Available online at: https://www.stroke.org/en/about-stroke/stroke-symptoms (accessed October 22, 2021).
13. Labiche LA, Chan W, Saldin KR, Morgenstern LB. Sex and acute stroke presentation. Ann Emerg Med. (2002) 40:453–60. doi: 10.1067/mem.2002.128682
14. Johnson CO. Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. (2019) 18:439–58. doi: 10.1016/S1474-4422(19)30034-1
15. Kapral MK, Fang J, Hill MD, Silver F, Richards J, Jaigobin C, et al. Sex differences in stroke care and outcomes: results from the Registry of the Canadian Stroke Network. Stroke. (2005) 36:809–14. doi: 10.1161/01.STR.0000157662.09551.e5
16. Li OL, Silver FL, Lichtman J, Fang J, Stamplecoski M, Wengle RS, et al. Sex differences in the presentation, care, and outcomes of transient ischemic attack: results from the ontario stroke registry. Stroke. (2016) 47:255–7. doi: 10.1161/STROKEAHA.115.010485
17. Eriksson M, Glader EL, Norrving B, Terént A, Stegmayr B et al. Sex differences in stroke care and outcome in the Swedish national quality register for stroke care. Stroke. (2009) 40:909–14. doi: 10.1161/STROKEAHA.108.517581
18. Béjot Y, Rouaud O, Jacquin A, Osseby GV, Durier J, Manckoundia P, et al. Stroke in the very old: incidence, risk factors, clinical features, outcomes and access to resources–a 22-year population-based study. Cerebrovasc Dis. (2010) 29:111–21. doi: 10.1159/000262306
19. Kaps M, Grittner U, Jungehulsing G, Tatlisumak T, Kessler C, Schmidt R, et al. Clinical signs in young patients with stroke related to FAST: results of the sifap1 study. BMJ Open. (2014) 4:e005276. doi: 10.1136/bmjopen-2014-005276
20. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. (2009) 42:377–81. doi: 10.1016/j.jbi.2008.08.010
21. Lisabeth LD, Brown DL, Hughes R, Majersik JJ, Morgenstern LB. Acute stroke symptoms: comparing women and men. Stroke. (2009) 40:2031–6. doi: 10.1161/STROKEAHA.109.546812
22. Govan L, Langhorne P, Weir CJ. Categorizing stroke prognosis using different stroke scales. Stroke. (2009) 40:3396–9. doi: 10.1161/STROKEAHA.109.557645
23. Araújo C, Laszczyńska O, Viana M, Melão F, Henriques A, Borges A, et al. Sex differences in presenting symptoms of acute coronary syndrome: the EPIHeart cohort study. BMJ Open. (2018) 8:e018798. doi: 10.1136/bmjopen-2017-018798
24. Shi H, Li W, Zhou X, Liu X, Liu J, Fan S, et al. Sex differences in prodromal symptoms and individual responses to acute coronary syndrome. J Cardiovasc Nurs. (2020) 35:545–9. doi: 10.1097/JCN.0000000000000643
25. Acciarresi M, De Luca P, Caso V, Agnelli G, D'Amore C, Alberti A, et al. Acute stroke symptoms: do differences exist between sexes? J Stroke Cerebrovasc Dis. (2014) 23:2928–33. doi: 10.1016/j.jstrokecerebrovasdis.2014.07.044
26. Gall SL, Donnan G, Dewey HM, Macdonell R, Sturm J, Gilligan A, et al. Sex differences in presentation, severity, and management of stroke in a population-based study. Neurology. (2010) 74:975–81. doi: 10.1212/WNL.0b013e3181d5a48f
27. Aziz ZA, Lee YY, Sidek NN, Ngah BA, Looi I, Hanip MR, et al. Gender disparities and thrombolysis use among patient with first-ever ischemic stroke in Malaysia. Neurol Res. (2016) 38:406–13. doi: 10.1080/01616412.2016.1178948
28. Roquer J, Campello AR, Gomis M. Sex differences in first-ever acute stroke. Stroke. (2003) 34:1581–5. doi: 10.1161/01.STR.0000078562.82918.F6
29. Jerath NU, Reddy C, Freeman WD, Jerath AU, Brown RD. Gender differences in presenting signs and symptoms of acute ischemic stroke: a population-based study. Gend Med. (2011) 8:312–9. doi: 10.1016/j.genm.2011.08.001
30. Mainz J, Andersen G, Valentin JB, Gude MF, Johnsen SP. Disentangling sex differences in use of reperfusion therapy in patients with acute ischemic stroke. Stroke. (2020) 51:2332–8. doi: 10.1161/STROKEAHA.119.028589
31. Iversen AB, Blauenfeldt RA, Johnsen SP, Sandal BF, Christensen B, Andersen G, et al. Understanding the seriousness of a stroke is essential for appropriate help-seeking and early arrival at a stroke centre: a cross-sectional study of stroke patients and their bystanders. Europ Stroke J. (2020) 5:351–61. doi: 10.1177/2396987320945834
32. Mellon L, Doyle F, Williams D, Brewer L, Hall P, Hickey A. Patient behaviour at the time of stroke onset: a cross-sectional survey of patient response to stroke symptoms. Emerg Med J. (2016) 33:396–402. doi: 10.1136/emermed-2015-204806
33. Soto-Camara R, Gonzalez-Santos J, Gonzalez-Bernal J, Martin-Santidrian A, Cubo E, Trejo-Gabriel-Galan JM. Factors associated with shortening of prehospital delay among patients with acute ischemic stroke. J Clin Med. (2019) 8:1712. doi: 10.3390/jcm8101712
34. Association TAH. Heart Attack Symptoms in Women. (2021). Available online at: https://www.heart.org/en/health-topics/heart-attack/warning-signs-of-a-heart-attack/heart-attack-symptoms-in-women (accessed October 22, 2021).
35. Nordanstig A, Palaszewski B, Asplund K, Norrving B, Wahlgren N, Wester P, et al. Evaluation of the Swedish National Stroke Campaign: a population-based time-series study. Int J Stroke. (2019) 14:862–70. doi: 10.1177/1747493019840939
36. Wolters FJ, Paul NL, Li L, Rothwell PM. Sustained impact of UK FAST-test public education on response to stroke: a population-based time-series study. Int J Stroke. (2015) 10:1108–14. doi: 10.1111/ijs.12484
37. Reeves M, Bhatt A, Jajou P, Brown M, Lisabeth L. Sex differences in the use of intravenous rt-PA thrombolysis treatment for acute ischemic stroke: a meta-analysis. Stroke. (2009) 40:1743–9. doi: 10.1161/STROKEAHA.108.543181
Keywords: stroke, signs and symptoms, sex diferences, prehospital delay time, behavior
Citation: Eddelien HS, Butt JH, Christensen T, Danielsen AK and Kruuse C (2022) Sex and Age Differences in Patient-Reported Acute Stroke Symptoms. Front. Neurol. 13:846690. doi: 10.3389/fneur.2022.846690
Received: 31 December 2021; Accepted: 11 February 2022;
Published: 21 March 2022.
Edited by:
Maurizio A. Leone, Home for Relief of Suffering (IRCCS), ItalyReviewed by:
Beata Sarecka-Hujar, Medical University of Silesia, PolandCopyright © 2022 Eddelien, Butt, Christensen, Danielsen and Kruuse. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Heidi S. Eddelien, aGVpZGkuc2hpbC5lZGRlbGllbi4wMUByZWdpb25oLmRr
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.