- 1Fondazione Policlinico Universitario “A. Gemelli” IRCCS, Rome, Italy
- 2Centro di Ricerca Sclerosi Multipla (CERSM), Università Cattolica, Rome, Italy
- 3MS Center, ASST Valle Olona, Gallarate Hospital, Gallarate, Italy
- 4Queen Square MS Centre, National Hospital for Neurology and Neurosurgery, London, United Kingdom
- 5Department of Neurology, Mellen Center, Neurologic Institute, Cleveland Clinic, Cleveland, OH, United States
- 6Department of Neurology, University Hospital Essen, Essen, Germany
- 7Lycalis sprl, Brussels, Belgium
Earlier diagnosis, access to disease-modifying therapies (DMTs), and improved supportive care have favorably altered the disease course of multiple sclerosis (MS), leading to an improvement in long-term outcomes for people with MS (PwMS). This success has changed the medical characteristics of the population seen in MS clinics. Comorbidities and the accompanying polypharmacy, immune senescence, and the growing number of approved DMTs make selecting the optimal agent for an individual patient more challenging. Glatiramer acetate (GA), a moderately effective DMT, interacts only minimally with comorbidities, other medications, or immune senescence. We describe here several populations in which GA may represent a useful treatment option to overcome challenges due to advanced age or comorbidities (e.g., hepatic or renal disease, cancer). Further, we weigh GA's potential merits in other settings where PwMS and their neurologists must base treatment decisions on factors other than selecting the most effective DMT, e.g., family planning, conception and pregnancy, or the need for vaccination.
Introduction
Glatiramer acetate (GA) was approved for the treatment of relapsing multiple sclerosis (MS) in 1996 in the US (1, 2), and 2001 in Europe (3), based on its beneficial effect on relapse rates in controlled trials (4–7). It is still widely prescribed as a safe and effective treatment after several million patient-years of exposure (8). Several generic alternatives have been developed (9, 10). GA is considered a platform therapy with modest effects on relapse-related clinical outcomes and no firmly established effect on delaying clinical progression or long-term disability (11). The continuing widespread use of this injectable agent despite newer, more efficacious DMTs may be attributable to its favorable safety profile with a lack of late adverse events and immunologic complications, or to its low level of interaction with comorbidities; however, part of the reason may also be the relatively few requirements for pre-treatment testing and on-treatment monitoring, the flexibility that it offers for family planning, or economic considerations. In this paper, we will discuss selected aspects and possible reasons for the enduring use of glatiramer acetate and its use in special populations of people with multiple sclerosis (PwMS).
MS, the most common chronic neuroinflammatory disease, causes demyelination, axonal degeneration, and gliosis, with focal inflammatory lesion activity usually predominating in the relapsing phase and diffuse inflammation and neurodegeneration becoming the main components for patient in the progressive phase (12). Although clinical distinctions are made between predominantly relapsing and progressive forms of MS (13, 14), the mechanisms underlying relapses and progression are present to varying degrees throughout the course of MS (13, 15–17). The prevalence of MS has increased in recent decades, and this may be due to the increasing sensitivity of radiographic methods and diagnostic criteria (18–22), and to longer survival (23).
Earlier diagnosis, access to disease-modifying therapies (DMTs), improved supportive care, and the fast-growing agency of PwMS have led to improvements in long-term outcomes (24–26). DMTs for MS suppress central nervous system (CNS) inflammation, reducing relapse rates and long-term disability. They work mainly during the predominantly relapsing phase by modulating the immune response, depleting immune cells or blocking their trafficking into the CNS (27). Important questions regarding treatment strategies include the optimal treatment approach in early MS, when and how to sequence treatments, including in patients with breakthrough activity, and when to de-escalate or discontinue treatment. The growing list of approved treatments for MS has made selecting the optimal agent for an individual patient more challenging. Current guidelines from the American Academy of Neurology (28), and European Committee for Treatment and Research in MS and the European Academy of Neurology (29) provide only limited guidance on starting, switching, and discontinuing, whereas treatment algorithms are provided in the National Health Service England guidelines (30), and in a recent position paper from the Multiple Sclerosis Treatment Consensus Group (17).
The two general approaches to treating MS involve either the early use of highly effective DMTs or the initial use of modestly effective DMTs, with escalation to highly effective DMTs when treatment response is inadequate (27, 31). Early highly-effective therapy maximizes anti-inflammatory effects early in the disease course, when they are most likely to be beneficial (32–35). Recent evidence from cohort studies suggests that early treatment with highly-effective therapy may be associated with a lower risk of disability progression (35, 36), and conversion to secondary progressive MS (34); however, this approach may expose some patients to an unnecessary risk of severe adverse effects such as infections, cardiac dysfunction, liver damage, or an increased risk of autoimmune diseases (37–40) (Table 1).
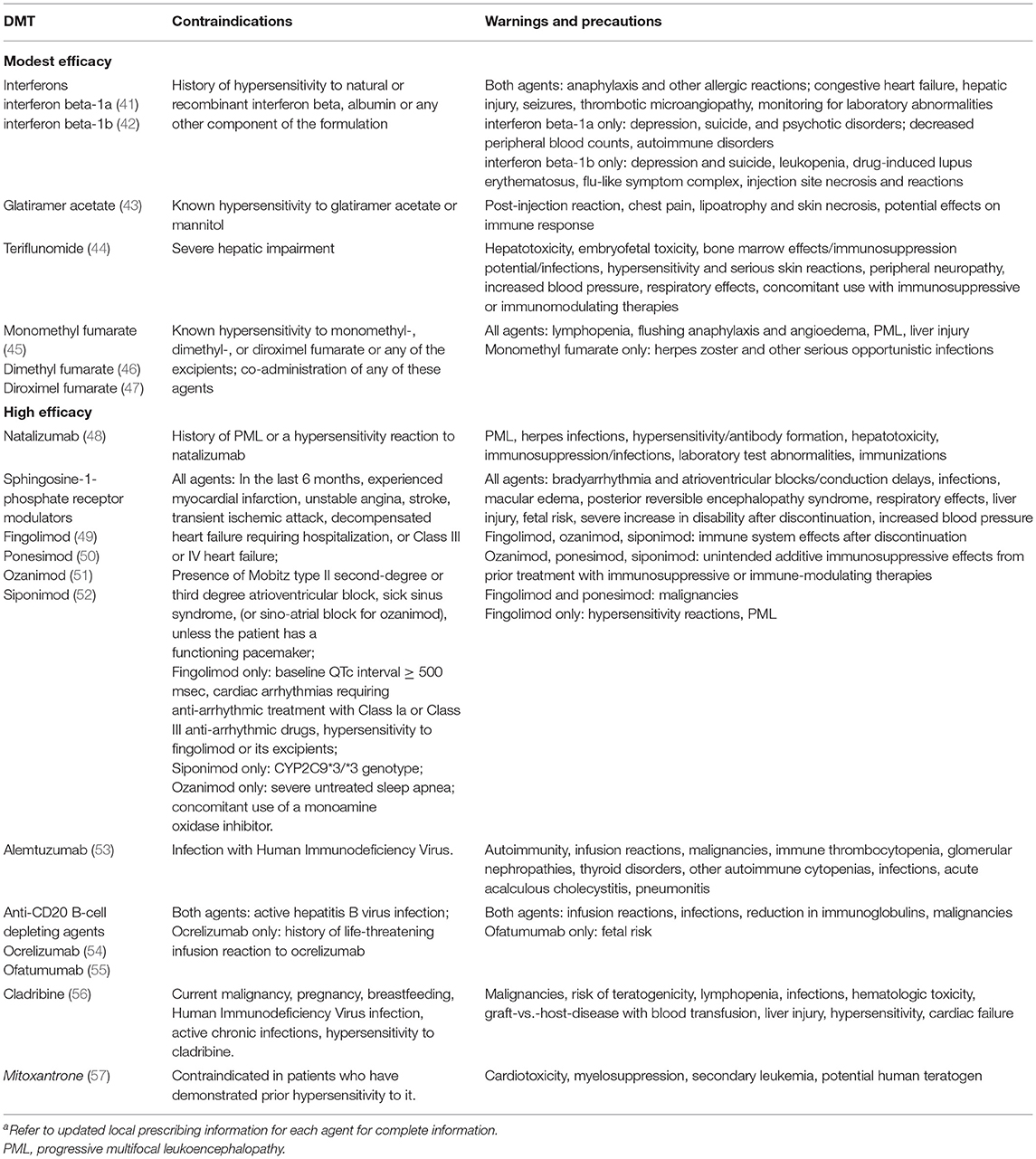
Table 1. US Food and Drug Administration contraindications, warnings and precautions for representative DMTs for MSa.
In the traditional escalation approach, determining the optimal timing of escalation is challenging and requires balancing the need to allow adequate time for a therapeutic effect to manifest with the need for timely response to ongoing disease activity. A low threshold for escalation in the face of breakthrough activity may reduce future disability (58, 59). The criteria of “No Evidence of Disease Activity” (NEDA) has been proposed to guide tight control of MS activity (60, 61). This outcome focuses on inflammatory demyelination that causes transient disability, but it has limitations (62), and its use may not influence long-term progression (63), due to the underlying diffuse inflammation and neurodegeneration that appear to drive long-term disability (16, 63). While fully validated biomarkers to guide treatment decisions in MS are lacking, cerebrospinal fluid and plasma levels of neurofilament light chain reflect axonal damage in a wide variety of neurological disorders (64). Recently, this marker has shown promise for monitoring disease activity and response to therapy in PwMS (65).
Ongoing controlled studies comparing escalation and early highly-effective treatment strategies may help to identify the most effective approach (NCT03500328, NCT03535298).
Shared decision making is a theme that should guide the relationship between PwMS and their neurologists (66, 67). The growing number of available DMTs with different potential benefits and risks makes it difficult to identify the most appropriate treatment for each patient. Communication between patient and clinician can be suboptimal (68, 69). In the context of shared decision making, clinicians should contribute the medical basics for suitable treatments, considering drug properties, disease characteristics and other factors, e.g., comorbidities, while patients may express their informed preferences based on expected benefits and their personal risk tolerance (70).
Glatiramer Acetate
Glatiramer acetate is an immunomodulating drug consisting of a complex polypeptide mixture (non-biological complex drug) obtained through the polymerization of the amino acids L-glutamic acid, L-alanine, L-lysine and L-tyrosine, followed by partial hydrolysis (43). It is administered by subcutaneous injection. Its mechanism of action is complex and not fully characterized but appears to involve effects on both innate and adaptive immune mechanisms. Briefly, it is thought to down-regulate myelin-specific T-cell activation and may compete with myelin basic protein peptides for binding to MHC class II molecules on antigen-presenting cells, leading to increased differentiation of T helper cells (Th)2, and T regulatory cells (Treg). Glatiramer acetate-reactive Th2 cells also suppress the activation of Th1 cells through “bystander suppression” and release neuroprotective factors, while the Treg cells reduce the secretion of proinflammatory cytokines by effector T cells. CD8+ T cells generated by antigen presentation of glatiramer acetate contribute to inhibiting myelin degradation (Figure 1) (71, 72).
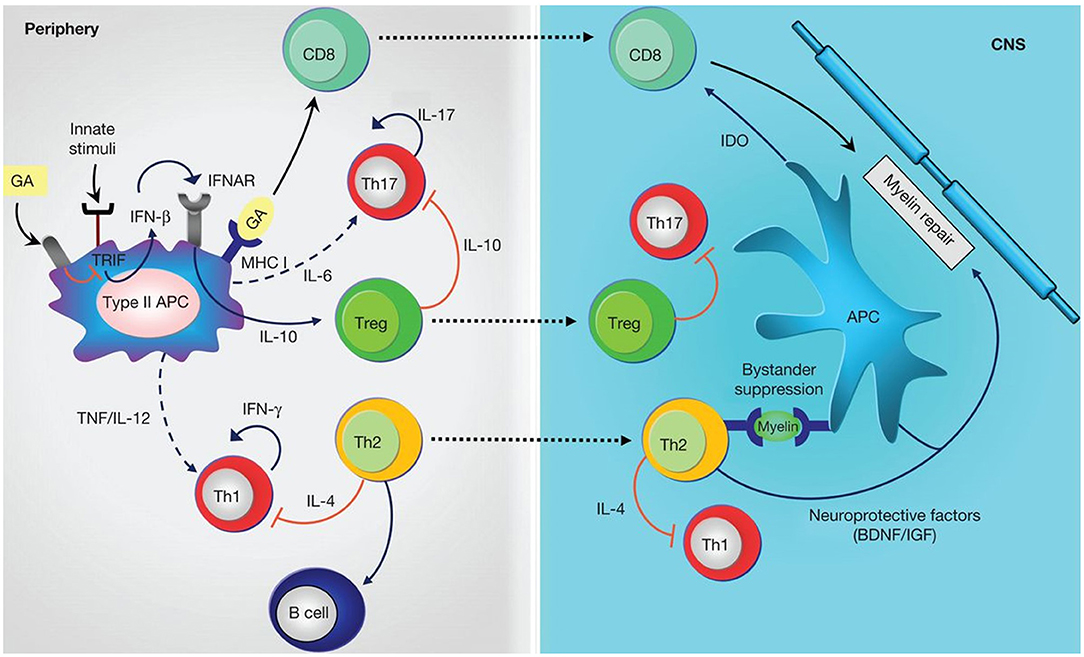
Figure 1. Anti-inflammatory mechanisms induced by glatiramer acetate (GA). GA treatment on antigen-presenting cells (APCs) leads to anti-inflammatory differentiation. Treatment modulates innate stimuli and is associated with down-regulation of type I interferon (IFN), increased T helper (Th)2, and regulatory T (Treg) cell differentiation. Reactivation of GA-reactive Th2 cells in periphery through presentation of myelin antigens is associated with bystander suppression. Th2 cells also modulate B-cell activation. Treg cells down-regulate secretion of proinflammatory cytokines by effector T (Teff) cells both in periphery and in the CNS. CD8+ T cells are generated by antigen presentation of GA in periphery and migrate to the CNS where they contribute to inhibiting myelin degradation. IL, Interleukin; TNF, tumor necrosis factor; IFNAR, interferon-receptor; MHC, major histocompatibility complex; BDNF, brain-derived neurotrophic factor; IGF, insulin-like growth factor; IDO, indoleamine-2,3-dioxygenase; solid lines, cytokines produced by the representative cells; dashed lines, reduced production of cytokines; red lines, inhibitory cytokines (72). [Figure from Prod92homme and Zamvil (72)].
Special Populations That May Benefit From GA Treatment
Patients With High Burden of Comorbidities
PwMS tend to have more comorbidities than the general population, and these can present specific challenges in treatment selection (73–75). The most common medical comorbidities include hyperlipidemia, hypertension, diabetes, gastrointestinal or thyroid diseases, which tend to increase with age (76). Diabetes, hypertension, and chronic obstructive pulmonary disease are associated with increased disability progression in PwMS (77). Vascular comorbidity correlates with poorer cognitive functioning and brain volume in PwMS (78).
Comorbidities increase the complexity of patient management by increasing the risk of drug-drug and drug-disease interactions. In addition to treatment for comorbidities, and for MS itself, PwMS often require pharmacotherapy to treat MS symptoms, such as fatigue, spasticity, pain, sleep disorders depression, urogenital, sexual and bowel dysfunction (79). A systematic literature review identified polypharmacy, defined as ≥ 5 prescription medications, in 15 to 59% of PwMS; rates increased with age, comorbidities, disability, cognitive deficits, and MS disease activity, and were associated with lower quality of life (80). Evidence to support treatment decisions in MS patients who have significant comorbidity is lacking. This knowledge gap can be attributed to the underrepresentation of such patients in clinical trials (81), limiting the possibility for evidence-based treatment. Thus, informed treatment decisions taking a patient's comorbidities and their accompanying essential medications into account rely mainly on empirical knowledge.
Patients at Risk for Infections and Virus Reactivation
Infections represent a major cause of morbidity and mortality in the setting of MS (82, 83). PwMS are intrinsically prone to various infections such as urinary tract infections secondary to neurogenic bladder (84). Immunosuppressive DMTs may increase this risk (85, 86). All DMTs, except glatiramer acetate and interferons, impair immune surveillance to some degree (39, 40); however, it is important to distinguish between immunosuppressive drugs that impair immune function generally, increasing the risk for a broad range of infections, and immune modulating agents that selectively inhibit specific aspects of the immune system, thereby predisposing patient to a more restricted set of pathogens (87). There are continuing concerns about increased susceptibility to severe SARS-CoV-2 infection; however, PwMS have an infection risk similar to that of the general population (88). Among DMTs, the risk of infections in general is lower in patients receiving interferons and glatiramer acetate (89). Progressive multifocal leukoencephalopathy (PML), which is caused by emerging of neuropathogenic and neurovirulent pool of John Cunningham virus undergoing sequential genomic rearrangements in immunocompromised subjects, is also a major concern (90). Among DMTs for MS, natalizumab is associated with the highest risk for PML, but it also been reported very rarely with ocrelizumab, fingolimod, and dimethyl fumarate.
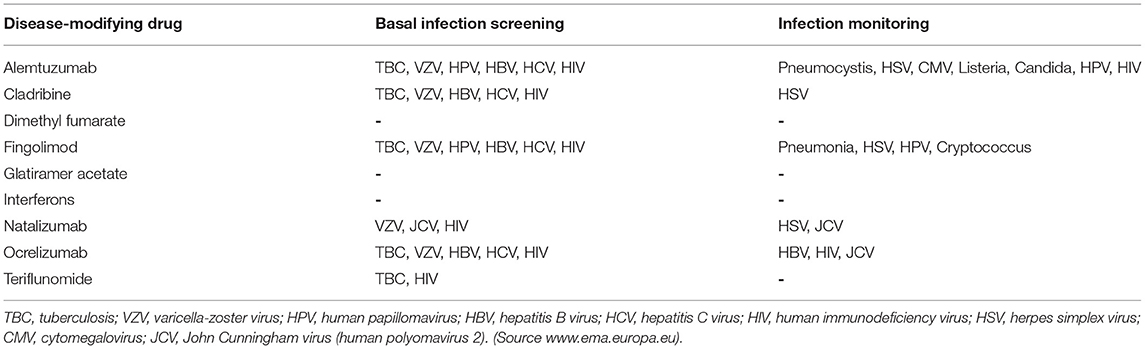
Evaluating the risk of infection has become one of the most important factors when choosing therapy or switching treatments (91), and monitoring for infections is an essential practice with some DMTs (Table 2).
The risk of hepatitis B virus reactivation with glatiramer acetate is equal to that of the general population (92), and screening for latent tuberculosis is not required before prescribing glatiramer.
Patients With Concomitant Liver Disease or History of Drug Induced Liver Damage
The risk of liver injury can limit a patient's treatment options. Several DMTs are associated with a risk of liver injury (alemtuzumab, fingolimod, interferons, mitoxantrone, teriflunomide) (93). Autoimmune hepatitis and reactivation of chronic liver infections can also occur during DMT treatment (93, 94). A retrospective Canadian study identified drug-induced liver injury in ~2% of MS patients treated with interferon beta (95). Baseline assessment of liver function is required for most DMTs, and several require periodic monitoring during treatment (Table 3).
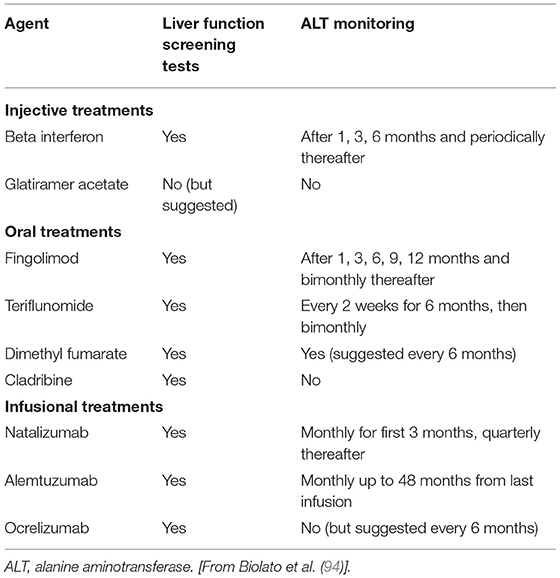
Table 3. Liver function testing requirements for disease-modifying treatments in MS (94).
Glatiramer acetate has a favorable overall liver safety profile (94). Sporadic reports of rare adverse liver effects with glatiramer acetate have included cases of suspected drug-induced liver injury and autoimmune hepatitis (93, 96), but no cases of hepatitis B virus or hepatitis C virus reactivation, or acute liver failure have been reported in patients treated with GA (94). Liver function testing is not required before initiating treatment with GA but the summary of product information suggests that patients be regularly monitored for signs of hepatic injury and instructed to seek immediate medical attention in case of symptoms of liver injury (97).
Elderly Patients
Peak MS incidence occurs in early adulthood, but prevalence peaks in late adulthood. Currently, the peak prevalence of PwMS is estimated to be between age 50 and 60 years (98, 99), meaning that many PwMS are older than the patient populations in pivotal trials for DMTs. The prevalence of MS in the elderly is increasing due to population aging, earlier diagnosis, access to DMTs, and improved supportive care (18–23). Moreover, about 5% of patients present with late-onset MS (onset at ≥ 50 years), often with motor dysfunction and a relatively poor prognosis (100–102).
The increased rate of comorbidities, with the accompanying polypharmacy, and immune senescence in elderly PwMS make selection of the optimal agent even more challenging. There is evidence that inflammatory lesion activity decreases with age in PwMS (103), and that the efficacy of DMTs decreases as well (28). There is no evidence to support differences in efficacy among DMTs in elderly patients (104). Meanwhile, some side effects of highly effective DMTs are more common/serious in elderly patients (105) (Table 4); therefore, the benefit-risk of a DMT may change as patients age, favoring a less effective DMT with a lower risk for adverse effects.
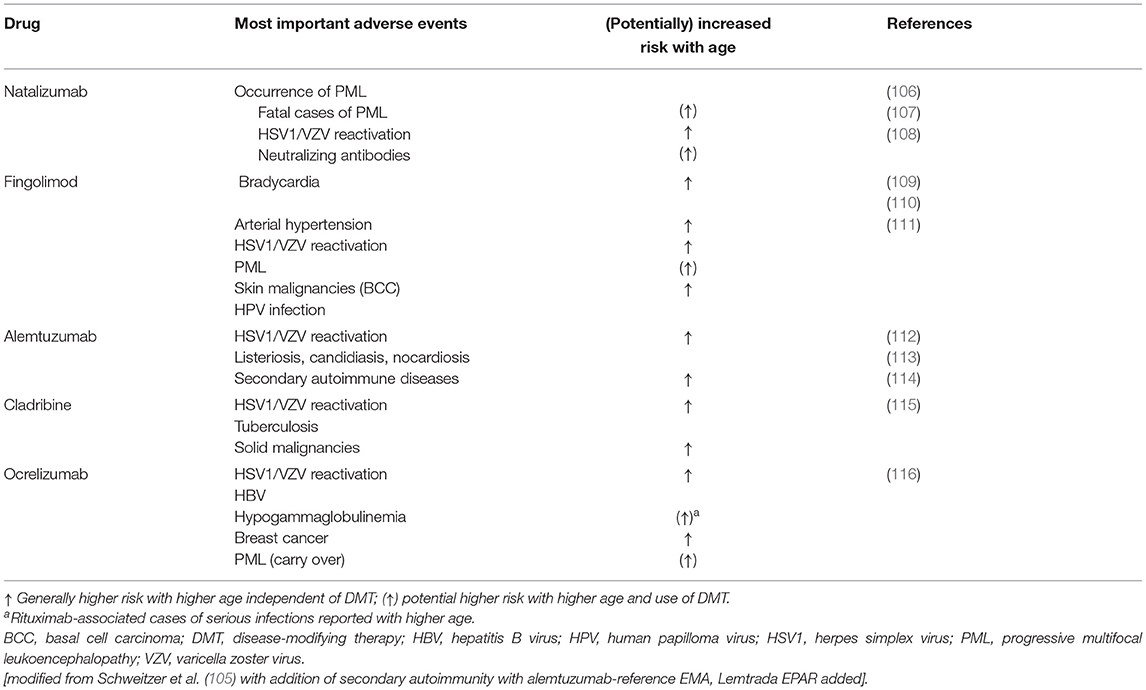
Table 4. High impact adverse events of DMTs and correlation with increasing age (105).
The pathological mechanisms underlying neurodegeneration during the progressive phase are thought to involve compartmentalized inflammation driving neurodegenerative tissue responses to chronic inflammatory injury (117). These mechanisms driving progressive disease do not appear to respond to DMT. Unless there is evidence of active inflammation, a high risk/high efficacy DMT might not be the right choice.
The absence of effective treatment or neuroprotective strategies for progressive disease in elderly patients, combined with the observed reduction in the efficacy of DMTs in the elderly (32, 104, 118), suggest a need to consider carefully the benefit-risk profile in this population (6). Thus, one of the unique challenges when treating elderly PwMS is determining the appropriateness/timing of DMT de-escalation or discontinuation in stable patients without clinical or radiological disease activity (119, 120). In a recent survey of 377 MS patients age ≥ 45 years who had been receiving DMT for ≥ 5 years, only 12% reported that they would consider discontinuing DMTs if they had no evidence of disease activity (121). Predictors of relapse/rebound included younger age, female sex, moderate disability, and a relapse within 1 year of discontinuation (122). Reactivation of MS disease activity after discontinuation of DMTs is independently associated with age at discontinuation, MRI activity at discontinuation, and the duration of clinical stability (123).
Pregnancy and Family Planning
The typical age at MS onset overlaps with childbearing years. Pregnancy is associated with lower MS disease activity (124), and may provide natural protection when DMT is suspended. Ideally, all agents except interferon or GA should be discontinued before attempting conception. An increasing number of pregnancies are conceived in women who are receiving DMT. Depending on the DMT, discontinuation in this situation may result in increased disease activity (125, 126). However, most of the safety data on exposure come from the first month after conception and focus on teratogenic risks and do not cover late term complications e.g., due to immunologic effects (126, 127). MS patient registries show that the injectable DMTs glatiramer acetate and interferon beta are indeed safe before conception and in patients with first trimester exposure, although limited data are available on their continuation throughout pregnancy (128–130). In light of the favorable safety evidence, both the US Food and Drug Administration and the European Medicines Agency have removed the restriction on GA use in pregnancy; however, as a precaution it is generally preferred to avoid exposure during pregnancy unless the benefit to the mother outweighs the risk to the fetus. When deemed necessary, administering a bridging therapy with a safer agent can provide coverage while trying to conceive (126). Switching treatments when planning a pregnancy in clinically stable patients is common practice (131); however, bridging therapy must be initiated early enough to be effective during the first trimester, when relapse risk is highest. Other strategies may include administering highly effective therapies that have long effect durations (e.g., ocrelizumab, alemtuzumab, cladribine) before pregnancy while observing the appropriate washout periods (126) or leveraging the fact that monoclonal antibodies do not cross the placenta until the second trimester (132).
Recent data from the German MS and Pregnancy Registry showed no evidence of adverse effects of GA exposure during breastfeeding on infant development, hospitalization, or the use of antibiotics (133). This has led to removal of the restriction on GA use while breastfeeding (134).
Vaccination
Vaccination does not appear to increase MS disease activity (135, 136). On the contrary, a variety of vaccine-preventable infections can exacerbate disease activity and trigger relapses (137–140); therefore, vaccination against preventable infections, for example influenza, can improve disease control. Guidelines from the American Academy of Neurology recommend following local vaccination schedules unless vaccination is contraindicated (e.g., PwMS already receiving immunosuppressive or immunomodulating therapy) or the patient is experiencing a relapse. In relapsing patients, vaccination should be delayed until resolution or until the relapse is no longer active or progressing (141). Moreover, PwMS should undergo a vaccination status assessment and updating of vaccinations soon after MS diagnosis to carefully plan and administer vaccinations early in the course of MS, before starting DMT (141, 142). Seroconversion after vaccination is attenuated in patients receiving anti-CD20 therapy, and the response to novel antigens (not encountered previously in life) is weakened (143), including after COVID-19 mRNA vaccination (144). Vaccines are less effective in the elderly, and immunodepleting therapy may further reduce the response to vaccines in this population.
In PwMS receiving GA, seroconversion was lower after the 2009 H1N1 pandemic influenza vaccine (n = 37) and the 2010 seasonal influenza vaccine (n = 12), compared to healthy controls (145). Because of this observation, the 2019 American Academy of Neurology guidelines stated that GA is “possibly” associated with a reduction in vaccine response. However, in a study of seroconversion after the 2010/2011 and 2011/2012 influenza vaccines, patients receiving GA (n = 26) had normal post-vaccination seroconversion rates for the 3 influenza antigens (H1N1 88.5%, H3N2 73.1%, B strain 80.8%, n = 26) (146). Response to the 2012/2013 seasonal influenza vaccine in patients receiving GA (n = 23) was also similar to healthy controls after 3, 6, and 12 months (147). Moreover, seroconversion after vaccination against SARS-CoV-2 is not attenuated in patients receiving GA (148–150). Live vaccinations are contraindicated in people receiving DMTs, except GA. Most oral DMTs interfere with response to hepatitis B virus vaccinations, whereas injectables therapies do not (151).
Conclusions
Given the difficulty of predicting the long-term course of MS at diagnosis, and although the early use of higher efficacy therapies may be warranted to prevent long-term disability, especially in patients with highly active disease, GA may be considered in scenarios where high efficacy therapies would pose more risk. GA may be appropriate later in the disease course in response to evolving patient conditions (e.g., aging, accumulating comorbidities, chronic treatment with corticosteroids and other immunosuppressants), or as a bridging therapy during conception, pregnancy, and breastfeeding. Similarly, it may be useful for vaccinations strategies, e.g., use of live or attenuated vaccines as well as vaccines against hepatitis B virus or SARS-CoV-2.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This study received funding from Viatris Inc. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of Interest
MM received consulting and/or speaking fees, research support or travel grants from Almirall, Bayer Schering, Biogen, CSL Behring, Sanofi-Genzyme, Merck, Novartis, Teva, Roche, Viatris (Mylan). JC received personal compensation for consulting for Biogen, Bristol-Myers Squibb, Convelo, Genentech, Janssen, NervGen, Novartis, and PSI; speaking for H3 Communications; and serving as an Editor of Multiple Sclerosis Journal. CW is a partner at Lycalis sprl. His organization has received compensation for consulting and speaking from Viatris (Mylan), Merck KAaG, Roche, Immunic, BMS Celgene, Novartis, Teva, Synthon, 2BBB, ICON, and Desitin. WB has received speaker honoraria and/or participated in advisory boards for Biogen, Celgene, Merck, Novartis, Roche, Sanofi and Viatris. PA received honoraria for lecturing and/or participation in advisory boards, and/or travel expenses for attending congresses and meetings from Almirall, Biogen, BMS-Celgene, Merck, Novartis, Roche, Sanofi-Genzyme, Teva Italia, and Viatris. CK received speaker honoraria and/or participated in advisory boards for Alexion, Biogen, Celgene, CSL Behring, Janssen-Cilag, MedDay, Merck, Novartis, Roche, Sanofi Genzyme, Teva and Viatris.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Editorial support and medical writing were provided by Ethos S.r.l.
References
1. US Food and Drug Administration. Glatopa (Glatiramer Acetate) Injection, 20 mg/mL. Approv Lett. (2014). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2015/090218Orig1s000ltr.pdf (accessed October 25, 2021).
2. U.S. Food and Drug Administration. Glatopa (Glatiramer Acetate) Injection, 40 mg/mL. Approv Lett. (2018). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2018/206921Orig1s000ltr.pdf (accessed October 25, 2021).
3. European Medicines Agency - Human Medicines Evaluation Division. List of nationally authorised medicinal products Active substance: glatiramer. (2018). Available online at: https://www.ema.europa.eu/en/documents/psusa/glatiramer-list-nationally-authorised-medicinal-products-psusa-00001529-202011_en.pdf (accessed October 25, 2021).
4. Bornstein MB, Miller AI, Teitelbaum D, Arnon R, Sela M. Multiple sclerosis: trial of a synthetic polypeptide. Ann Neurol. (1982) 11:317–9. doi: 10.1002/ana.410110314
5. Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology. (1995) 45:1268–76. doi: 10.1212/WNL.45.7.1268
6. Johnson KP, Brooks BR, Cohen JA, Ford CC, Goldstein J, Lisak RP, et al. Extended use of glatiramer acetate (Copaxone) is well tolerated and maintains its clinical effect on multiple sclerosis relapse rate and degree of disability. Copolymer 1 Multiple Sclerosis Study Group. Neurology. (1998) 50:701–8. doi: 10.1212/WNL.50.3.701
7. Comi G, Filippi M, Wolinsky JS. European/Canadian multicenter, double-blind, randomized, placebo-controlled study of the effects of glatiramer acetate on magnetic resonance imaging–measured disease activity and burden in patients with relapsing multiple sclerosis. Ann Neurol. (2001) 49:290–7. doi: 10.1002/ana.64
8. Wynn DR. Enduring Clinical Value of Copaxone® (Glatiramer Acetate) in Multiple Sclerosis after 20 Years of Use. Mult Scler Int. (2019) 2019:7151685. doi: 10.1155/2019/7151685
9. Cohen J, Belova A, Selmaj K, Wolf C, Sormani MP, Oberyé J, et al. Equivalence of Generic Glatiramer Acetate in Multiple Sclerosis: A Randomized Clinical Trial. JAMA Neurol. (2015) 72:1433–41. doi: 10.1001/jamaneurol.2015.2154
10. Bell C, Anderson J, Ganguly T, Prescott J, Capila I, Lansing JC, et al. Development of Glatopa® (Glatiramer Acetate): The First FDA-Approved Generic Disease-Modifying Therapy for Relapsing Forms of Multiple Sclerosis. J Pharm Pract. (2018) 31:481–8. doi: 10.1177/0897190017725984
11. La Mantia L, Munari LM, Lovati R. Glatiramer acetate for multiple sclerosis. Cochrane Database Syst Rev. (2010) CD004678. doi: 10.1002/14651858.CD004678.pub2
12. Reich DS, Lucchinetti CF, Calabresi PA. Multiple Sclerosis. N Engl J Med. (2018) 378:169–80. doi: 10.1056/NEJMra1401483
13. Lublin FD, Reingold SC, Cohen JA, Cutter GR, Sørensen PS, Thompson AJ, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. (2014) 83:278–86. doi: 10.1212/WNL.0000000000000560
14. Lublin FD, Coetzee T, Cohen JA, Marrie RA, Thompson AJ. The 2013 clinical course descriptors for multiple sclerosis: a clarification. Neurology. (2020) 94:1088–92. doi: 10.1212/WNL.0000000000009636
15. Lassmann H. Pathogenic Mechanisms Associated With Different Clinical Courses of Multiple Sclerosis. Front Immunol. (2019) 9:3116. doi: 10.3389/fimmu.2018.03116
16. Kappos L, Wolinsky JS, Giovannoni G, Arnold DL, Wang Q, Bernasconi C, et al. Contribution of Relapse-Independent Progression vs Relapse-Associated Worsening to Overall Confirmed Disability Accumulation in Typical Relapsing Multiple Sclerosis in a Pooled Analysis of 2 Randomized Clinical Trials. JAMA Neurol. (2020) 77:1132–40. doi: 10.1001/jamaneurol.2020.1568
17. Wiendl H, Gold R, Berger T, Derfuss T, Linker R, Mäurer M, et al. Multiple Sclerosis Therapy Consensus Group (MSTCG): position statement on disease-modifying therapies for multiple sclerosis (white paper). Ther Adv Neurol Disord. (2021) 14:17562864211039648. doi: 10.1177/17562864211039648
18. McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. (2001) 50:121–7. doi: 10.1002/ana.1032
19. Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Ann Neurol. (2005) 58:840–6. doi: 10.1002/ana.20703
20. Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. (2011) 69:292–302. doi: 10.1002/ana.22366
21. Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. (2018) 17:162–73. doi: 10.1016/S1474-4422(17)30470-2
22. Schwenkenbecher P, Wurster U, Konen FF, Gingele S, Sühs KW, Wattjes MP, et al. Impact of the McDonald Criteria 2017 on Early Diagnosis of Relapsing-Remitting Multiple Sclerosis. Front Neurol. (2019) 10:188. doi: 10.3389/fneur.2019.00188
23. Vaughn CB, Jakimovski D, Kavak KS, Ramanathan M, Benedict RHB, Zivadinov R, et al. Epidemiology and treatment of multiple sclerosis in elderly populations. Nat Rev Neurol. (2019) 15:329–42. doi: 10.1038/s41582-019-0183-3
24. Brønnum-Hansen H, Koch-Henriksen N, Stenager E. Trends in survival and cause of death in Danish patients with multiple sclerosis. Brain J Neurol. (2004) 127:844–50. doi: 10.1093/brain/awh104
25. Marrie RA, Elliott L, Marriott J, Cossoy M, Blanchard J, Leung S, et al. Effect of comorbidity on mortality in multiple sclerosis. Neurology. (2015) 85:240–7. doi: 10.1212/WNL.0000000000001718
26. Ekestern E, Lebhart G. Mortality from multiple sclerosis in Austria 1970-2001: dynamics, trends, and prospects. Eur J Neurol. (2004) 11:511–20. doi: 10.1111/j.1468-1331.2004.00818.x
27. Hauser SL, Cree BAC. Treatment of Multiple Sclerosis: A Review. Am J Med. (2020) 133:1380-1390.e2. doi: 10.1016/j.amjmed.2020.05.049
28. Rae-Grant A, Day GS, Marrie RA, Rabinstein A, Cree BAC, Gronseth GS, et al. Practice guideline recommendations summary: Disease-modifying therapies for adults with multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. (2018) 90:777–88. doi: 10.1212/WNL.0000000000005347
29. Montalban X, Gold R, Thompson AJ, Otero-Romero S, Amato MP, Chandraratna D, et al. ECTRIMS/EAN Guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler. (2018) 24:96–120. doi: 10.1177/1352458517751049
30. NHS England. Treatment Algorithm for Multiple Sclerosis Disease-Modifying Therapies NHS England Reference: 170079ALG Treatment Algorithm for Multiple Sclerosis Disease-modifying Therapies Contents. (2018). Available online at: https://www.england.nhs.uk/commissioning/wp-content/uploads/sites/12/2019/03/Treatment-Algorithm-for-Multiple-Sclerosis-Disease-Modifying-Therapies-08-03-2019-1.pdf (accessed September 29, 2021).
31. Rieckmann P. Concepts of induction and escalation therapy in multiple sclerosis. J Neurol Sci. (2009) 277 (Suppl):S42–5. doi: 10.1016/S0022-510X(09)70012-7
32. Weideman AM, Tapia-Maltos MA, Johnson K, Greenwood M, Bielekova B. Meta-analysis of the Age-Dependent Efficacy of Multiple Sclerosis Treatments. Front Neurol. (2017) 8:577. doi: 10.3389/fneur.2017.00577
33. Granqvist M, Boremalm M, Poorghobad A, Svenningsson A, Salzer J, Frisell T, et al. Comparative Effectiveness of Rituximab and Other Initial Treatment Choices for Multiple Sclerosis. JAMA Neurol. (2018) 75:320–7. doi: 10.1001/jamaneurol.2017.4011
34. Brown JWL, Coles A, Horakova D, Havrdova E, Izquierdo G, Prat A, et al. Association of Initial Disease-Modifying Therapy With Later Conversion to Secondary Progressive Multiple Sclerosis. JAMA. (2019) 321:175–87. doi: 10.1001/jama.2018.20588
35. Harding K, Williams O, Willis M, Hrastelj J, Rimmer A, Joseph F, et al. Clinical Outcomes of Escalation vs Early Intensive Disease-Modifying Therapy in Patients With Multiple Sclerosis. JAMA Neurol. (2019) 76:536–41. doi: 10.1001/jamaneurol.2018.4905
36. He A, Merkel B, Brown JWL, Zhovits Ryerson L, Kister I, Malpas CB, et al. Timing of high-efficacy therapy for multiple sclerosis: a retrospective observational cohort study. Lancet Neurol. (2020) 19:307–16. doi: 10.1016/S1474-4422(20)30067-3
37. Klotz L, Havla J, Schwab N, Hohlfeld R, Barnett M, Reddel S, et al. Risks and risk management in modern multiple sclerosis immunotherapeutic treatment. Ther Adv Neurol Disord. (2019) 12:1756286419836571. doi: 10.1177/1756286419836571
38. Jalkh G, Abi Nahed R, Macaron G, Rensel M. Safety of Newer Disease Modifying Therapies in Multiple Sclerosis. Vaccines. (2020) 9:12. doi: 10.3390/vaccines9010012
39. Luna G, Alping P, Burman J, Fink K, Fogdell-Hahn A, Gunnarsson M, et al. Infection Risks Among Patients With Multiple Sclerosis Treated With Fingolimod, Natalizumab, Rituximab, and Injectable Therapies. JAMA Neurol. (2020) 77:184–91. doi: 10.1001/jamaneurol.2019.3365
40. Wijnands JMA, Zhu F, Kingwell E, Fisk JD, Evans C, Marrie RA, et al. Disease-modifying drugs for multiple sclerosis and infection risk: a cohort study. J Neurol Neurosurg Psychiatry. (2018) 89:1050–6. doi: 10.1136/jnnp-2017-317493
41. US Food Drug Administration. Prescribing information: AVONEX (interferon beta-1a). (2020). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/103628s5263lbl.pdf (accessed October 26, 2021).
42. US Food Drug Administration. Prescribing information: BETASERON (interferon beta-1b). (2016). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/103471s5157lbl.pdf (accessed October 26, 2021).
43. US Food Drug Administration. Copaxone Highlights of Prescribing Information. (2018). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/020622s102lbl.pdf (accessed October 26, 2021).
44. US Food Drug Administration. Prescribing Information: AUBAGIO. (2019). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/202992s006lbl.pdf (accessed October 26, 2021).
45. US Food Drug Administration. Prescribing information: BafiertamTM (monomethyl fumarate). (2020). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/210296s000lbl.pdf (accessed September 29, 2021).
46. US Food Drug Administration. Prescribing information: TECFIDERA® (dimethyl fumarate). (2017). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/204063s017lbl.pdf (accessed September 29, 2021).
47. US Food Drug Administration. Prescribing information: Vumerity (diroximel fumarate). (2019). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211855s000lbl.pdf (accessed September 29, 2021).
48. US Food Drug Administration. TYSABRI Highlights of Prescribing Information. (2017). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/1215104s959lbl.pdf (accessed September 29, 2021).
49. US Food Drug Administration. Prescribing information Gilenya (fingolimod). (2019). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022527s26lbl.pdf (accessed October 26, 2021).
50. US Food Drug Administration. Prescribing information. PONVORY (ponesimod). (2021). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/213498s000lbl.pdf (accessed October 26, 2021).
51. US Food Drug Administration. Prescribing information: ZEPOSIA (ozanimod). (2020). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/209899s000lbl.pdf (accessed October 26, 2021).
52. US Food Drug Administration. Prescribing information: MAYZENT (siponimod). (2019). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/209884s000lbl.pdf (accessed October 26, 2021).
53. US Food Drug Administration. LEMTRADA® HIGHLIGHTS OF PRESCRIBING INFORMATION. (2017). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/103948s5158lbl.pdf (accessed September 29, 2021).
54. US Food Drug Administration. Prescribing information: OCREVUS (ocrelizumab). (2020). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761053s023lbl.pdf (accessed October 26, 2021).
55. US Food Drug Administration. Prescribing information: KESIMPTA (ofatumumab). (2020). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125326s070lbl.pdf (accessed October 26, 2021).
56. US Food Drug Administration. Mavenclad Highlights of Prescribing Information. (2019). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022561s000lbl.pdf (accessed September 29, 2021).
57. US Food Drug Administration. NOVANTRONE ® mitoXANTRONE Label. (2009). Available online at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/019297s030s031lbl.pdf (accessed September 29, 2021).
58. Castillo-Trivino T, Mowry EM, Gajofatto A, Chabas D, Crabtree-Hartman E, Cree BA, et al. Switching multiple sclerosis patients with breakthrough disease to second-line therapy. PLoS ONE. (2011) 6:e16664. doi: 10.1371/journal.pone.0016664
59. Giovannoni G, Butzkueven H, Dhib-Jalbut S, Hobart J, Kobelt G, Pepper G, et al. Brain health: time matters in multiple sclerosis. Mult Scler Relat Disord. (2016) 9 (Suppl 1):S5–S48. doi: 10.1016/j.msard.2016.07.003
60. Havrdova E, Galetta S, Stefoski D, Comi G. Freedom from disease activity in multiple sclerosis. Neurology. (2010) 74 (Suppl 3):S3–7. doi: 10.1212/WNL.0b013e3181dbb51c
61. Giovannoni G, Turner B, Gnanapavan S, Offiah C, Schmierer K, Marta M. Is it time to target no evident disease activity (NEDA) in multiple sclerosis? Mult Scler Relat Disord. (2015) 4:329–33. doi: 10.1016/j.msard.2015.04.006
62. Hegen H, Bsteh G, Berger T. “No evidence of disease activity” - is it an appropriate surrogate in multiple sclerosis? Eur J Neurol. (2018) 25:1107–e101. doi: 10.1111/ene.13669
63. Cree BAC, Hollenbach JA, Bove R, Kirkish G, Sacco S, Caverzasi E, et al. Silent progression in disease activity-free relapsing multiple sclerosis. Ann Neurol. (2019) 85:653–66. doi: 10.1002/ana.25463
64. Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. (2018) 14:577–89. doi: 10.1038/s41582-018-0058-z
65. Cai L, Huang J. Neurofilament light chain as a biological marker for multiple sclerosis: a meta-analysis study. Neuropsychiatr Dis Treat. (2018) 14:2241–54. doi: 10.2147/NDT.S173280
66. Rahn AC, Solari A, Beckerman H, Nicholas R, Wilkie D, Heesen C, et al. Rehabilitation in Multiple Sclerosis (RIMS) Special Interest Group on Patient Autonomy. “I Will Respect the Autonomy of My Patient”: A Scoping Review of Shared Decision Making in Multiple Sclerosis. Int J MS Care. (2020) 22:285–93. doi: 10.7224/1537-2073.2020-027
67. Bansback N, Chiu JA, Carruthers R, Metcalfe R, Lapointe E, Schabas A, et al. Development and usability testing of a patient decision aid for newly diagnosed relapsing multiple sclerosis patients. BMC Neurol. (2019) 19:173. doi: 10.1186/s12883-019-1382-7
68. Tintoré M, Alexander M, Costello K, Duddy M, Jones DE, Law N, et al. The state of multiple sclerosis: current insight into the patient/health care provider relationship, treatment challenges, and satisfaction. Patient Prefer Adherence. (2017) 11:33–45. doi: 10.2147/PPA.S115090
69. Oreja-Guevara C, Potra S, Bauer B, Centonze D, Giambastiani M-P, Giovannoni G, et al. Joint Healthcare Professional and Patient Development of Communication Tools to Improve the Standard of MS Care. Adv Ther. (2019) 36:3238–52. doi: 10.1007/s12325-019-01071-9
70. Fox RJ, Cosenza C, Cripps L, Ford P, Mercer M, Natarajan S, et al. survey of risk tolerance to multiple sclerosis therapies. Neurology. (2019) 92:e1634–42. doi: 10.1212/WNL.0000000000007245
71. Johnson KP. Glatiramer acetate and the glatiramoid class of immunomodulator drugs in multiple sclerosis: an update. Expert Opin Drug Metab Toxicol. (2010) 6:643–60. doi: 10.1517/17425251003752715
72. Prod'homme T, Zamvil SS. The Evolving Mechanisms of Action of Glatiramer Acetate. Cold Spring Harb Perspect Med. (2019) 9:a029249. doi: 10.1101/cshperspect.a029249
73. Marrie RA, Cohen J, Stuve O, Trojano M, Sørensen PS, Reingold S, et al. systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: overview. Mult Scler. (2015) 21:263–81. doi: 10.1177/1352458514564491
74. Hauer L, Perneczky J, Sellner J. A global view of comorbidity in multiple sclerosis: a systematic review with a focus on regional differences, methodology, and clinical implications. J Neurol. (2020) 268:4066–77. doi: 10.1007/s00415-020-10107-y
75. Kern DM, Cepeda MS. Treatment patterns and comorbid burden of patients newly diagnosed with multiple sclerosis in the United States. BMC Neurol. (2020) 20:296. doi: 10.1186/s12883-020-01882-2
76. Edwards NC, Munsell M, Menzin J, Phillips AL. Comorbidity in US patients with multiple sclerosis. Patient Relat Outcome Meas. (2018) 9:97–102. doi: 10.2147/PROM.S148387
77. Magyari M, Sorensen PS. Comorbidity in Multiple Sclerosis. Front Neurol. (2020) 11:851. doi: 10.3389/fneur.2020.00851
78. Fitzgerald KC, Damian A, Conway D, Mowry EM. Vascular comorbidity is associated with lower brain volumes and lower neuroperformance in a large multiple sclerosis cohort. Mult Scler. (2021) 27:1914–23. doi: 10.1177/1352458520984746
79. Rommer PS, Eichstädt K, Ellenberger D, Flachenecker P, Friede T, Haas J, et al. Symptomatology and symptomatic treatment in multiple sclerosis: Results from a nationwide MS registry. Mult Scler. (2019) 25:1641–52. doi: 10.1177/1352458518799580
80. Frahm N, Hecker M, Zettl UK. Polypharmacy among patients with multiple sclerosis: a qualitative systematic review. Expert Opin Drug Saf. (2020) 19:139–45. doi: 10.1080/14740338.2020.1720646
81. Boyd CM, Vollenweider D, Puhan MA. Informing evidence-based decision-making for patients with comorbidity: availability of necessary information in clinical trials for chronic diseases. PLoS ONE. (2012) 7:e41601. doi: 10.1371/journal.pone.0041601
82. Manouchehrinia A, Tanasescu R, Tench CR, Constantinescu CS. Mortality in multiple sclerosis: meta-analysis of standardised mortality ratios. J Neurol Neurosurg Psychiatry. (2016) 87:324–31. doi: 10.1136/jnnp-2015-310361
83. Kingwell E, Zhu F, Evans C, Duggan T, Oger J, Tremlett H. Causes that Contribute to the Excess Mortality Risk in Multiple Sclerosis: A Population-Based Study. Neuroepidemiology. (2020) 54:131–9. doi: 10.1159/000504804
84. Medeiros Junior WLG de, Demore CC, Mazaro LP, de Souza MFN, Parolin LF, Melo LH, et al. Urinary tract infection in patients with multiple sclerosis: An overview. Mult Scler Relat Disord. (2020) 46:102462. doi: 10.1016/j.msard.2020.102462
85. Nelson RE, Xie Y, DuVall SL, Butler J, Kamauu AWC, Knippenberg K, et al. Multiple Sclerosis and Risk of Infection-Related Hospitalization and Death in US Veterans. Int J MS Care. (2015) 17:221–30. doi: 10.7224/1537-2073.2014-035
86. Lechner-Scott J, Waubant E, Levy M, Hawkes C, Giovannoni G. Is multiple sclerosis a risk factor for infections? Mult Scler Relat Disord. (2020) 41:102184. doi: 10.1016/j.msard.2020.102184
87. Winkelmann A, Loebermann M, Reisinger EC, Hartung HP, Zettl UK. Disease-modifying therapies and infectious risks in multiple sclerosis. Nat Rev Neurol. (2016) 12:217–33. doi: 10.1038/nrneurol.2016.21
88. Richter D, Faissner S, Bartig D, Tönges L, Hellwig K, Ayzenberg I, et al. Multiple sclerosis is not associated with an increased risk for severe COVID-19: a nationwide retrospective cross-sectional study from Germany. Neurol Res Pract. (2021) 3:42. doi: 10.1186/s42466-021-00143-y
89. Reder AT, Centonze D, Naylor ML, Nagpal A, Rajbhandari R, Altincatal A, et al. COVID-19 in Patients with Multiple Sclerosis: Associations with Disease-Modifying Therapies. CNS Drugs. (2021) 35:317–30. doi: 10.1007/s40263-021-00804-1
90. Cortese I, Reich DS, Nath A. Progressive multifocal leukoencephalopathy and the spectrum of JC virus-related disease. Nat Rev Neurol. (2021) 17:37–51. doi: 10.1038/s41582-020-00427-y
91. Celius EG. Infections in patients with multiple sclerosis: Implications for disease-modifying therapy. Acta Neurol Scand. (2017) 136 Suppl:34–6. doi: 10.1111/ane.12835
92. Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology. (2015) 148:221–244.e3. doi: 10.1053/j.gastro.2014.10.038
93. Antonazzo IC, Poluzzi E, Forcesi E, Riise T, Bjornevik K, Baldin E, et al. Liver injury with drugs used for multiple sclerosis: A contemporary analysis of the FDA Adverse Event Reporting System. Mult Scler. (2019) 25:1633–40. doi: 10.1177/1352458518799598
94. Biolato M, Bianco A, Lucchini M, Gasbarrini A, Mirabella M, Grieco A. The Disease-Modifying Therapies of Relapsing-Remitting Multiple Sclerosis and Liver Injury: a narrative review. CNS Drugs. (2021) 35:861–80. doi: 10.1007/s40263-021-00842-9
95. Kowalec K, Kingwell E, Yoshida EM, Marrie RA, Kremenchutzky M, Campbell TL, et al. Characteristics associated with drug-induced liver injury from interferon beta in multiple sclerosis patients. Expert Opin Drug Saf. (2014) 13:1305–17. doi: 10.1517/14740338.2014.947958
96. Almeida J, Solà-Valls N, Pose E, Blanco Y, Sepúlveda M, Llufriu S, et al. Liver injury and glatiramer acetate, an uncommon association: case report and literature review. Ther Adv Neurol Disord. (2017) 10:367–72. doi: 10.1177/1756285617722352
97. Electronic Medicines Compendium (emc). Copaxone 20 mg/ml solution for injection in pre-filled syringe - Summary of Product Characteristics (2021). Available online at: https://www.medicines.org.uk/emc/product/183/smpc (accessed October 26, 2021).
98. Wallin MT, Culpepper WJ, Campbell JD, Nelson LM, Langer-Gould A, Marrie RA, et al. The prevalence of MS in the United States: a population-based estimate using health claims data. Neurology. (2019) 92:e1029–40. doi: 10.1212/WNL.0000000000007035
99. GBD 2016 Multiple Sclerosis Collaborators. Global, regional, and national burden of multiple sclerosis 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. (2019)18:269–85. doi: 10.1016/S1474-4422(18)30443-5
100. Martinelli V, Rodegher M, Moiola L, Comi G. Late onset multiple sclerosis: clinical characteristics, prognostic factors and differential diagnosis. Neurol Sci. (2004) 25 Suppl 4:S350–5. doi: 10.1007/s10072-004-0339-8
101. Guillemin F, Baumann C, Epstein J, Kerschen P, Garot T, Mathey G, et al. Older Age at Multiple Sclerosis Onset Is an Independent Factor of Poor Prognosis: a population-based cohort study. Neuroepidemiology. (2017) 48:179–87. doi: 10.1159/000479516
102. Naseri A, Nasiri E, Sahraian MA, Daneshvar S, Talebi M. Clinical Features of Late-Onset Multiple Sclerosis: a Systematic Review and Meta-analysis. Mult Scler Relat Disord. (2021) 50:102816. doi: 10.1016/j.msard.2021.102816
103. Scalfari A, Lederer C, Daumer M, Nicholas R, Ebers GC, Muraro PA. The relationship of age with the clinical phenotype in multiple sclerosis. Mult Scler. (2016) 22:1750–8. doi: 10.1177/1352458516630396
104. Zhang Y, Gonzalez Caldito N, Shirani A, Salter A, Cutter G, Culpepper W 2nd, et al. Aging and efficacy of disease-modifying therapies in multiple sclerosis: a meta-analysis of clinical trials. Ther Adv Neurol Disord. (2020) 13:1756286420969016. doi: 10.1177/1756286420969016
105. Schweitzer F, Laurent S, Fink GR, Barnett MH, Reddel S, Hartung HP, et al. Age and the risks of high-efficacy disease modifying drugs in multiple sclerosis. Curr Opin Neurol. (2019) 32:305–12. doi: 10.1097/WCO.0000000000000701
106. Dong-Si T, Gheuens S, Gangadharan A, Wenten M, Philip J, McIninch J, et al. Predictors of survival and functional outcomes in natalizumab-associated progressive multifocal leukoencephalopathy. J Neurovirol. (2015) 21:637–44. doi: 10.1007/s13365-015-0316-4
107. Blankenbach K, Schwab N, Hofner B, Adams O, Keller-Stanislawski B, Warnke C. Natalizumab-associated progressive multifocal leukoencephalopathy in Germany. Neurology. (2019) 92:e2232–9. doi: 10.1212/WNL.0000000000007451
108. Bachelet D, Hässler S, Mbogning C, Link J, Ryner M, Ramanujam R, et al. Occurrence of anti-drug antibodies against interferon-beta and natalizumab in multiple sclerosis: a collaborative cohort analysis. PLoS ONE. (2016) 11:e0162752. doi: 10.1371/journal.pone.0162752
109. Lebrun C, Rocher F. Cancer Risk in Patients with Multiple Sclerosis: Potential Impact of Disease-Modifying Drugs. CNS Drugs. (2018) 32:939–49. doi: 10.1007/s40263-018-0564-y
110. Berger JR, Cree BA, Greenberg B, Hemmer B, Ward BJ, Dong VM, et al. Progressive multifocal leukoencephalopathy after fingolimod treatment. Neurology. (2018) 90:e1815–21. doi: 10.1212/WNL.0000000000005529
111. Ritter C, Svačina MKR, Bobylev I, Joshi A, Schneider T, Lehmann HC. Impact of Age and Polytherapy on Fingolimod Induced Bradycardia: a Preclinical Study. J Neuroimmune Pharmacol. (2017) 12:204–9. doi: 10.1007/s11481-017-9727-8
112. Huhn K, Bayas A, Doerck S, Frank B, Gerbershagen K, Hellwig K, et al. Alemtuzumab as rescue therapy in a cohort of 50 relapsing-remitting MS patients with breakthrough disease on fingolimod: a multi-center observational study. J Neurol. (2018) 265:1521–7. doi: 10.1007/s00415-018-8871-2
113. Pfeuffer S, Schmidt R, Straeten FA, Pul R, Kleinschnitz C, Wieshuber M, et al. Efficacy and safety of alemtuzumab versus fingolimod in RRMS after natalizumab cessation. J Neurol. (2019) 266:165–73. doi: 10.1007/s00415-018-9117-z
114. European Medicines Agency. LEMTRADA epar-product-information. Available online at: https://www.ema.europa.eu/en/documents/product-information/lemtrada-epar-product-information_en.pdf (accessed September 30, 2021).
115. European Medicines Agency. Mavenclad, INN-cladribine (2017). Available online at: https://www.ema.europa.eu/en/documents/product-information/mavenclad-epar-product-information_en.pdf (accessed October 26, 2021).
116. European, Medicines Agency. Ocrevus European Public Assessment Report. Available online at: https://www.ema.europa.eu/en/documents/product-information/ocrevus-epar-product-information_en.pdf (accessed September 30, 2021).
117. Absinta M, Lassmann H, Trapp BD. Mechanisms underlying progression in multiple sclerosis. Curr Opin Neurol. (2020) 33:277–85. doi: 10.1097/WCO.0000000000000818
118. Dahlke F, Arnold DL, Aarden P, Ganjgahi H, Häring DA, Cuklina J, et al. Characterisation of MS phenotypes across the age span using a novel data set integrating 34 clinical trials (NO.MS cohort): Age is a key contributor to presentation. Mult Scler. (2021) 27:2062–76. doi: 10.1177/1352458520988637
119. Ostolaza A, Corroza J, Ayuso T. Multiple sclerosis and aging: comorbidity and treatment challenges. Mult Scler Relat Disord. (2021) 50:102815. doi: 10.1016/j.msard.2021.102815
120. Hartung HP, Meuth SG, Miller DM, Comi G. Stopping disease-modifying therapy in relapsing and progressive multiple sclerosis. Curr Opin Neurol. (2021) 34:598–603. doi: 10.1097/WCO.0000000000000960
121. McGinley MP, Cola PA, Fox RJ, Cohen JA, Corboy JJ, Miller D. Perspectives of individuals with multiple sclerosis on discontinuation of disease-modifying therapies. Mult Scler. (2020) 26:1581–9. doi: 10.1177/1352458519867314
122. Kister I, Spelman T, Patti F, Duquette P, Trojano M, Izquierdo G, et al. Predictors of relapse and disability progression in MS patients who discontinue disease-modifying therapy. J Neurol Sci. (2018) 391:72–6. doi: 10.1016/j.jns.2018.06.001
123. Bsteh G, Hegen H, Riedl K, Altmann P, Auer M, Berek K, et al. Quantifying the risk of disease reactivation after interferon and glatiramer acetate discontinuation in multiple sclerosis: the VIAADISC score. Eur J Neurol. (2021) 28:1609–16. doi: 10.1111/ene.14705
124. Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med. (1998) 339:285–91. doi: 10.1056/NEJM199807303390501
125. Alroughani R, Alowayesh MS, Ahmed SF, Behbehani R, Al-Hashel J. Relapse occurrence in women with multiple sclerosis during pregnancy in the new treatment era. Neurology. (2018) 90:e840–6. doi: 10.1212/WNL.0000000000005065
126. Krysko KM, Bove R, Dobson R, Jokubaitis V, Hellwig K. Treatment of Women with Multiple Sclerosis Planning Pregnancy. Curr Treat Options Neurol. (2021) 23:11. doi: 10.1007/s11940-021-00666-4
127. Nguyen AL, Havrdova EK, Horakova D, Izquierdo G, Kalincik T, van der Walt A, et al. Incidence of pregnancy and disease-modifying therapy exposure trends in women with multiple sclerosis: a contemporary cohort study. Mult Scler Relat Disord. (2019) 28:235–43. doi: 10.1016/j.msard.2019.01.003
128. Herbstritt S, Langer-Gould A, Rockhoff M, Haghikia A, Queisser-Wahrendorf A, Gold R, et al. Glatiramer acetate during early pregnancy: a prospective cohort study. Mult Scler. (2016) 22:810–6. doi: 10.1177/1352458515623366
129. Thiel S, Langer-Gould A, Rockhoff M, Haghikia A, Queisser-Wahrendorf A, Gold R, et al. Interferon-beta exposure during first trimester is safe in women with multiple sclerosis-a prospective cohort study from the German Multiple Sclerosis and Pregnancy Registry. Mult Scler. (2016) 22:801–9. doi: 10.1177/1352458516634872
130. Sandberg-Wollheim M, Neudorfer O, Grinspan A, Weinstock-Guttman B, Haas J, Izquierdo G, et al. Pregnancy Outcomes from the Branded Glatiramer Acetate Pregnancy Database. Int J MS Care. (2018) 20:9–14. doi: 10.7224/1537-2073.2016-079
131. Almouzain L, Stevenson F, Chard D, Rahman NA, Hamilton F. Switching treatments in clinically stable relapsing remitting multiple sclerosis patients planning for pregnancy. Mult Scler J Exp Transl Clin. (2021) 7:20552173211001571. doi: 10.1177/20552173211001571
132. DeSesso JM, Williams AL, Ahuja A, Bowman CJ, Hurtt ME. The placenta, transfer of immunoglobulins, and safety assessment of biopharmaceuticals in pregnancy. Crit Rev Toxicol. (2012) 42:185–210. doi: 10.3109/10408444.2011.653487
133. Ciplea A, Kurzeja A, Thiel S, Haben S, Alexander J, Adamus E, et al. Safety analysis of offspring breastfed by mothers on glatiramer acetate therapy for relapsing multiple sclerosis. Eur J Neurol. (2021) 28:201–2.
134. COPAXONE PRESCRIBING INFORMATION (2022). Available online at: https://www.copaxone.com/globalassets/copaxone/prescribing-information.pdf (accessed February 15, 2022).
135. Confavreux C, Suissa S, Saddier P, Bourdès V, Vukusic S. Vaccinations and the risk of relapse in multiple sclerosis. Vaccines in Multiple Sclerosis Study Group. N Engl J Med. (2001) 344:319–26. doi: 10.1056/NEJM200102013440501
136. Di Filippo M, Cordioli C, Malucchi S, Annovazzi P, Cavalla P, Torri Clerici V, et al. mRNA COVID-19 vaccines do not increase the short-term risk of clinical relapses in multiple sclerosis. J Neurol Neurosurg Psychiatry. (2021) 93:448–50. doi: 10.1136/jnnp-2021-327200
137. Buljevac D, Flach HZ, Hop WCJ, Hijdra D, Laman JD, Savelkoul HFJ, et al. Prospective study on the relationship between infections and multiple sclerosis exacerbations. Brain J Neurol. (2002) 125:952–60. doi: 10.1093/brain/awf098
138. Correale J, Fiol M, Gilmore W. The risk of relapses in multiple sclerosis during systemic infections. Neurology. (2006) 67:652–9. doi: 10.1212/01.wnl.0000233834.09743.3b
139. Vollmer T. The natural history of relapses in multiple sclerosis. J Neurol Sci. (2007) 256 Suppl:S5–13. doi: 10.1016/j.jns.2007.01.065
140. Steelman AJ. Infection as an Environmental Trigger of Multiple Sclerosis Disease Exacerbation. Front Immunol. (2015) 6:520. doi: 10.3389/fimmu.2015.00520
141. Farez MF, Correale J, Armstrong MJ, Rae-Grant A, Gloss D, Donley D, et al. Practice guideline update summary: Vaccine-preventable infections and immunization in multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. (2019) 93:584–94. doi: 10.1212/WNL.0000000000008157
142. Smets I, Reyes S, Baker D, Giovannoni G. Blunted vaccines responses after ocrelizumab highlight need for immunizations prior to treatment. Mult Scler Relat Disord. (2021) 50:102851. doi: 10.1016/j.msard.2021.102851
143. Bar-Or A, Calkwood JC, Chognot C, Evershed J, Fox EJ, Herman A, et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: The VELOCE study. Neurology. (2020) 95:e1999–2008. doi: 10.1212/WNL.0000000000010380
144. Ali A, Dwyer D, Wu Q, Wang Q, Dowling CA, Fox DA, et al. Characterization of humoral response to COVID mRNA vaccines in multiple sclerosis patients on disease modifying therapies. Vaccine. (2021) 39:6111–6. doi: 10.1016/j.vaccine.2021.08.078
145. Olberg HK, Cox RJ, Nostbakken JK, Aarseth JH, Vedeler CA, Myhr KM. Immunotherapies influence the influenza vaccination response in multiple sclerosis patients: an explorative study. Mult Scler. (2014) 20:1074–80. doi: 10.1177/1352458513513970
146. Metze C, Winkelmann A, Loebermann M, Hecker M, Schweiger B, Reisinger EC, et al. Immunogenicity and predictors of response to a single dose trivalent seasonal influenza vaccine in multiple sclerosis patients receiving disease-modifying therapies. CNS Neurosci Ther. (2019) 25:245–54. doi: 10.1111/cns.13034
147. Olberg HK, Eide GE, Cox RJ, Jul-Larsen Å, Lartey SL, Vedeler CA, Myhr KM. Antibody response to seasonal influenza vaccination in patients with multiple sclerosis receiving immunomodulatory therapy. Eur J Neurol. (2018) 25:527–34. doi: 10.1111/ene.13537
148. Ciampi E, Uribe-San-Martin R, Soler B, García L, Guzman J, Pelayo C, et al. Safety and humoral response rate of inactivated and mRNA vaccines against SARS-CoV-2 in patients with Multiple Sclerosis. Mult Scler Relat Disord. (2022) 59:103690–103690. doi: 10.1016/j.msard.2022.103690
149. Bock H, Juretzek T, Handreka R, Ruhnau J, Löbel M, Reuner K, et al. Humoral and cellular immune responses to SARS CoV-2 vaccination in People with Multiple Sclerosis and NMOSD patients receiving immunomodulatory treatments. Mult Scler Relat Disord. (2022) 59:103554–103554. doi: 10.1016/j.msard.2022.103554
150. Sabatino JJJ, Mittl K, Rowles WM, McPolin K, Rajan JV, Laurie MT, et al. Multiple sclerosis therapies differentially affect SARS-CoV-2 vaccine-induced antibody and T cell immunity and function. JCI Insight. (2022) 7:doi: 10.1172/jci.insight.156978
Keywords: disease modifying treatment, glatiramer acetate, special populations, multiple sclerosis, comorbidities
Citation: Mirabella M, Annovazzi P, Brownlee W, Cohen JA, Kleinschnitz C and Wolf C (2022) Treatment Challenges in Multiple Sclerosis – A Continued Role for Glatiramer Acetate? Front. Neurol. 13:844873. doi: 10.3389/fneur.2022.844873
Received: 28 December 2021; Accepted: 09 March 2022;
Published: 15 April 2022.
Edited by:
Emilio Portaccio, Careggi University Hospital, ItalyReviewed by:
Maria Celica Ysrraelit, Fundación Para la Lucha Contra las Enfermedades Neurológicas de la Infancia (FLENI), ArgentinaRiley Bove, University of California, San Francisco, United States
Copyright © 2022 Mirabella, Annovazzi, Brownlee, Cohen, Kleinschnitz and Wolf. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Massimiliano Mirabella, bWFzc2ltaWxpYW5vLm1pcmFiZWxsYUBwb2xpY2xpbmljb2dlbWVsbGkuaXQ=; orcid.org/0000-0002-7783-114X
 Massimiliano Mirabella
Massimiliano Mirabella Pietro Annovazzi3
Pietro Annovazzi3 Jeffrey A. Cohen
Jeffrey A. Cohen Christoph Kleinschnitz
Christoph Kleinschnitz Christian Wolf
Christian Wolf